Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives
Abstract
:1. Introduction
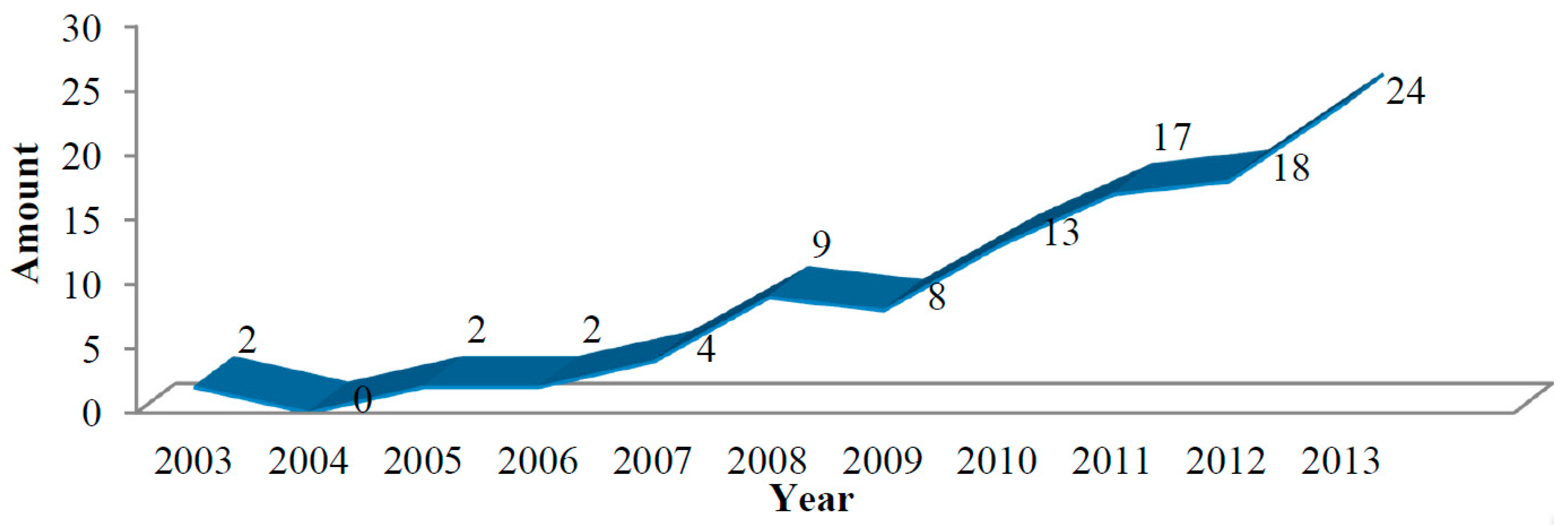
2. The Evolution of Spectrum-Effect Relationships
2.1. The Fingerprint
2.2. The Spectrum-Effect Relationship
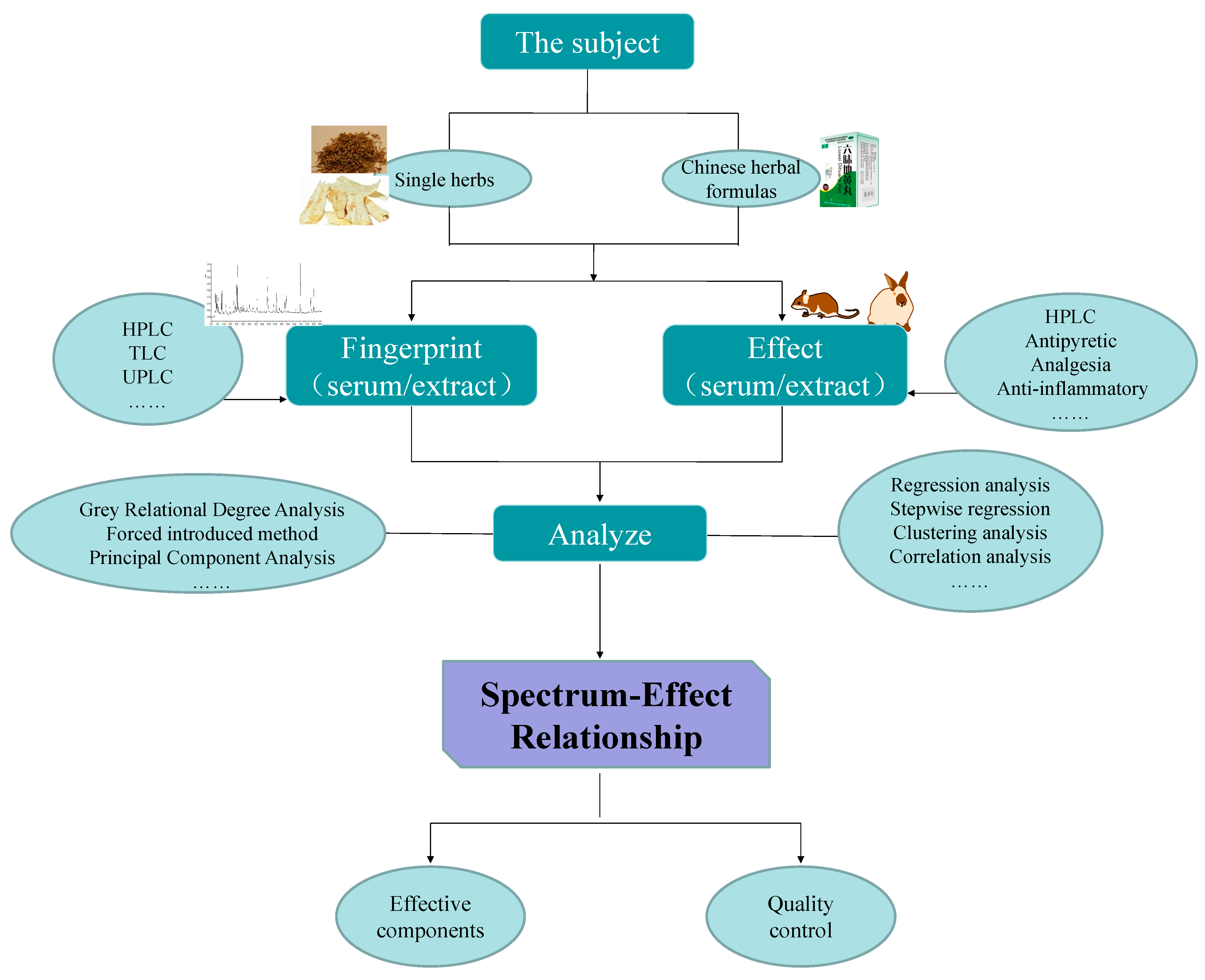
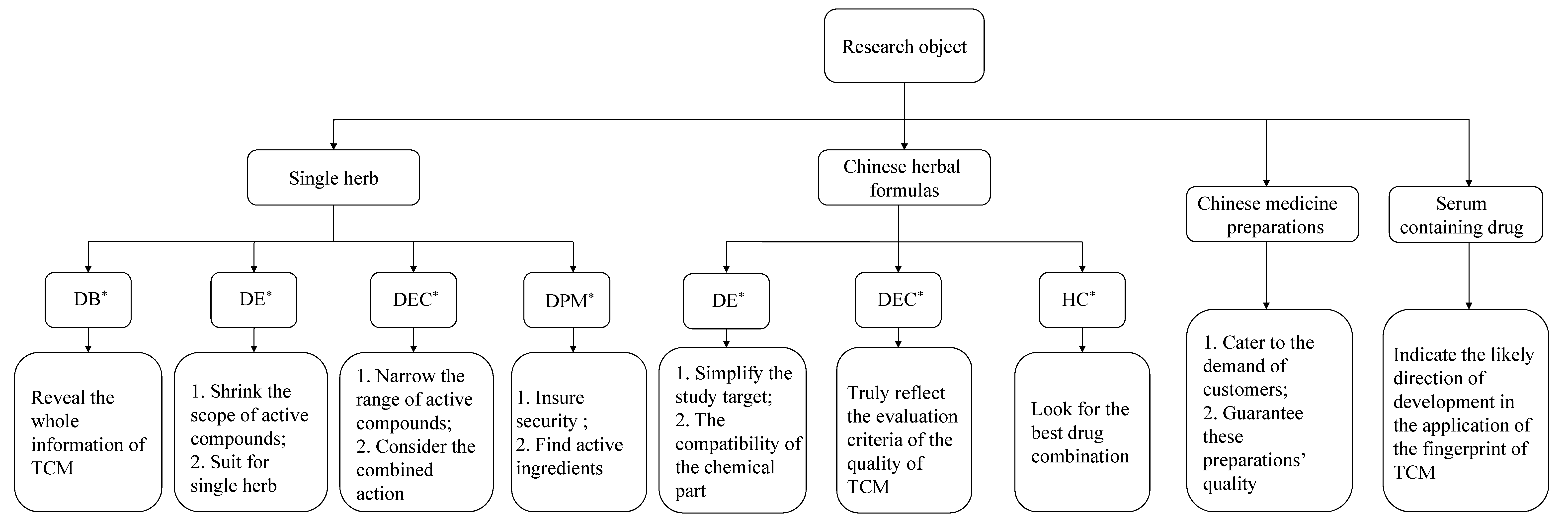
3. The Research Objects of the Spectrum-Effect Relationship Method
3.1. Single Herbs
3.2. Chinese Herbal Formulas
| Chinese Herb Medicine | Processing Method | Fingerprint | Effects | Experimental Model | Analytical Method |
|---|---|---|---|---|---|
| Vaccinium ashei Reade | DB | HPLC | Antioxidant effect | Scavenge DPPH radical | HCA [11]; |
| Bostaurus domesticus Gmelin. | DB DE | UPLC | Antibacterial effect | Escherichia coli | HCA, OMLR, PCA [12,13]; |
| Isatis indigotica Fort. | DB | HPLC | Antibacterial effect | Escherichia coli | HCA, PCA, OMLR [14,15]; |
| Psoralea corylifolia Linn. | DB | HPLC | Antioxidant effect | Scavenge DPPH radical | CA [19]; |
| Angelica sinensis (Oliv.) Diles | DB DPM | HPLC | Reinforcing Qi, Replenishing blood Scavenging free radicals | Mice Fenton reaction | GRDA [20]; OMLR [21]; |
| Cureuma kwangsiensis S. G. Lee et C. F. Liang | DB | GC | Antitumor effect | Nasopharygeal carcinoma cells | GRDA [22]; |
| C.kwangsiensis S. G. Lee et C. F. Liang stir-baked with vinegar | DB | HPLC | Dispersing blood stasis effect | Mice | GRDA [23]; |
| Polygoni cuspidati Sieb. et Zucc. | DB DE | HPLC | Antibacterial effect Anticancer effect | Scavenge DPPH radical Mice, Rat, Leukemia cell line K 562, Lung cancer cell line A 549 | BCA [24] PLSR, GRDA [25,26,27,28,29,30]; |
| Morina nepalensis D. Don var. alba (Hand. -Mazz.) Y. C. Tang | DB | HPLC | Anti-inflammatory effect | RAW 264.7 cells | PLSR [31]; |
| Juglansmandshurica | DE | TLC | Antitumor effect | BGC 803 cancer cells | CA [32]; |
| Paeoniae lactiflora Pall. | DE | HPLC | Cooling blood effect | Rat alveolar macrophage NR 8383 | PLSR [33,34]; |
| Rheum palmatum L. | DB DPM | UPLC HPLC | Anti-HIV-1 effect Hemostatic effect | HIV-1 reverse transcriptase Mice | PCA [35]; GRDA [36]; |
| Rheum officinale Baill. | DE | HPLC | Tyrosinase inhibitor | Tyrosinase | CA [37]; |
| Salvia miltiorrhiza Bunge | DB DE | HPLC | Anti-oxidation effect | Fenton reaction Mice | BCA [38]; PLSR [39]; |
| Erigeron breviscapus (Vant.) Hand-Mazz. | DEC | HPLC | Neuroprotective effects | SH-SY5Y cells | BCA [40]; |
| Euphorbia humifusa Willd. | DE | HPLC | Antifungal effect | NCCLS M 38-A | GRDA [41]; |
| Cordyceps sinesis (Berk.) Sacc. | DB | HPLC | Anti-hepatic fibrosis effect | LX-2 hepatic stellate cells | BCA [42]; |
| Alpinia officinarum Hance. | DE | HPLC | Promotion of melanogenesis effect | Melanoma B 16 cells | GRDA [43]; |
| Pogostemon cahlin (Blanco) Benth. | DE | HPLC | Anti-gastrointestinal propulsion effect | Mice | GRDA [44]; |
| Polygonum orientale L. | DEC | UPLC | Protective effect on myocardial cells | Myocardial cells | BCA [45]; |
| Coptis chinensis Franch. | DB DPM | HPLC UPLC | Ameliorating insulin resistanc Antibacterial effect | 3T3-L1 preadipocyte ATP bioluminescence | PCA,CA,GRDA [46,47]; HCA [48]; OMLR [49]; |
| Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao | DB DEC | HPLC | Improving immunity effect Anti-gastric ulcer effect Diuretic effect Antifatigue effect | Mice | GRDA [50]; PLSR,GRDA [41,42,43,44,45,46,47,48,49,50,51,52,53]; GRDA [54]; |
| Scutellaria baicalensis Georgi | DB | HPLC | Antipyretic effect Antibaterial effect | Rat
Staphylococcus aureus | GRDA [55]; GRNN [56]; |
| Bupleurum chinense DC. | DE | HPLC | Hepatoprotective effect | Mice | HCA, TCA [57]; |
| Tinospora sagittata (Oliv.) Gagnep. | DB DE | HPLC | Anti-inflammatory effect Analgesia effect | Mice | CA [58]; |
| Lonicera japonica Thunb. | DB DE | HPLC | Anti-influenza virus effect Anti-inflammatory effect; Analgesia effect | MDCK cells Mice | OMLR [59,60]; |
| Murraya exotica L. | DE | HPLC | Anti-inflammatory effect | Mice | GRDA [61]; |
| Zanthoxylum nitidum (Roxb.) DC. | DB | IR | Antitumor effect Antineoplastic effect | 7901, Hela cells | OMLR [62,63,64] |
| Kalimeris indica (L.) Sch-Bip | DB | HPLC | Anti-inflammatory effect | Mice | GRDA [65] |
| Citrus grandis (L.) Osbeck | DE | HPL C | Antioxidant effect | KMnO4 | GRDA, CA, GRNN [66]; |
| Paeonia suffruticosa Andr. | DE | HPLC | Promote blood circulation Remove blood stasis | Mice | OMLR [67]; |
| Cnidium monnieri L.Cuss. | DE | HPLC | Sedative-hypnotic effect | Mice | CA [68]; |
| Artemisia capillaries Thunb. | DE | HPLC | Hepatoprotective effect | Mice | CA [69]; |
| Crataegus pinnatifida Bge. | DB | HPLC | Antioxidant effect | Rat | CA [70]; |
| Peucedunum harrysmithii var. subglabrum (shan et sheh) | DB | HPLC | Eliminate phlegm effect | Mice | GRDA [71]; |
| Evodia ruatecarpa (Juss.) Bneht. Var. bodinieri (Dode) Huang | DE | HPLC | Alleviate intestinal cramps effect | Rabbit | CA [72]; |
| Pseudostellaria heterophylla (Miq.) Pax | DE | HPLC | Cytotoxic effect | MGC 80-3, RKO, HepG2 cells | CA [73]; |
| Panax notoginseng (Burk.) F.H.Chen | DB | HPLC | Anti-myocardial ischemia effect | Rat | CA, FMA, PCA [74]; |
| Aconitum carmichaelii Debx. | DPM | UPLC | Mitochondria growth promoting effect | Rat | CA [75]; |
| Aconitum L. | DB | UPLC | Antibacterial effect | Escherichia coli | CA [76]; |
| Names | Involved TCMs | Fingerprint | Effects | Experimental Model | Analytical Method |
|---|---|---|---|---|---|
| Baihu Tang | Anemarrhena asphodeloides Bge. Gypsum fibrosum Glycyrrhiza uralensis Fisch. Rice | HPLC | Anti-inflammatory effect | Rat | BCA [77] |
| Danggui Chuanxiong | A. sinensis (Oliv.) Diels. Ligusticum chuanxiong Hort. | HPLC | Anti-myocardial ischaemia effect | Rat | BCA, OMLR [78] |
| Mongolian Preparation Sendeng-4 Decoction | Xanthoceras sorbifoliae Bunge. Melia toosendan Sieb.et Zucc Terminalia chebula Retz. Gardenia jasminoides Ellis. | HPLC | Anti-inflammatory and analgesic effect | Mice | OMLR [79] |
| Compound Wuren chun Capsules | Schisandra chinensis (Turcz.) Baill. B. chinense DC. P.notoginseng (Burk.) F. H. Chen Phyllanthus urinaria L. | HPLC | Liver protection | Rat | CA [80] |
| Gushu Dan | Epimedium brevicornu Maxim. Drynaria fortunei(Kunze) J. Sm. C. monnieri (L.) Cuss. S. miltiorrhiza Bge. | HPLC | The proliferative effect of osteoblast-like cells | Osteoblast-like cells | BCA, OMLR [81] |
| Tongsaimai Pellet | A. membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao G. uralensis Fisch. Lonicerae japonicae Thunb. Scrophularia ningpoensis Hemsl. Dendrobium nobile Lindl. A. sinensis (Oliv.) Diels. | UPLC | Brain protection Vasodilatation effect PC12 cell injury protection; | Rat, Rabbit, PC 12 cells | HCA [82] |
| Xiaoyao Wan | B. chinense DC. G. uralensis Fisch. A. sinensis (Oliv.) Diels. Paeonia lactiflora Pall. Atractylodes macrocephala Koidz. Poria cocos (Schw.) Wolf Mentha haplocalyx Briq. Zingiber officinale Rosc. | HPLC GC | Anti-tyrosinase effect Anti-depression effect | B 16 melanoma cells Mice | BCA [83,84] |
| Jia Wei Si Miao Decoction | Atractylodes lancea (Thunb.) DC. Phellodendron chinense Schneid. Achyranthes bidentata Blume Coix lacryma-jobi L. var. mayuen. (Roman.) Stapf | GC HPLC | Anti-inflammatory effect Analgesia effect Decrease blood uric acid | Mice | OMLR, CA [85,86] |
| Ling Gui Shu Gan Tang | P. cocos (Schw.) Wolf Cinnamomum cassia Presl A. macrocephala Koidz. G. uralensis Fisch. | HPLC | Diuretic effect Anti-hypoxic effect | Mice | OMLR [87] |
| Shaoyao Gancao formulas | P. lactiflora Pall. G. uralensis Fisch. | HPLC | Analgesic effect | Mice | CA [88] |
| Qi Zhi Wei Tong | B. chinense DC. Corydalis yanhusuo W.T.Wang Citrus aurantium L. Cyperus rotundus L. P. lactiflora Pall. G. uralensis Fisch. | HPLC | Anti-inflammatory effect | RAW 264. 7 cells | GRDA, GRNN [89] |
| Sheng Hua Tang | A. sinensis (Oliv.) Diels L. chuanxiong Hort. Prunus persica (L.) Batsch Zingiber offcinale Rosc. G. uralensis Fisch. | HPLC | Invigorate the circulation of Qi | Rat | CA [90] |
| Tao Hong Si Wu Tang | P. persica (L.) Batsch Carthamus tinctorius L. A. sinensis (Oliv.) Diels. Paeonia veitchii Lynch Rehmannia glutinosa Libosch. L. chuanxiong Hort. | GC | Analgesic effect | Mice | OMLR, CA [91] |
| Wu Zhu Yu Tang | Evodia rutaecarpa (Juss.) Benth Panax ginseng C. A. Mey. Z. officinale Rosc. Ziziphus zizyphus Mill. | HPLC | Analgesic effect Anti-nausea effect | Mice | OMLR [92] |
| Xie Bai San | Morus alba L. Lycium chinesnse Mill. G. uralensis Fisch. | HPLC | Anti-inflammatory effect Expectorant effect | Mice | OMLR, BCA [93] |
| Zuo Jin Wan | C. chinensis Franch. E. rutaecarpa (Juss.) Benth. | HPLC | Biothermo-logical effect | Escherichia coli | CA [94] |
| Da Cheng Qi Tang | R. palmatum L. Magnolia officinalis Rehd. et Wils. C. aurantium L. | HPLC | Purgative effect | Mice | HCA [95] |
3.3. Chinese Medicine Preparations
3.4. Drug-Containing Serum
| Names | Type | Fingerprint | Effects | Experimental Model | Analytical Method |
|---|---|---|---|---|---|
| Radix Astragali Injection | Chinese medicine preparation | HPLC | Antioxidant effect | Scavenge DPPH radical | PLSR [98]; |
| San Huang Preparation | Chinese medicine preparation | HPLC | Improve insulin resistance Antiendotoxin effect | Rat, The 3T3-L1 preadipocytes cells | OMLR, BCA, PCA [49,99]; |
| Xiang Dan Injection | Chinese medicine preparation | HPLC | Anti-myocardial ischemia effect | Rat | GRNN [100]; |
| Bu Zhong Yi Qi Wan | Serum containing drug | HPLC | Blood enriching effect | Mice | GRDA [101]; |
| Xiao Yao Fang | Chinese medicine preparation | HPLC | Anti-depression effect | Rat | GRDA [102]; |
| H. cordata Injection | Chinese medicine preparation | GC | Anti-inflammatory effect | Rat, Mice | HCA [96]; |
| Carthamus tinctorius L. | Serum containing drug | CE | Increase the coronary artery flow Enhance the heart stroke amplitude Decrease the heart rate | Rabbit Guinea pig | CA [103]. |
4. Methods for Establishing Fingerprints for Spectrum-Effect Relationships
5. Pharmacodynamics Studies for Spectrum-Effect Relationships
5.1. The Pharmacodynamics Studies of TCM Extracts
5.2. Pharmacodynamics Studies of Drug-Containing Serum
6. Data Processing Methods of Spectrum-Effect Relationships
7. Conclusions and Future Perspectives
| Method | Purpose | Advantages | Limits |
|---|---|---|---|
| CA | Study close degree between variable | Determine the relativity degree, significant extent and direction of change | Cannot explain the combined effect of the various peaks corresponding components to the pharmacodynamic indicators |
| HCA | Study the problem of classification, also known as group analysis | Intuitive, concise and achieve the classification | Cannot evaluate the correlation magnitude and the direction between fingerprint peaks and pharmacodynamic indicators |
| OMLR | Study a linear function to clarify the relationship between one dependent and two or more independent variables | Most commonly used method to study the intrinsic link | Not be able to see the contribution of peaks to the efficacy and not suitable for multiple correlation independent variables |
| PLSR | Allow the condition of the number of samples is less than that of variables to do regression modeling | Strong practicality and stability includes; Include all the original peaks of fingerprints | Abstract and difficult to understand; only suitable for qualitative analysis but not to determine the precise quantitative relationship between them |
| GRDA | Analyze the association degree of the various factors in system | Can use the known information to reveal unknown information | Difficult to describe overall contribution of the various peaks corresponding components through pharmacodynamic indicators |
| PCA | Elect fewer important variables from multiple variables through a linear transformation | Without loss of characteristic value number and information of sample | The amount of information after variable dimension reduction maintaining at a high level; The extracted principal component number being less than the original number of variables |
7.1. The Application of Advanced Modern Analytical Technology
7.2. Selecting Pharmacodynamic Models Close to the Clinical Efficacy
7.3. Choosing the Proper Data Processing Method
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Yan, J.B.; Liu, X.M.; Ye, Z.G.; Yang, X.H.; Meyboome, R.; Chan, K.; Shaw, D.; Duez, P. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: Current status and future perspective. J. Ethnopharmacol. 2012, 140, 519–525. [Google Scholar] [CrossRef]
- Li, R.; Yan, Z.Y.; Li, W.J.; Xu, T.; Tan, R.A.; Pan, L.; Li, Y.M.; Ma, Y.L. The establishment of chromatographic pharmacodynamics. Educ. Chin. Med. 2002, 21, 62. [Google Scholar]
- Liang, Y.Z.; Xie, P.S.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Yuan, R.; Yuan, L. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol. Ther. 2000, 86, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, G.; Dejaegher, B.; Smeyers-Verbeke, J.; Vander Heyden, Y. Recent Developments in Chromatographic Fingerprints from Herbal Products: Set-Up and Data Analysis. Comb. Chem. High Throughput Screen. 2010, 13, 900–922. [Google Scholar] [CrossRef]
- Li, P.; Qi, L.W.; Liu, E.H.; Zhou, J.L.; Wen, X.D. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Anal. Chem. 2008, 27, 66–77. [Google Scholar] [CrossRef]
- Xie, P.S. A feasible strategy for applying chromatography fingerprint to assess quality of Chinese herbal medicine. Tradit. Chin. Drug Res. Clin. Pharmacol. 2001, 3, 141–151. [Google Scholar]
- Li, G.S.; Liu, C.H.; Wang, H.S.; Zhang, L.J. Assaying of content of catalpol in Rehmanniaglutinosa from different origins. Chin. Tradit. Herb. Drugs 2002, 33, 126–128. [Google Scholar]
- Pan, R.J.; Guo, F.Q.; Lu, H.M.; Feng, W.W.; Liang, Y.Z. Development of the chromatographic fingerprint of Scutellaria barbata D. Don by GC–MS combined with Chemometrics methods. J. Pharm. Biomed. Anal. 2011, 55, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Dan, M.; Wu, J.B.; Yang, H.Z.; Huang, H.; Qi, Y.; Wei, S.D.; Toru, O.; Kaoru, N. Study on the chromatographic fingerprinting of Schisandra chinensis (Turcz.) Baill. by LC coupled with principal component analysis. Chromatographia 2008, 68, 101–104. [Google Scholar] [CrossRef]
- Sun, L.Q.; Ding, X.P.; Qi, J.; Yu, H.; He, S.A.; Zhang, J.; Ge, H.X.; Yu, B.Y. Antioxidant anthocyanins screening through spectrum-effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2012, 132, 759–765. [Google Scholar] [CrossRef]
- Kong, W.J.; Wang, J.B.; Zang, Q.C.; Xing, X.Y.; Zhao, Y.L.; Liu, W.; Jin, C.; Li, Z.L.; Xiao, X.H. Fingerprint-efficacy study of artificial Calculus bovis in quality control of Chinese material medica. Food Chem. 2011, 127, 1342–1347. [Google Scholar] [CrossRef]
- Zang, Q.C.; Wang, J.B.; Kong, W.J.; Jin, C.; Ma, Z.J.; Cheng, J.; Gong, Q.F.; Xiao, X.H. Searching for the main anti-bacterial components in artificial Calculus bovis using UPLC and microcalorimetry coupled with multi-linear regression analysis. J. Sep. Sci. 2011, 34, 3330–3338. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhao, Y.L.; Shan, L.M.; Xiao, X.H.; Guo, W.Y. Investigation on the spectrum-effect relationships of EtOAc extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J. Chromatogr. B 2008, 871, 109–114. [Google Scholar]
- Hu, X.Y.; Liu, M.H.; Sun, Q.; Zhang, S.J.; Jiang, L. Spectrum-effect relationship of antibacterial extracts from Isatidis Radix. Chin. Tradit. Herb. Drugs 2013, 44, 1615–1620. [Google Scholar]
- Li, Y.; Ma, L.; Shen, P.; Gong, M.X. Spectrum-effect relationships between HPLC fingerprints and antiendotoxin activity of chloroform extract from Isatidis Radix. Chin. Pharm. J. 2011, 46, 741–744. [Google Scholar]
- Zhao, Y.L.; Cao, L.; Wang, J.B.; Jin, C.; Xiao, X.H. Study on the n-BuOH extracts fingerprint chromatography and bacteriostatic activity of Radix Isatis and correlation analysis. Chin. Tradit. Pat. Med. 2005, 28, 1079–1082. [Google Scholar]
- Sun, Q.; Ma, L.; Li, L.; Hu, X.Y.; Jiang, L.; Xiao, X.H. Spectrum-activity relationship of hemagglutination components in Isatidis Radix. Chin. Tradit. Herb. Drugs 2012, 43, 125–130. [Google Scholar]
- Chang, Y.X.; Zhu, Z.W.; Li, J.; Zhang, Q.H.; Kang, L.Y.; Zhang, B.L. Research on antioxidant activity fingerprint of Psoralea corylifolia L. Tianjin J. Tradit. Chin. Med. 2011, 28, 158–160. [Google Scholar]
- Yang, Y.L.; Hu, F.; Liu, X.F.; Guo, L.; Yang, T.; Li, Y.D.; Feng, S.L. Spectrum-effect relationship of active fraction from Angelicae Sinensis Radix with effect of reinforcing Qi. Chin. Tradit. Herb. Drugs 2013, 44, 3346–3351. [Google Scholar]
- Guo, Y.S.; Hua, Y.L.; Deng, H.J.; Wei, Y.M. Relationship between HPLC fingerprint chromatogram and scavenging free radicals of different processed products of Radix Angelicae sinensi. Chin. Tradit. Pat. Med. 2010, 32, 2107–2111. [Google Scholar]
- Zeng, J.H.; Mo, X.Y.; Dai, P.; Huang, F.X.; Liao, Y.; Wang, J.H.; Chen, X. Study on spectrum-effect relationship between fingerprint of essential oil and of anti-tumor effect from curcuma kwangsiensis. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 91–94. [Google Scholar]
- Liao, W.; Zhang, J.M.; Fu, S.; Wang, J.S.; Fu, C.M. Chromatography-activity relation of stagnation of vital energy and blood stasis syndrome influencing hemorheology by stir-baked Curcumae Rhizoma with vinegar. Chin. Tradit. Pat. Med. 2013, 35, 330–334. [Google Scholar]
- Luo, Z.J.; Xu, Y.; Wu, J.Y.; Zhang, Z.F. Spectrum-effect relationship between HPLC fingerprints and antioxidant activity of Polygonum cospidatum. J. Southwest Univ. 2012, 34, 138–142. [Google Scholar]
- Peng, J.B.; Li, Q.; Lei, H.M.; Long, H.L.; Zhou, Y.X. Effective compositions of Polygoni Cuspidati study (I): Study on spectrum-effect relationship about clotting time. China J. Tradit. Chin. Med. 2011, 26, 1737–1739. [Google Scholar]
- Peng, J.B.; Li, Q.; Si, Y.C.; Long, H.L.; Lei, H.M.; Xiao, H.B. Study on the effective compositions of Polygoni Cuspidati II: Spectrum-effect relationship studies about pharmacodynamic of whole blood viscosity and HPLC. China J. Tradit. Chin. Med. 2011, 26, 1964–1966. [Google Scholar]
- Long, H.L.; Li, Q.; Si, Y.C.; Peng, J.B.; Lei, H.M.; Xiao, H.B. Spectrum-effect relationship study on influence of effective compositions research III of Polygoni Cuspidati on plasma viscosity. Mod. Med. Health 2011, 20, 3080–3082. [Google Scholar]
- Long, H.L.; Lei, H.M.; Li, Q.; Peng, J.B.; Si, Y.C. Effective compositions of Polygoni Cuspidati study (IV): Study on spectrum-effect relationship about fibrinogen. Guide China Med. 2012, 33, 4–5. [Google Scholar]
- Li, Q.; Xia, X.H.; Lei, H.M.; Xiao, H.B.; Song, Y.B. Effective Compositions of Polygoni Cuspidati (V): Study on spectrum-effect relationship of blood sedimentation rate. Acta Chin. Med. Pharmacol. 2011, 39, 63–65. [Google Scholar]
- Chen, H.G.; Zhao, H.B.; Zhao, C.; Zhou, X. HPLC fingerprint analysis of the extract from Polygonum cuspidatum and associated pharmacodynamics. China Pharm. 2010, 21, 1775–1776. [Google Scholar]
- Luo, P.; Liu, Y.; Lv, L.Y.; Zhang, Z.F. Spectrum-effect correlation analysis of traditional Tibetan medicine “Morinanepalensis” on nitric oxide production inhibition. China J. Chin. Mater. Med. 2013, 17, 2882–2885. [Google Scholar]
- Zhang, L.J.; Guan, J.; Liu, L.J. Preliminary exploration of antitumor pprofile-effect of fresh rejuvenated fruits of Juglans Mandshurica. Prog. Mod. Biomed. 2010, 10, 751–752. [Google Scholar]
- Song, Y.C.; Ma, H.B.; Cui, X.R.; Zhang, Q.; Lian, C.J.; Li, Q.; Lei, H.M. Spectrum-effect relationship between LC-MS fingerprint chromatogram and cell inhibitory rate of Paeoniae Radix Rubra and Paeoniae Radix Alba. Drug Clin. 2012, 27, 103–106. [Google Scholar]
- Song, Y.C.; Cui, X.R.; Lian, C.J.; Ma, H.B.; Zhang, Q.; Li, Q.; Lei, H.M. Comparative study on cooling blood between Radix Paeniae Rubra and Radix Paeoniae Alba (II): Spectrum-effect analysis on inhibitory apoptosis of LPS-stimulated rat alveolar macrophages. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 181–184. [Google Scholar]
- Ma, P.; Zhang, X.Y.; Xu, L.J.; Wang, Z.; Xiao, P.G. Spectrum-effect relationship between anti-HIV 1 activities and ultra-performance liquid chromatography fingerprints of Rheum species. China J. Chin. Mater. Med. 2013, 15, 2434–2437. [Google Scholar]
- Zhu, S.T.; Lei, P.; Li, X.Z.; Li, Y.L. Grey relational analysis fingerprint and hemostatic function of Rheum palmatum L. Cent. South Pharm. 2009, 7, 55–58. [Google Scholar]
- Liu, Y.J.; Wang, Q.; Jiang, M.; Li, H.Y.; Zou, M.J.; Bai, G. Screening of effective components for inhibition of tyrosinase activity in rhubarb based on spectrum-efficiency-structure-activity relationship. Chin. Tradit. Herb. Drugs 2012, 43, 2120–2126. [Google Scholar]
- Sun, G.X.; Gao, Y.N.; Sun, J.S. Correlation between quantified HPLC fingerprint and its anti-oxidation activity of Salviae miltiorrhizae Radix Et Rhizoma. Cent. South Pharm. 2013, 11, 535–538. [Google Scholar]
- Ni, L.J.; He, J.; Zhang, L.G.; Bi, Y.J. Relationship between HPLC fingerprints and anti-oxidation activity of different salvianolic acid extracts. Chin. Tradit. Pat. Med. 2011, 33, 2029–2033. [Google Scholar]
- Huang, Y.; Qi, X.L.; Guan, Z.Z.; Wang, Y.L.; Wang, A.M.; Li, C.B.; Chi, M.Y. Study on fingerprints correlated with pharmacodynamic of constituents in Herba Erigerontis against neurotoxicity induced by beta-amyloid peptide. China J. Chin. Mater. Med. 2010, 35, 1038–1041. [Google Scholar]
- Li, Z.J.; Zhou, L.; Gulnar, D.W.; Siafu, A.B. Grey relational analysis on fingerprint characteristics of different eluted parts of Euphorbia humifusa and antifungal effect. China J. Chin. Mater. Med. 2012, 37, 580–584. [Google Scholar]
- Wu, S.T.; Bai, J.X.; Wang, Z.; Dai, L.; Lv, Y.Q.; Yu, C.; Han, J.; Han, J.; Yuan, H.L. Spectrum-effect relationship on anti-hepatic fibrosis efficacy of Cordyceps sinesis and its cultured mycelia. Glob. Tradit. Chin. Med. 2013, 6, 801–805. [Google Scholar]
- Huo, S.X.; Kang, Y.T.; Peng, X.M.; Gao, L.; Yan, M. Spectrum-effect relationship of extract from rhizome of Alpinia officinarum on promotion of melanogenesis. Chin. Tradit. Herb. Drugs 2013, 44, 995–1002. [Google Scholar]
- Mei, Q.H.; Chen, Y.; Lan, S.M.; Fan, C.Q. Construction of pharmacodynamics fingerprint and analysis of spectrum effect relationship on anti-gastrointestinal propulsive of Herba Pogostemonis. China Med. Her. 2013, 10, 17–20. [Google Scholar]
- Zheng, L.; Li, J.; Chen, H.; Wang, Y.L.; Wang, A.M.; Huang, Y. Study on fingerprint-pharmacology correlation of protective effect of Polygonum orientale on myocardial cell oxidative injury induced by H2O2. China J. Chin. Mater. Med. 2012, 37, 2585–2588. [Google Scholar]
- Tang, G.X.; Huang, Y.J.; Zhang, Y.; Meng, X.L.; Luo, W.Z.; Li, J.C.; Geng, Z.P. Correlation between HPLC fingerprints and activity of Rhizoma Coptidis in amelliorating insulin resistance in vitro. World Sci. Technol./Mod. Tradit. Chin. Med. Mater. Med. 2009, 11, 828–833. [Google Scholar]
- Ma, X.X.; Liu, J.H.; Yu, B.Y. Spectrum-effect relationship of antibacterial activities in Coptidis Rhizoma. Drug Eval. Res. 2013, 36, 171–175. [Google Scholar]
- Kong, W.J.; Zhao, Y.L.; Xiao, X.H.; Wang, J.B.; Li, H.B.; Li, Z.L.; Jin, C.; Liu, Y. Spectrum-effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizoma coptidis. Anal. Chim. Acta 2009, 634, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.L.; Zhang, Y.; Zheng, H.J.; Deng, C.; Fan, G.; Zhou, L.; Meng, X.L. Study on effective substances of San Huang preparation and its single herbs by serum pharmacochemistry. World Sci. Technol./Mod. Tradit. Chin. Med. Mater. Med. 2010, 12, 666–670. [Google Scholar]
- Huang, X.H.; Liang, J.; Ren, Y.; Wei, X.Y.; Feng, D.M.; Feng, S.L. Spectrum-effect relationship in effect of improving immunity of Astragali Radix. J. Chin. Med. Mater. 2012, 35, 1978–1981. [Google Scholar]
- Liu, X.H.; Liang, J.; Liang, J.D.; Dang, Z.L.; Feng, S.L. Spectrum-effect relationship on anti-gastric ulcer effect of Astragali Radix. Chin. Tradit. Herb. Drugs 2012, 43, 2448–2452. [Google Scholar]
- Deng, S.H.; Song, L.; Duan, X.J.; Zhang, D.L.; Nie, L. Correlation analysis between HPLC fingerprint of Astragali Radix extracts and antifatigue effect. J. Chin. Med. Mater. 2013, 36, 260–264. [Google Scholar]
- Liu, X.H.; Zhao, L.G.; Liang, J.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Component analysis and structure identification of active substances for anti-gastric ulcer effects in Radix Astragali by liquid chromatography and tandem mass spectrometry. J. Chromatogr. B 2014, 960, 43–51. [Google Scholar] [CrossRef]
- Liu, X.H.; Lin, X.Y.; Liang, J.; Chen, Y.L.; Ren, Y.; Feng, S.L. Spectrum-effect relationship in diuretic effect of Astragali Radix. Chin. J. Mod. Appl. Pharm. 2013, 30, 491–495. [Google Scholar]
- Meng, Q.G.; Wang, W.; Li, Q.; Xu, S. Spectrum-effect relationship in antipyretic effect of Huangqin (Radix Scutellariae). J. Beijing Univ. Tradit. Chin. Med. 2011, 34, 379–383. [Google Scholar]
- Zhao, B.N.; Yu, Z.Y.; Ding, X.Y.; Lv, L.; Li, K.; Li, S.B. Spectrum-efficient correlation pattern for quality evaluation of Scutellariae Radix. Chin. Tradit. Herb. Drugs 2011, 42, 380–383. [Google Scholar]
- Zhang, H.F.; Liu, J.; Zhang, J.; Yang, Y.; Wei, B.; Wang, Q.S. Spectrum-effect relationship of Bupleurum chinense for hepatoprotective effect based on cluster analysis and typical correlation analysis. Chin. Tradit. Herb. Drugs 2013, 44, 2696–2702. [Google Scholar]
- Wang, L.B.; Jia, X.S.; Chen, X.F.; Huang, Y.Q.; Sun, J.P. Sdudys on spectrum of diterpenoids constituents of Radix Tinosporae and relavant its pharmacology. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 83–86. [Google Scholar]
- Liang, S.W.; Cui, Y.X.; Wang, S.M.; Wu, M.X. Pharmacodynamic fingerprint of Flos Lonicerea by HPLC. Chin. Tradit. Herb. Drugs 2006, 37, 1489–1493. [Google Scholar]
- Gao, Y.; Zhao, B.N.; Yu, Z.Y.; Li, K.; Wang, B.L.; Yin, J. Study on the spectrum-efficient correlation pattern for quality evaluation of Lonicerae Japonicae Flos on anti-influenza virus. China J. Tradit. Chin. Med. Pharm. 2013, 28, 3508–3511. [Google Scholar]
- Wu, L.H.; Wen, H.L.; Jin, Q.; Cheng, Q.L.; Chenggen, J.S. Gray relational analysis on fingerprint of Murraya exotica and anti-inflammation effects. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 338–342. [Google Scholar]
- Mao, X.L.; Qin, Y.; Cai, J.; Zheng, J.M.; YE, Y.H.; Liu, H.G.; Huang, S.S. Infrared fingerprint of Zathoxylum nitidum and its effect on inhibition of tumor cell. J. Infrared Millim. Waves 2013, 32, 91–96. [Google Scholar]
- Wang, H.H.; Liu, H.G.; Huang, H.X.; Liu, L.M.; Shen, Q.R.; Cao, L.J. Studies on chromatogram-effect relation of Zanthoxylum nitidum (Roxb.) DC. on tumor cell line HeLa. Pharmacol. Clin. Chin. Mater. Med. 2011, 27, 84–89. [Google Scholar]
- Shen, Q.R.; Huang, H.X.; Wang, H.H.; Cao, L.J.; Feng, K.; Ye, Y.H.; Liu, H.G. Study on chromatography-efficacy relation of Zanthoxylum nitidum on gastric cancer cells. China J. Chin. Mater. Med. 2011, 36, 2693–2696. [Google Scholar]
- Chen, H.G.; Zhou, X.; Zhao, C.; Yang, S.L.; Yi, Z.X. Dose-response and HPLC spectrum-activity relationships of the anti-inflammatory active site of Kalimeris indica. Chin. Pharm. J. 2013, 48, 1190–1193. [Google Scholar]
- Chen, N.D.; Fang, M.Y.; Yu, C.F.; Lin, L.; Zhao, H.Y. Research of spectral efficiency relationship between total flavonoids fingerprints and antioxidant activity of Exocarpium Citri Grandis. J. Guangzhou Univ. Tradit. Chin. Med. 2012, 29, 702–706. [Google Scholar]
- Zhou, L.Y.; Liang, S.W.; Wang, S.M.; Wu, M.X.; Chen, C.Y. Studies on the pharmacodynamic fingerprint of Cortex Moutan. Lishizhen Med. Mater. Med. Res. 2008, 19, 1337–1339. [Google Scholar]
- Tong, L.G.; Song, M.Q.; Feng, M.L.; Jia, L.L.; Wu, Y.P.; Niu, Y.Y. Spectrum analysis of sedative-hypnotic material in Fructus Cnidii. Shanxi J. Tradit. Chin. Med. 2011, 27, 52–53. [Google Scholar]
- Wu, F.Y.; Chen, Y.X.; Zhou, R.L.; Jiang, B.; Zeng, Y.E.; Zhang, J. Evaluation of refinement process for extracting Herba Artemisiae with macroporous resin by the method of pharmacodynamics combining with fingerprint. Tradit. Chin. Drug Res. Clin. Pharmacol. 2009, 20, 166–168. [Google Scholar]
- Liu, R.H.; Chen, L.Y.; Yu, B.Y.; Huang, H.L.; Shao, F.; Ren, G. Exploring study on inhibition on O2− and correlative chemical characteristic fingerprint of Crataegi Folium. Chin. Tradit. Herb. Drugs. 2010, 41, 1173–1178. [Google Scholar]
- Liang, J.D.; Zhao, L.G.; Liu, X.H.; Li, W.; Dang, Z.L.; Liang, J.; Feng, S.L. Spectrum-effect relationship of reducing phlegm effect of Peucedanum harrysmithii var. subglabrum. China J. Chin. Mater. Med. 2012, 37, 2894–2897. [Google Scholar]
- He, Q.S.; Yang, W.P.; Zhang, L.Y.; Wang, H.; Mang, R.S. Study on the spectrum efficiency relationship of the Evodia ruatecarpa extracts ease the rabbit In vitro intestinal muscle spasm. Lishizhen Med. Mater. Med. Res. 2012, 23, 1108–1110. [Google Scholar]
- Lin, S.; Cai, Q.Y.; Zeng, J.W.; Zhu, X.Q.; Wu, J.Z. Correlation between cytotoxic activity and HPLC fingerprint chromatogram of the effective fractions of Radix Pseudostellariae. Nat. Prod. Res. Dev. 2012, 24, 349–352. [Google Scholar]
- Liu, X.; Li, M.C.; Xu, X.; Ji, S.G.; Xu, J.P.; Li, X.Z. Study on associativity between fingerprint of Panax Notoginseng and curative effect on myocardial ischemia in rat. Chin. J. Mod. Appl. Pharma. 2013, 30, 819–823. [Google Scholar]
- Zheng, Q.F.; Zhao, Y.L.; Wang, J.B.; Liu, T.T.; Zhang, B.; Gong, M.; Li, J.W.; Liu, H.H.; Han, B.; Zhang, Y.M. Spectrum-effectrelationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014, 153, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Zhao, Y.L.; Liu, T.T.; Sun, X.J.; Li, R.S.; Zhang, P.; Xiao, X.H. Spectrum-effect relationships between UPLC fingerprints and bioactivities of five Aconitum L. plants. Thermochim. Acta 2013, 558, 61–66. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, F.; Sun, L.L. Investigation on formula compatibility and spectrum-effect relationship of Bai-Hu-Tang. Pharm. Clin. Res. 2010, 18, 226–230. [Google Scholar]
- Zhang, J.; Yang, Y.F.; Wu, C.Z.; Fan, S.Y.; Xiao, W.; Wang, Z.Z. Spectrum-activity relationship of Angelicae Sinensis Radix-Chuanxiong Rhizoma supercritical fluid extraction with Carthami Flos against myocardial ischemia. Chin. Tradit. Herb. Drugs 2013, 44, 1944–1950. [Google Scholar]
- Xu, L.; Bi, K. Application of multiple linear regression analytical method to spectrum-activity relationship analysis of Mongolian preparation Sendeng-4 decoction. Comput. Appl. Chem. 2008, 25, 1189–1192. [Google Scholar]
- Dou, Z.H.; Luo, L.; Ding, A.W.; Wang, L.J.; Zhang, B.; Li, M. Profile-effect between fingerprint and liver protection of serum containing compound Wurenchun capsules. J. Fourth Mil. Med. Univ. 2008, 29, 116–118. [Google Scholar]
- Liu, M.; Liu, H.P.; Liang, M.X.; Xiong, Z.L.; Li, F.M. HPLC fingerprint analysis of Gushudan prescription and the correlation with its activity. J. Shenyang Pharm. Univ. 2007, 24, 758–762. [Google Scholar]
- Chen, J.; Wang, H.; Wang, H.H.; Yun, F.; Di, L.Q.; Zhao, X.L.; Cai, B.C. Tongsaimai pellets spectral efficiency related fingerprint establishment based on “Technology-fingerprint-effect” model research. J. Nanjing Univ. Tradit. Chin. Med. 2012, 28, 259–264. [Google Scholar]
- Zhang, N.; Li, H.J.; Kuang, Y.H.; Chen, Q.Y.; Wang, X.J. TCM serum pharmacochemistry-based analysis of the effective components of Xiaoyao San for the therapy of chloasma. World Sci. Technol./Mod. Trad. Chin. Med. 2010, 12, 643–646. [Google Scholar]
- Jia, G.C.; Zhou, Y.Z.; Ren, Y.L.; Qin, X.M. Study on the spectrum efficiency relationship of the antidepressant effective parts compatibility of Xiaoyao San. Chin. J. Pharmacol. Toxicol. 2010, 25, 97. [Google Scholar]
- Yi, L.; Qian, J. Jiaweisimiao effective parts of GC fingerprint spectrum ralationship and compatibility change research. Chin. Tradit. Pat. Med. 2007, 29, 634–637. [Google Scholar]
- Yi, L.; Qian, J. Jiaweisimiao’s HPLC fingerprint and associated pharmacodynamics. World Sci. Technol./Mod. Trad. Chin. Med. 2007, 19, 40–45. [Google Scholar]
- Song, Z.H.; Feng, D.; Xu, J.B. Study on the com patibility and therapeutical basis of composite herbal medicines of Lingguishugan decoction. Chin. Tradit. Pat. Med. 2003, 25, 114–118. [Google Scholar]
- Gu, Y.; Feng, Y.; Xu, D.S. Correlation between drug effects and serum HPLC fingerprint of active compositions of Radix paeoniae alba and Radix et Rhizowa Glycyrrhizae. Chin. Tradit. Pat. Med. 2008, 30, 6–10. [Google Scholar]
- Xu, W.W.; Wang, S.; Meng, X.S.; Bao, Y.R. Study on chromatography-efficacy relationship of anti-inflammatory activity of QizhiWeitong particle compound herbs with neural network and gray correlation method. China J. Chin. Mater. Med. 2013, 38, 1806–1811. [Google Scholar]
- Chen, Y.G.; Liu, X.; Tang, D.Q.; Yin, X.X.; Shi, X.D.; Chang, Y.H.; Du, Q. Primary investigation on chromatogram-pharmacodynamics relationship of Shenghua decoction. J. Chengdu Med. Coll. 2010, 5, 126–131. [Google Scholar]
- Li, P.; Li, X.; Chen, J.W.; Wu, L.L. Spectrum-effect relationship of active components from Taohong Siwu tang in dysmenorrheal model mice. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 144–149. [Google Scholar]
- Ning, L.L.; Bi, K.S.; Wang, R.; Che, Z.T.; Wang, X.; Luo, X. Methodological study on the material basis for the efficacy of the traditional Chinese medicine Wuzhuyu decoction. Acta Pharm. Sin. 2000, 35, 131–134. [Google Scholar]
- Lin, L.; Liu, X.Q. Primary investigation on spectrum-activity relationship of Xiebai Powder. Mod. Chin. Med. 2009, 11, 35–38. [Google Scholar]
- Kong, W.J.; Zhao, Y.L.; Shan, L.M.; Xiao, X.H.; Guo, W.Y. Spectrum-effect relationships between HPLC fingerprints and biothermological activity of Zuojin Wan and its similar formulaes. Acta Chim. Sin. 2008, 66, 2533–2538. [Google Scholar]
- Xie, R.F.; Zhou, X.; Shi, Z.N.; Li, Y.M.; Li, Z.C. Study on spectrum-effect relationship of Rhizoma Rhei, Cortex Magnoliae Officinalis, Fructus Aurantii Immaturus and their formula. J. Chromatogr. Sci. 2013, 51, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.M.; Liang, Y.Z.; Qian, P. Profile-effect on quality control of Houttuynia cordata injection. Acta Pharm. Sin. 2005, 40, 1147–1150. [Google Scholar]
- Tashiro, S. “Serum pharmacology” and “serum pharmacochemistry”—use Kampo pharmacology to create a new world for the determination of drug concentration in blood. Jpn. J. Ther. Drug Monit. Res. 1988, 5, 54. (In Japanese) [Google Scholar]
- Zhang, L.; Nie, L.; Wang, W.H. Correlation analysis between HPLC fingerprint of Radix Astragali injection and antioxidant activity. J. Chin. Med. Mater. 2009, 32, 1757–1760. [Google Scholar]
- Deng, C.; Zhang, Y.; Meng, X.L.; Wang, Z.; Fan, G. Spectrum efficiency relationship research on anti-endotoxin activity of San-Huang Formula. Lishizhen Med. Mater. Med. Res. 2011, 22, 525–527. [Google Scholar]
- Yin, Y.Q.; Zhu, J.F.; Shen, Z.B.; Tang, C.P.; Yang, C.Y.; Sun, Y. Neural network analysis of active ingredients of Xiang Dan injection against myocardial ischemia spectral correlation studies. Chin. Tradit. Herb. Drugs 2009, 40, 1284–1287. [Google Scholar]
- Hu, F.; Yang, Y.L.; Liu, X.H.; Li, C.; Zhu, R.J.; Feng, S.L. The double-efficacy to evaluate the spectrum-effect relationship of blood enriching function of Buzhongyiqi Wan. Pharmacol. Clin. Chin. Mater. Med. 2013, 29, 14–18. [Google Scholar]
- Wang, Y.H.; Li, B.; Cai, G.X.; Meng, P.; Yang, Y.H. Investigation on chromatogram-pharmacodynamics relationship of three kinds of Xiao-Yao Formula on the effect of Shu-Gan-Jie-Yu. World Sci. Technol./Mod. Trad. Chin. Med. 2012, 14, 2018–2021. [Google Scholar]
- Sui, Y.; Sun, Y.; Guo, T.; Li, M.; Li, F.M. Studying the effect of Flos Carthami on the guineapigs’ hearts in vitro by serum pharmacology. J. Shenyang Pharm. Univ. 2003, 20, 373–376. [Google Scholar]
- Wang, X.J. Study on serum pharmacochemistry of traditional Chinese medicine. World Sci. Technol./Mod. Trad. Chin. Med. 2002, 4, 1–4. [Google Scholar]
- Zhou, J.L.; Qi, L.W.; Li, P. Quality control of Chinese herbal medicines with chromatographic fingerprints. Chin. J. Chromatogr. 2008, 26, 153–159. [Google Scholar] [CrossRef]
- Zhao, X.P.; Fan, X.H.; Yu, J.; Cheng, Y.Y. A method for predicting activity of traditional Chinese medicine based on quantitative composition-activity relationship of neural network model. China J. Chin. Mater. Med. 2004, 29, 1082–1085. [Google Scholar]
- Hazarika, P.; Russell, D.A. Advances in fingerprint analysis. Angew. Chem. 2012, 15, 3524–3531. [Google Scholar] [CrossRef]
- Li, Y.F.; Cheng, Y.Y.; Fan, X.H. A strategy for multidimensional spectrum-effect relationship of traditional Chinese medicine. Chin. J. Nat. Med. 2010, 8, 167–170. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.-L.; Xie, M.; Yang, X.-Y.; Song, Y.; Yan, C.; Yang, Y.; Zhang, X.; Liu, Z.-Z.; Tian, Y.-X.; Wang, Y.; et al. Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives. Molecules 2014, 19, 17897-17925. https://doi.org/10.3390/molecules191117897
Xu G-L, Xie M, Yang X-Y, Song Y, Yan C, Yang Y, Zhang X, Liu Z-Z, Tian Y-X, Wang Y, et al. Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives. Molecules. 2014; 19(11):17897-17925. https://doi.org/10.3390/molecules191117897
Chicago/Turabian StyleXu, Guan-Ling, Meng Xie, Xiao-Yan Yang, Yan Song, Cheng Yan, Yue Yang, Xia Zhang, Zi-Zhen Liu, Yu-Xin Tian, Yan Wang, and et al. 2014. "Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives" Molecules 19, no. 11: 17897-17925. https://doi.org/10.3390/molecules191117897
APA StyleXu, G.-L., Xie, M., Yang, X.-Y., Song, Y., Yan, C., Yang, Y., Zhang, X., Liu, Z.-Z., Tian, Y.-X., Wang, Y., Jiang, R., Liu, W.-R., Wang, X.-H., & She, G.-M. (2014). Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives. Molecules, 19(11), 17897-17925. https://doi.org/10.3390/molecules191117897




