Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection
Abstract
:1. Introduction
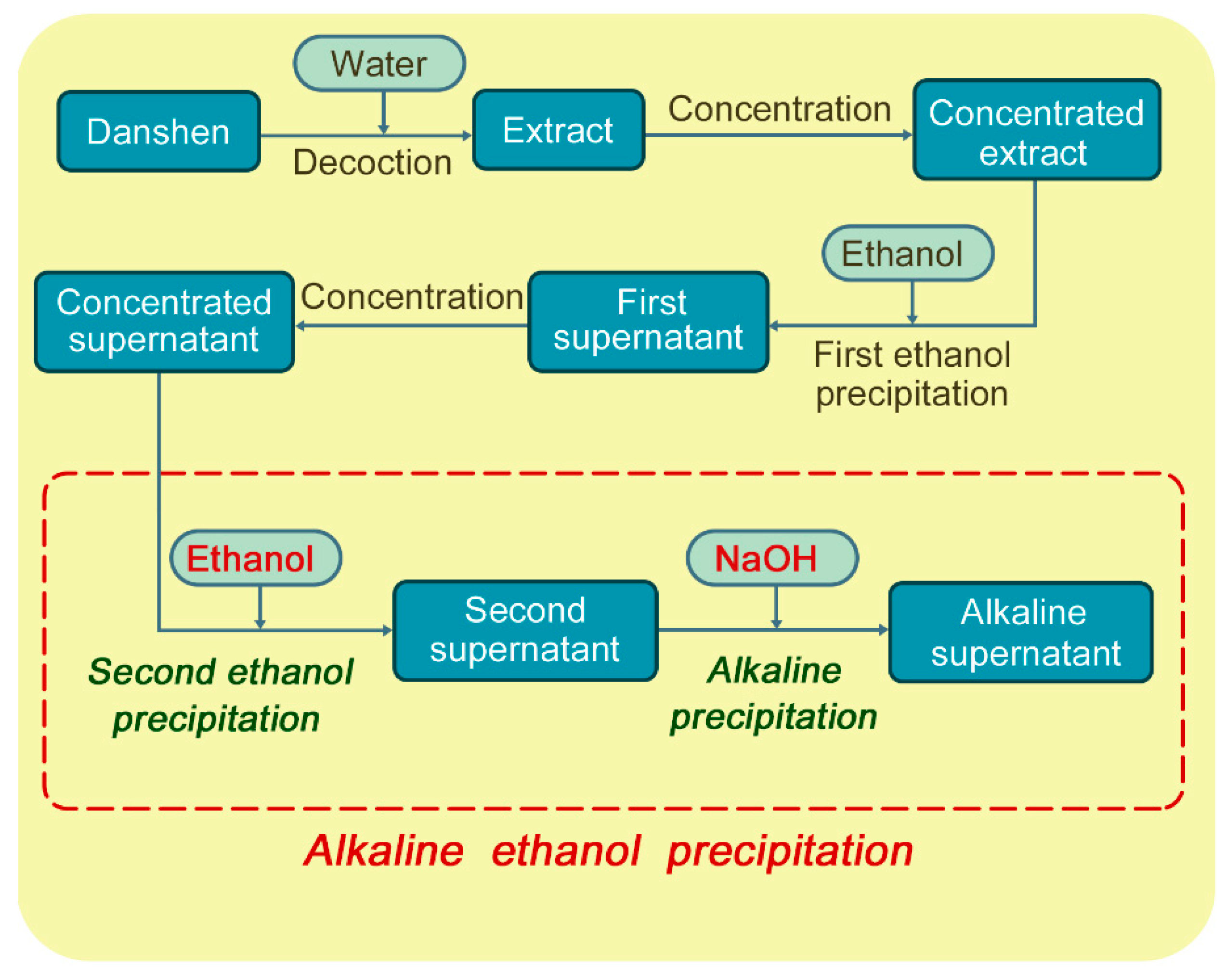
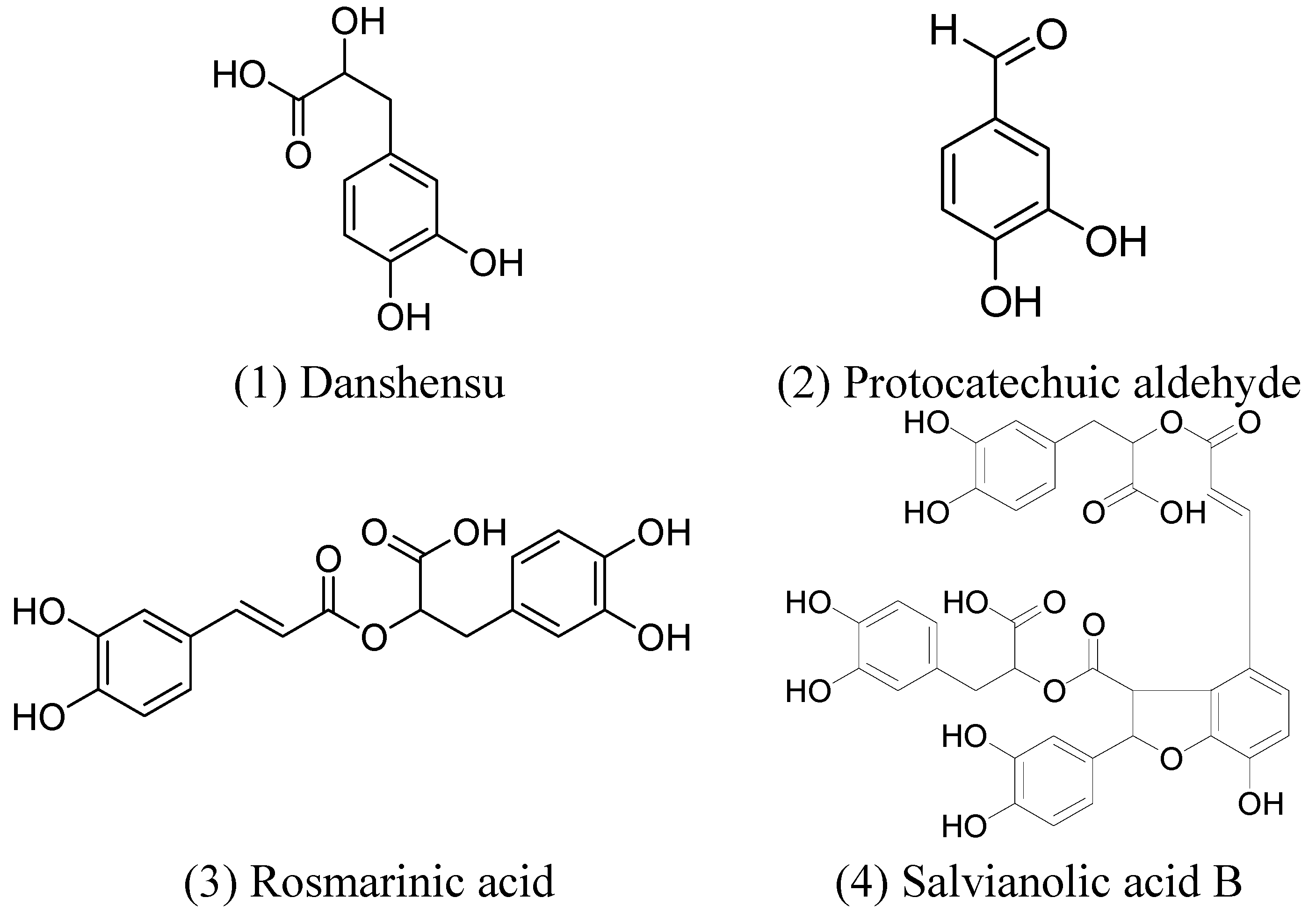
2. Results and Discussion
2.1. Composition of the Concentrated Supernatant
| Composition | Content (mg/g) | Composition | Content (mg/g) |
|---|---|---|---|
| Dry matter | 593.7 | Water content | 365.1 |
| DSS | 27.2 | PA | 4.18 |
| RA | 5.02 | SaB | 23.1 |
| Total tannin | 5.73 |
2.2. Effects of Refrigeration Time
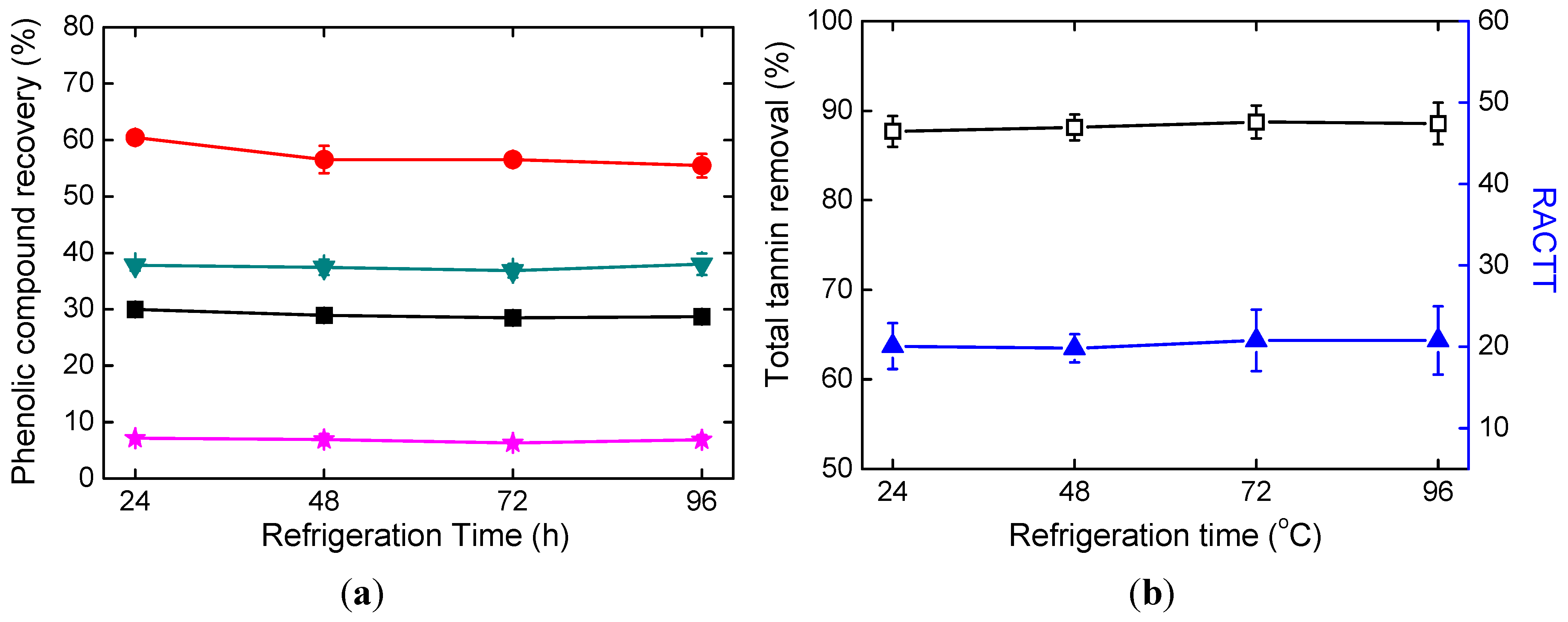
| Parameters | Refrigeration Time | Refrigeration Temperature | Ethanol Amount | NaOH Solution Amount |
|---|---|---|---|---|
| TTR | 0.899 | 0.001 a | 0.000 a | 0.000 a |
| PCRDSS | 0.245 | 0.000 a | 0.000 a | 0.000 a |
| PCRPA | 0.030 | 0.000 a | 0.002 a | 0.000 a |
| PCRRA | 0.769 | 0.001 a | 0.000 a | 0.000 a |
| PCRSaB | 0.567 | 0.000 a | 0.000 a | 0.000 a |
| RECTT | 0.975 | 0.006 a | 0.156 | 0.003 a |
2.3. Effects of Refrigeration Temperature
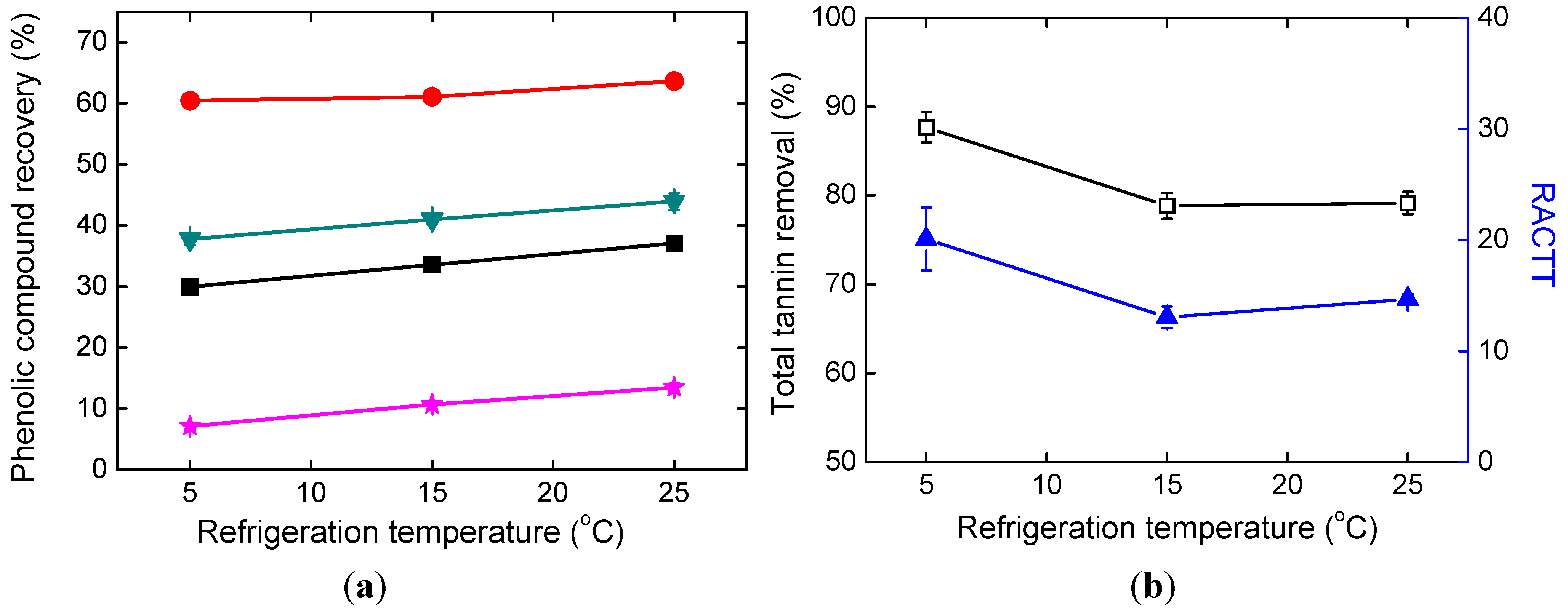
2.4. Effects of Alkali Addition
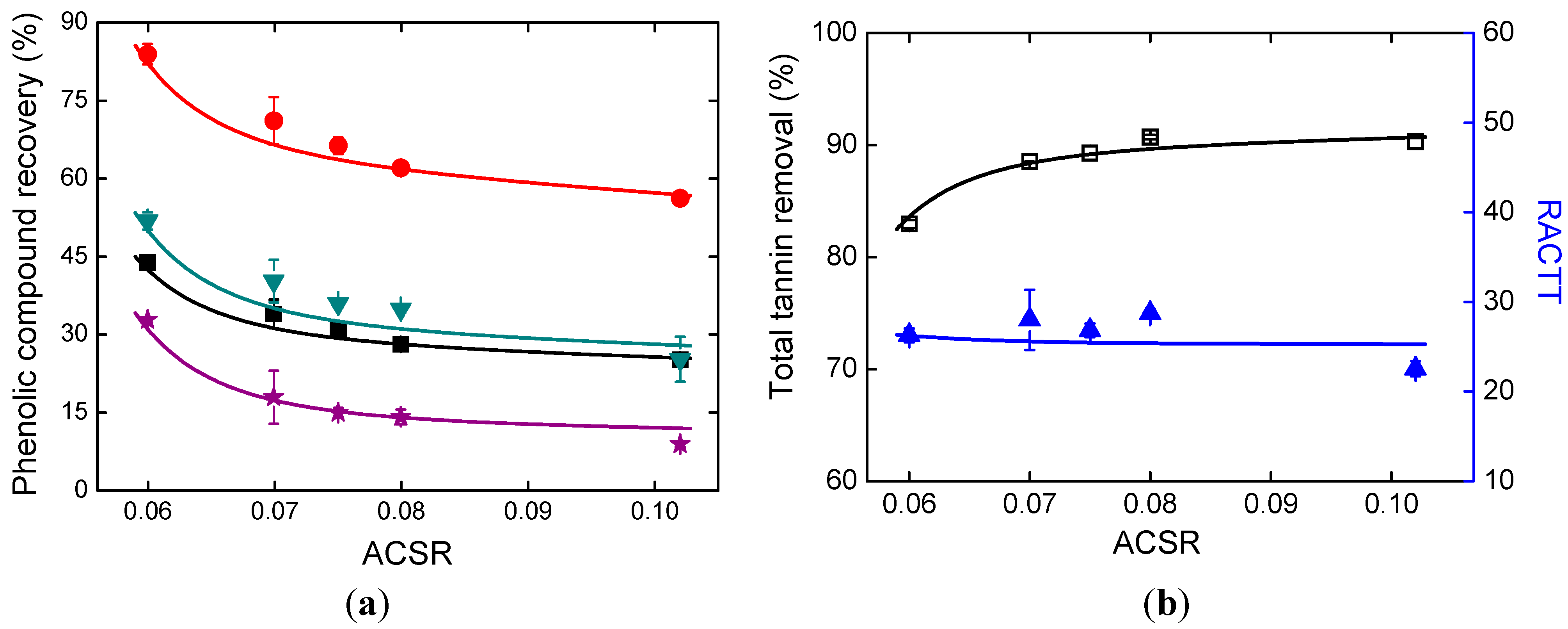
2.5. Effects of Ethanol Addition
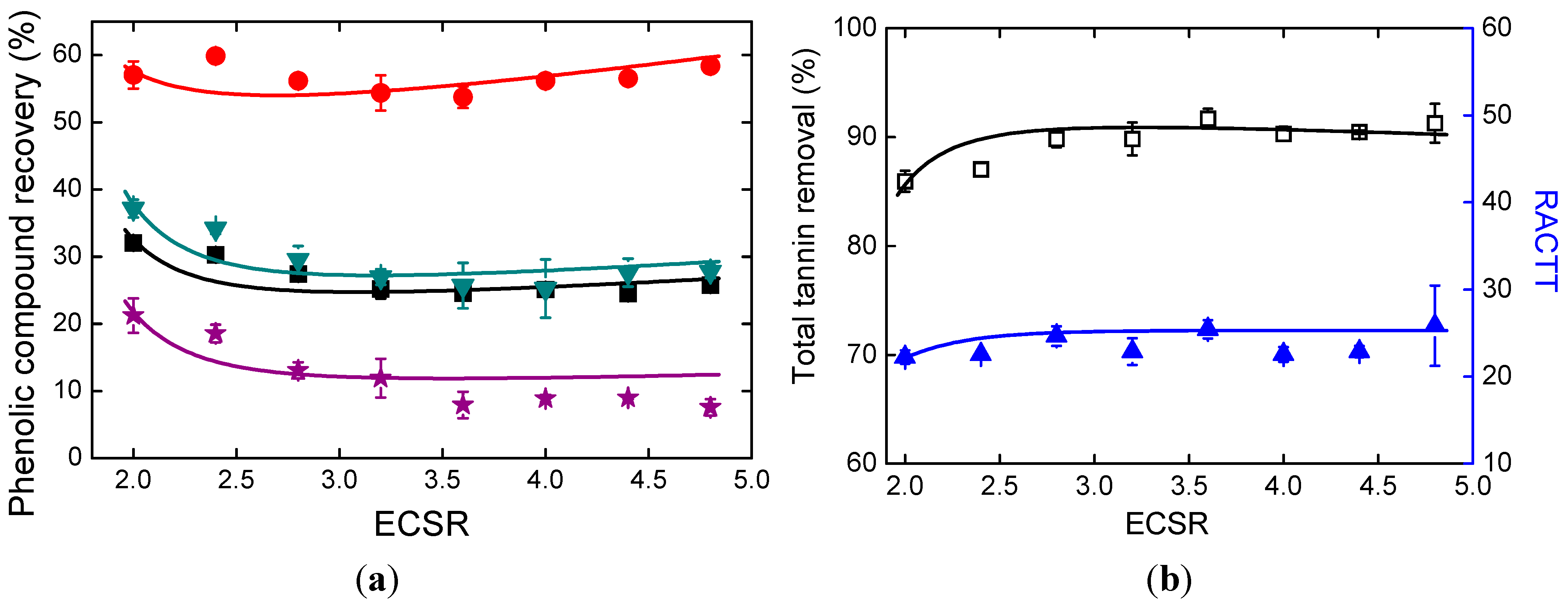
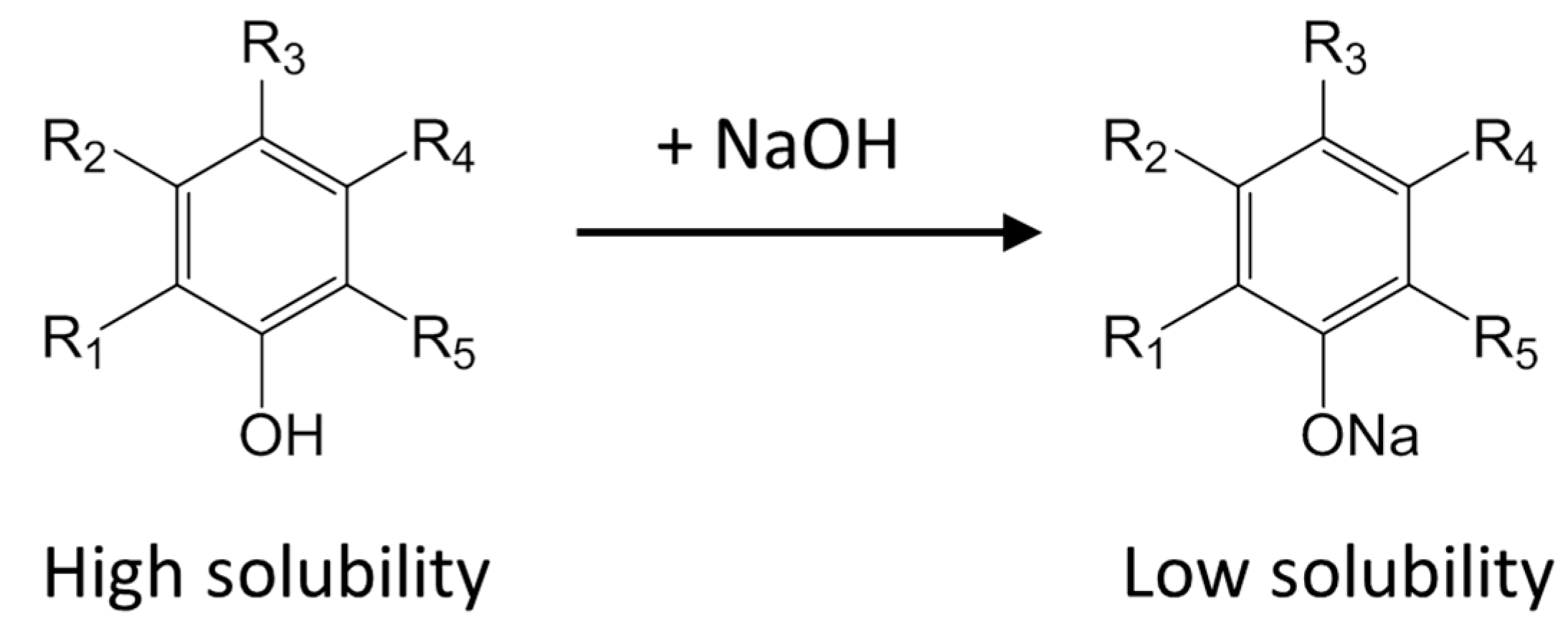
2.6. Mechanism of Phenolic Compound Loss and Tannin Removal
| NO. | ECSR | Refrigeration Temperature (°C) | Refrigeration Time (h) | ACSR | Alkaline Supernatant pH Value | MSPCS (g/g) |
|---|---|---|---|---|---|---|
| 1 | 2.0 | 5 | 24 | 0.102 | 8.614 ± 0.614 | 2.418 ± 0.001 |
| 2 | 2.4 | 5 | 24 | 0.102 | 8.658 ± 0.031 | 2.753 ± 0.005 |
| 3 | 2.8 | 5 | 24 | 0.102 | 8.937 ± 0.044 | 3.026 ± 0.003 |
| 4 | 3.2 | 5 | 24 | 0.102 | 9.024 ± 0.175 | 3.257 ± 0.062 |
| 5 | 3.6 | 5 | 24 | 0.102 | 9.185 ± 0.119 | 3.511 ± 0.045 |
| 6 | 4.0 | 5 | 24 | 0.102 | 9.101 ± 0.067 | 3.827 ± 0.018 |
| 7 | 4.4 | 5 | 24 | 0.102 | 9.046 ± 0.041 | 4.054 ± 0.052 |
| 8 | 4.8 | 5 | 24 | 0.102 | 9.150 ± 0.089 | 4.482 ± 0.082 |
| 9 | 4.0 | 5 | 24 | 0.060 | 7.350 ± 0.022 | 4.416 ± 0.016 |
| 10 | 4.0 | 5 | 24 | 0.070 | 8.036 ± 0.134 | 4.289 ± 0.053 |
| 11 | 4.0 | 5 | 24 | 0.075 | 8.282 ± 0.115 | 4.227 ± 0.026 |
| 12 | 4.0 | 5 | 24 | 0.080 | 8.413 ± 0.097 | 4.167 ± 0.034 |
| 13 | 4.0 | 5 | 24 | 0.082 | 8.483 ± 0.042 | 4.170 ± 0.005 |
| 14 | 4.0 | 5 | 48 | 0.082 | 8.532 ± 0.070 | 4.156 ± 0.089 |
| 15 | 4.0 | 5 | 72 | 0.082 | 8.413 ± 0.047 | 4.248 ± 0.028 |
| 16 | 4.0 | 5 | 96 | 0.082 | 8.412 ± 0.096 | 4.214 ± 0.058 |
| 17 | 4.0 | 15 | 24 | 0.082 | 8.550 ± 0.047 | 4.293 ± 0.013 |
| 18 | 4.0 | 25 | 24 | 0.082 | 8.369 ± 0.014 | 4.425 ± 0.020 |
| Phenolic Compounds | RPPC (%) | PCR + RPPC (%) |
|---|---|---|
| DSS | 67.3 ± 6.5 | 97.2 ± 6.2 |
| PA | 29.3 ± 3.0 | 89.8 ± 3.4 |
| RA | 59.7 ± 6.4 | 97.5 ± 6.4 |
| SaB | 91.6 ± 8.9 | 98.7 ± 9.1 |
2.7. Modeling
| Phenolic Compounds | DSS | PA | RA | SaB | TT |
|---|---|---|---|---|---|
| Ka,Eth | 4.78 | 5.20 | 4.88 | 5.28 | 4.52 |
| α | 14.7 | 10.5 | 14.6 | 14.2 | 16.7 |
| β (mg/g) | 10.9 | 3.75 | 2.20 | 4.27 | 0.836 |
| AAD (mg/g) | 0.102 | 0.0198 | 0.0307 | 0.119 | 0.0132 |
| ARD (%) | 4.37 | 2.71 | 6.77 | 18.4 | 7.32 |
| Parameters | Values | P value | R2adj |
|---|---|---|---|
| a0 | 2.456 | 0.000 | 0.996 |
| a1 | 0.704 | 0.000 | |
| a2 | −14.04 | 0.000 | |
| b0 | 12.05 | 0.000 | 0.980 |
| b1 | −2.02 | 0.000 | |
| b2 | −0.249 | 0.000 |
3. Experimental Section
3.1. Materials and Chemicals
3.2. Procedures
3.3. Analytical Methods
4. Conclusions
Abbreviations
| A | activity |
| ACSR | the mass ratio of NaOH solution and the concentrated supernatant |
| AAD | the average absolute deviations [mg/g] |
| ARD | the average relative deviations [%] |
| DSS | Danshensu |
| ECSR | the mass ratio of ethanol and the concentrated supernatant |
| Ka | dissociation equilibrium |
| MAS | mass of supernatant [g] |
| MCS | mass of the concentrated supernatant [g] |
| MPRE | the mass of precipitation [g] |
| MSPCS | the mass of supernatant obtained from per gram concentrated supernatant [g/g] |
| NED | the number of experimental data |
| PA | protocatechuic aldehyde |
| PC | phenolic compound content [mg/g] |
| PCR | phenolic compound recovery [%] |
| QbD | quality by design |
| RA | rosmarinic acid |
| RACTT | the mass ratio of total active constituent and total tannin |
| RPPC | the ratio of precipitated phenolic compound [%] |
| RPTT | the ratio of precipitated tannins [%] |
| S | the solubility of HIPC [mg/g] |
| SaB | Salvianolic acid B |
| TAC | total active constituent [mg/g] |
| TT | total tannin content [mg/g] |
| TTR | the total tannin removal [%] |
| TTS | total tannin remained in supernatant [%] |
| w1 | water mass fraction in 95% (v/v) ethanol [%] |
| w2 | water mass fraction in the concentrated supernatant [%] |
| w3 | water mass fraction in NaOH solution [%] |
| w4 | ethanol mass fraction in the concentrated supernatant [%] |
| a0 | parameter calibrated to correlate MSPCS |
| a1 | parameter calibrated to correlate MSPCS |
| a2 | parameter calibrated to correlate MSPCS |
| b0 | parameter calibrated to correlate pH |
| b1 | parameter calibrated to correlate pH |
| b2 | parameter calibrated to correlate pH |
Greek Symbols
| α | slope parameter for pKa calculation |
| β | slope parameter for solubility calculation [mg/g] |
| γ | activity coefficient |
| Ø | water mass fraction in the mixture of ethanol and water of alkaline supernatant [%] |
Subscripts
| AS | alkaline supernatant |
| Cal | calculated values |
| CS | the concentrated supernatant |
| Eth | ethanol |
| Exp | experimental values |
| IF | ionized phenolic compounds |
| PRE | precipitation |
| UF | unionized phenolic compounds |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.D.; Li, J. Analysis of adverse reactions of Danshen injection preparations. China J. Chin. Mat. Med. 2004, 29, 270–272. [Google Scholar]
- Wang, Z.Y.; Wang, Y.Q.; Li, M. Analysis of 302 ADR cases caused by Danshen injection. China Pharm. 2010, 21, 3035–3037. [Google Scholar]
- Meng, R.; Ren, X.Y.; Lan, P.Y. Analysis on problems of TCM injection included in national essential drugs list: A case study of Danshen injection. China Pharm. 2010, 21, 2606–2608. [Google Scholar]
- Sun, Y.; Li, A.M.; Zhang, Q.X.; Chen, J.L.; Fu, D.F.; Wang, S.H. Adsorptive separation of tannic acid from aqueous solution by polymeric resins. Sep. Sci. Technol. 2008, 43, 389–402. [Google Scholar] [CrossRef]
- Buso, A.; Balbo, L.; Giomo, M.; Farnia, G.; Sandona, G. Electrochemical removal of tannins from aqueous solutions. Ind. Eng. Chem. Res. 2000, 39, 494–499. [Google Scholar] [CrossRef]
- Murugananthan, M.; Raju, G.B.; Prabhakar, S. Removal of tannins and polyhydroxy phenols by electro-chemical techniques. J. Chem. Technol. Biot. 2005, 80, 1188–1197. [Google Scholar] [CrossRef]
- Gnanamani, A.; Sekaran, G.; Babu, M. Removal of tannin from cross-linked and open chain polymeric tannin substrates using heme peroxidases of Phanerochaete chrysosporium. Bioproc. Biosyst. Eng. 2001, 24, 211–217. [Google Scholar] [CrossRef]
- Boye, B.; Farnia, G.; Sandona, G.; Buso, A.; Giomo, M. Removal of vegetal tannins from wastewater by electroprecipitation combinedwith electrogenerated Fenton oxidation. J. Appl. Electrochem. 2005, 35, 369–374. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Suchithra, P.S. Adsorptive characteristics of tannin removal from aqueous solutions and coir industry effluents using calcined and uncalcined hydrotalcites. Ind. Eng. Chem. Res. 2007, 46, 4606–4613. [Google Scholar] [CrossRef]
- Sreeram, K.J.; Saravanabhavan, S.; Rao, J.R.; Nair, B.U. Use of chromium-collagen wastes for the removal of tannins from wastewaters. Ind. Eng. Chem. Res. 2004, 43, 5310–5317. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of tannin from aqueous solutions by cationic surfactant-modified bentonite clay. J. Colloid Interf. Sci. 2006, 299, 116–124. [Google Scholar] [CrossRef]
- Liao, X.P.; Shi, B. Selective removal of tannins from medicinal plant extracts using a collagen fiber adsorbent. J. Sci. Food Agr. 2005, 85, 1285–1291. [Google Scholar] [CrossRef]
- Ma, L. Quality Evaluation and Study on Quality Standards of Guanxinning Injection. Master’s Thesis, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Zhang, L.; Gong, X.; Qu, H. Optimizing the alcohol precipitation of Danshen by response surface methodology. Sep. Sci. Technol. 2013, 48, 977–983. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, B.J.; Gong, X.C.; Yu, L.X.; Qu, H.B. Application of quality by design to the process development of botanical drug products: A case study. Aaps PharmSciTech 2013, 14, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.A.G.; Vaz, A.F.M.; Correia, M.T.S.; Pessoa, A.; Carneiro-da-Cunha, M.G. Purification of bromelain from pineapple wastes by ethanol precipitation. Sep. Purif. Technol. 2012, 98, 389–395. [Google Scholar] [CrossRef]
- Gong, X.C.; Wang, S.S.; Qu, H.B. Comparison of Two Separation Technologies Applied in the Manufacture of Botanical Injections: Second Ethanol Precipitation and Solvent Extraction. Ind. Eng. Chem. Res. 2011, 50, 7542–7548. [Google Scholar] [CrossRef]
- Golunski, S.; Astolfi, V.; Carniel, N.; de Oliveira, D.; di Luccio, M.; Mazutti, M.A.; Treichel, H. Ethanol precipitation and ultrafiltration of inulinases from Kluyveromyces marxianus. Sep. Purif. Technol. 2011, 78, 261–265. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Guo, Z.; Qu, H. Control the effects caused by noise parameter fluctuations to improve pharmaceutical process robustness: A case study of design space development for an ethanol precipitation process. Sep. Purif. Technol. 2014, 132, 126–137. [Google Scholar] [CrossRef]
- Vogt, F.G.; Kord, A.S. Development of Quality-By-Design Analytical Methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Han, J.; Li, H.F.; Fan, L.; Liu, A.H.; Guo, D.A. Analysis on the stability of total phenolic acids and salvianolic acid B from Salvia miltiorrhiza by HPLC and HPLC-MSn. Nat. Prod. Commun. 2008, 3, 669–676. [Google Scholar]
- Xia, X.H.; Liu, M.; Zhang, Z.M.; Wu, Z.J.; Pan, S.Y. Antioxidant activity of hydrophilic components of danshen root in vitro. Chin. Arch. .Trad. Chin. Med. 2009, 27, 1085–1087. [Google Scholar]
- Cheng, T.O. Cardiovascular effects of Danshen. Int. J. Cardiol. 2007, 121, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Han, D.E.; Li, N.; Lu, Y.; Li, T.T.; Yang, S.Y.; He, J.K.; Chen, X.J. Simultaneous determination of six phenolic constituents of Danshen injection in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. Eur. J. Mass Spectrom. 2011, 17, 395–403. [Google Scholar] [CrossRef]
- Zhou, G.J.; Wang, W.; Xie, X.M.; Qin, M.J.; Kuai, B.K.; Zhou, T.S. Post-harvest induced production of salvianolic acids and significant promotion of antioxidant properties in roots of Salvia miltiorrhiza (Danshen). Molecules 2014, 19, 7207–7222. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Son, W.C.; Ryu, J.E.; Koo, B.A.; Kim, Y.S. Standardized Salvia miltiorrhiza Extract Suppresses Hepatic Stellate Cell Activation and Attenuates Steatohepatitis Induced by a Methionine-Choline Deficient Diet in Mice. Molecules 2014, 19, 8189–8211. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Shen, J.; Cheng, Y.Y.; Qu, H.B. Simultaneous detection of seven phenolic acids in Danshen injection using HPLC with ultraviolet detector. J. Zhejiang Univ. Sci. B 2008, 9, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.C.; Wang, S.S.; Li, Y.; Qu, H.B. Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep. Purif. Technol. 2013, 107, 273–280. [Google Scholar] [CrossRef]
- Getaz, D.; Butte, A.; Morbidelli, M. Model-based design space determination of peptide chromatographic purification processes. J. Chromatogr. A 2013, 1284, 80–87. [Google Scholar] [CrossRef]
- Espinosa, S.; Bosch, E.; Roses, M.; Valko, K. Change of mobile phase pH during gradient reversed-phase chromatography with 2,2,2-trifluoroethanol-water as mobile phase and its effect on the chromatographic hydrophobicity index determination. J. Chromatogr. A 2002, 954, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.C.; Yan, A.Y.; Qu, H.B. Optimization for the Ethanol Precipitation Process of Botanical Injection: Indicator Selection and Factor Influences. Sep. Sci. Technol. 2014, 49, 619–626. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Li, Y.; Qu, H. Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection. Molecules 2014, 19, 18705-18720. https://doi.org/10.3390/molecules191118705
Gong X, Li Y, Qu H. Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection. Molecules. 2014; 19(11):18705-18720. https://doi.org/10.3390/molecules191118705
Chicago/Turabian StyleGong, Xingchu, Yao Li, and Haibin Qu. 2014. "Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection" Molecules 19, no. 11: 18705-18720. https://doi.org/10.3390/molecules191118705
APA StyleGong, X., Li, Y., & Qu, H. (2014). Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection. Molecules, 19(11), 18705-18720. https://doi.org/10.3390/molecules191118705





