A New Phenolic Glycoside from the Barks of Cinnamomum cassia
Abstract
:1. Introduction
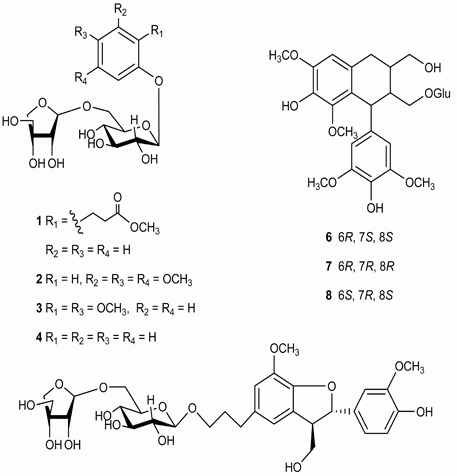
2. Results and Discussion
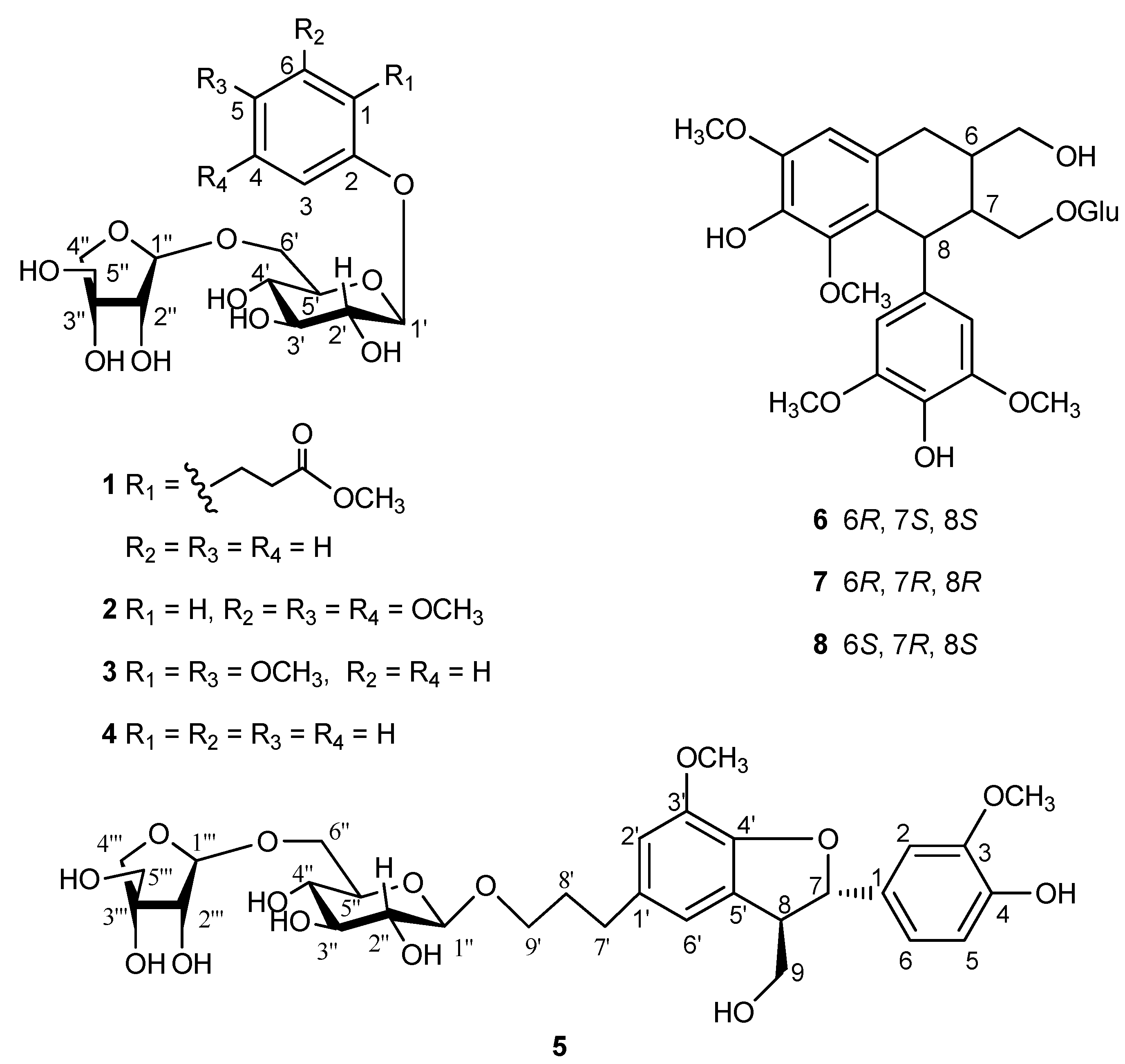
| Position | δH | δC |
|---|---|---|
| 1 | 131.3 | |
| 2 | 157.0 | |
| 3 | 7.21 dd (8.1, 1.2) | 128.9 |
| 4 | 7.18 ddd (8.1, 7.9, 1.9) | 116.7 |
| 5 | 6.96 ddd (7.9, 7.9, 1.2) | 123.5 |
| 6 | 7.17 dd (7.9, 1.9) | 131.0 |
| 7 | 3.00 m | 26.9 |
| 8 | 2.68 t (7.7) | 35.3 |
| 9 | 175.9 | |
| OMe | 3.66 s | 52.0 |
| 1′ | 4.88 d (7.8) | 102.8 |
| 2′ | 3.50 m | 75.0 |
| 3′ | 3.49 m | 78.2 |
| 4′ | 3.38 m | 71.6 |
| 5′ | 3.60 m | 77.0 |
| 6′a | 4.04 d (10.6) | 68.8 |
| 6′b | 3.64 m | |
| 1′′ | 5.00 d (2.3) | 111.0 |
| 2′′ | 3.93 d (2.3) | 78.1 |
| 3′′ | 80.0 | |
| 4′′a | 3.77 d (9.7) | 75.0 |
| 4′′b | 3.97 d (9.7) | |
| 5′′ | 3.61 s | 65.6 |
| Compounds | Concentration (μM) | Inhibitory/Enhanced Rate (%) b | |
|---|---|---|---|
| ConA−Induced T Cell Proliferation | LPS−Induced B Cell Proliferation | ||
| 1 | 200 | −56.7 | 10.3 |
| 100 | −61.2 | c25.2 | |
| 50 | −35.4 | 25.6 | |
| 25 | −22.1 | 30.1 | |
| 12.5 | 15.2 | 7.8 | |
| 2 | 200 | −45.2 | −35.1 |
| 100 | −38.8 | −5.8 | |
| 50 | −8.5 | −12.5 | |
| 25 | −12.5 | 10.3 | |
| 12.5 | −20.1 | 6.2 | |
| 5 | 200 | −27.9 | 28.4 |
| 100 | −5.4 | 22.2 | |
| 50 | 6.5 | 10.7 | |
| 25 | 11.7 | −1.5 | |
| 12.5 | 2.8 | 13.8 | |
| 6 | 200 | −47.7 | −13.2 |
| 100 | −58.0 | 20.5 | |
| 50 | −23.6 | 41.4 | |
| 25 | −47.5 | 36.1 | |
| 12.5 | −11.3 | −12.5 | |
| 7 | 200 | −10.4 | −22.7 |
| 100 | −13.8 | 78.5 | |
| 50 | 2.5 | 62.4 | |
| 25 | 18.7 | 58.4 | |
| 12.5 | 35.1 | 12.5 | |
| 8 | 200 | −80.1 * | −46.7 |
| 100 | −58.3 | −10.3 | |
| 50 | −19.5 | 25.6 | |
| 25 | 27.8 | 57.8 | |
| 12.5 | 14.5 | 52.2 | |
| CsA | 200 | −109.8 ** | −106.1 ** |
| 100 | −106.0 ** | −94.8 ** | |
| 50 | −106.1 ** | −75.8 * | |
| 25 | −97.1 ** | −60.1 | |
| 12.5 | −84.5 ** | −54.5 | |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis
3.5. Lymphocyte Proliferation Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 7, p. 102. [Google Scholar]
- Choi, J.; Lee, K.T.; Ka, H.; Jung, W.T.; Jung, H.J.; Park, H.J. Constituents of the essential oil of the Cinnamomum cassia stem bark and the biological properties. Arch. Pharm. Res. 2001, 24, 418–423. [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, 2010 ed.; Chemical Industry Press: Beijing, China, 2010; Part I; p. 128. [Google Scholar]
- Zeng, X.Y.; Chen, X.F.; Wei, B.W. Study on immunosuppressive activity of Cinnamomum cassia extract. Guangxi Yixue 1984, 6, 62–64. [Google Scholar]
- Zeng, J.F.; Xue, Y.B.; Shu, P.H.; Qian, H.Q.; Sa, R.J.; Xiang, M.; Li, X.N.; Luo, Z.W.; Yao, G.M.; Zhang, Y.H. Diterpenoids with Immunosuppressive Activities from Cinnamomum cassia. J. Nat. Prod. 2014, 77, 1948–1954. [Google Scholar]
- Tran, M.N.; Nguyen, M.K.; Do, T.H.; Nguyen, X.N.; Bui, H.T.; Dao, V.D.; Hoang, L.; Doan, C.S.; KiHwan, B. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorg. Med. Chem. Lett. 2012, 22, 4625–4628. [Google Scholar]
- Sunghwa, F.; Koketsu, M. Phenolic and bis-iridoid glycosides from Strychnos cocculoides. Nat. Prod. Res. 2009, 23, 1408–1415. [Google Scholar]
- Watanabe, S.; Hashimoto, I.; Hayashi, K.; Yagi, K.; Asai, T.; Knapp, H.; Straubinger, M.; Winterhalter, P.; Watanabe, N. Isolation and identification of 2-phenylethyl disaccharide glycosides and mono glycosides from rose flowers, and their potential role in scent formation. Biotechnol. Biochem. 2001, 65, 442–445. [Google Scholar]
- Xiao, H.H.; Dai, Y.; Wong, M.S.; Yao, X.S. New lignans from the bioactive fraction of Sambucus williamsii Hance and proliferation activities on osteoblastic-like UMR106 cells. Fitoterapia 2014, 94, 29–35. [Google Scholar]
- Yang, Y.L.; Chang, F.R.; Wu, Y.C. Squadinorlignoside: A novel 7, 9′-dinorlignan from the stems of Annona squamosal. Helv. Chim. Acta 2005, 88, 2731–2737. [Google Scholar]
- Nie, T.T.; Zhao, H.X.; Bai, H. Chemical constituents of Pittosporum glabratum. Chin. J. Nat. Prod. 2011, 9, 180–184. [Google Scholar]
- Yagi, A.; Tokubuchi, N.; Nohara, T.; Nonaka, G.; Nishioka, I.; Koda, A. The constituents of cinnamomi cortex. I. structures of cinncassiol A and its glucoside. Chem. Pharm. Bull. 1980, 28, 1432–1436. [Google Scholar]
- Lin, H.C.; Lee, S.S. Dibenzocycloheptanoids from the leaves of Cinnamomum subavenium. J. Nat. Prod. 2012, 75, 1735–1743. [Google Scholar]
- Kawaguchi, T.; Matsumoto, I.; Osawa, T.J. Studies on hemagglutinins from Maackia amurensis seeds. Biol. Chem. 1974, 249, 2786–2792. [Google Scholar]
- Yamamoto, Y.; Mochida, J.; Sakai, D.; Nakai, T.; Nishimura, K.; Kawada, H.; Hotta, T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: Significance of direct cell-to-cell contact in coculture system. Spine 2004, 29, 1508–1514. [Google Scholar]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Xue, Y.; Lai, Y.; Yao, G.; Luo, Z.; Zhang, Y.; Zhang, J. A New Phenolic Glycoside from the Barks of Cinnamomum cassia. Molecules 2014, 19, 17727-17734. https://doi.org/10.3390/molecules191117727
Zeng J, Xue Y, Lai Y, Yao G, Luo Z, Zhang Y, Zhang J. A New Phenolic Glycoside from the Barks of Cinnamomum cassia. Molecules. 2014; 19(11):17727-17734. https://doi.org/10.3390/molecules191117727
Chicago/Turabian StyleZeng, Junfen, Yongbo Xue, Yongji Lai, Guangmin Yao, Zengwei Luo, Yonghui Zhang, and Jinwen Zhang. 2014. "A New Phenolic Glycoside from the Barks of Cinnamomum cassia" Molecules 19, no. 11: 17727-17734. https://doi.org/10.3390/molecules191117727
APA StyleZeng, J., Xue, Y., Lai, Y., Yao, G., Luo, Z., Zhang, Y., & Zhang, J. (2014). A New Phenolic Glycoside from the Barks of Cinnamomum cassia. Molecules, 19(11), 17727-17734. https://doi.org/10.3390/molecules191117727