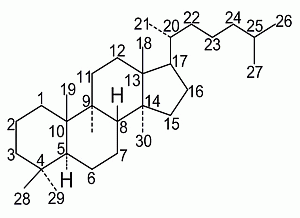
A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp.
Abstract
:1. Introduction
2. Ganoderma Triterpenes
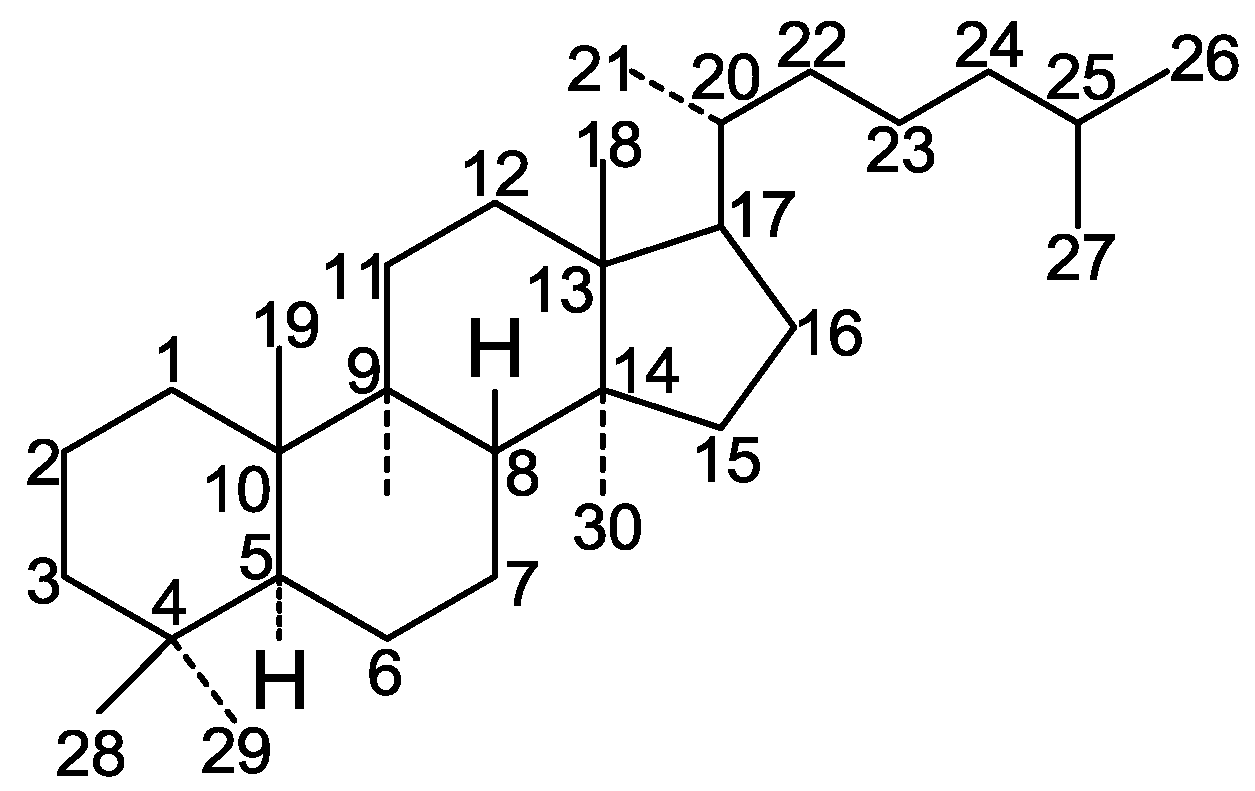
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 1 | n-Butyl ganoderate H (n-butyl 12β-acetoxy-3β-hydroxy-7,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [16] |
| 2 | Butyl ganoderate A | G. lucidum (fruit bodies) | [17] |
| 3 | Butyl ganoderate B | G. lucidum (fruit bodies) | [17] |
| 4 | Ganoderic acid α (12β-acetoxy-3β, 15β-dihydroxy-7,11,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (fruit bodies) | [18] |
| 5 | Ganolucidic acid A | G. lucidum (gill surface) | [19] |
| 6 | Methyl ganolucidate A (methyl 15α-hydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum (gill surface) | [19,20] |
| 7 | Ganolucidic acid B | G. lucidum (gill surface) | [19] |
| 8 | Methyl ganolucidate B | G. lucidum (gill surface) | [19,20] |
| 9 | Ganoderic acid A (7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [9,21] |
| 10 | Methyl ganoderate A (methyl 7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [21] |
| 11 | Ganoderic acid B (3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 12 | Methyl ganoderate B (methyl 3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [9,21] |
| 13 | Ganoderic acid C (3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 14 | Methyl ganoderate C | G. lucidum | [21] |
| 15 | Ganoderic acid D (7β-hydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 16 | Methyl ganoderate D | G. lucidum | [21] |
| 17 | Methyl ganoderate C2 (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [22] |
| 18 | Methyl ganoderate K | G. lucidum (gills) | [22,23] |
| 19 | Compound B8 | G. lucidum (gills) | [22] |
| 20 | Compound B9 | G. lucidum (gills) | [22] |
| 21 | 3β-Oxo-formyl-7β, 12β-dihydroxy-5α-lanost-11,15,23-trioxo-8-en(E)-26-oic acid | G. lucidum (fruit bodies) | [24] |
| 22 | Ganoderic acid B8 | G. lucidum (fruit bodies) | [25] |
| 23 | Ganoderic acid C1 | G. lucidum (fruit bodies) | [25] |
| 24 | 12β-Acetoxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid ethyl ester | G. lucidum | [26] |
| 25 | 3β, 7β-Dihydroxy-12β-acetoxy-11,15,23-trioxo-5α-lanosta-8-en-26-oic acid methyl ester | G. lucidum | [27] |
| 26 | 3β-Hydroxy-7,11,12,15,23-pentaoxolanost-8-en-26-oic acid | G. lucidum (fruit bodies) | [28] |
| 27 | Ganoderic acid Df (7β, 11β-dihydroxy-3,15,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum | [29] |
| 28 | Ganoderic acid H | G. lucidum (gill surface) | [30] |
| 29 | Ganoderic acid F (12β-acetoxy-3,7,11,15-pentaoxo-5α-lanost-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 30 | Ganoderic acid E (3,7,11,15,23-pentaoxo-5α-lanost-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 31 | Ganoderic acid K | G. lucidum (fruit bodies) | [32] |
| 32 | Ganoderic acid AM1 | G. lucidum (fruit bodies) | [32] |
| 33 | Ganoderic acid J | G. lucidum (fruit bodies) | [32] |
| 34 | Ganoderic acid C2 (3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (gills) | [22] |
| 35 | Ganoderic acid G (3β, 7β, 15β-trihydroxy-11,15,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 36 | 7β, 12β-Dihydroxy-3,11,15,23-tetraoxo-5α-lanosta-8-en-26-oic acid | G. lucidum | [26] |
| 37 | 12β-Hydroxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid | G. lucidum | [26] |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
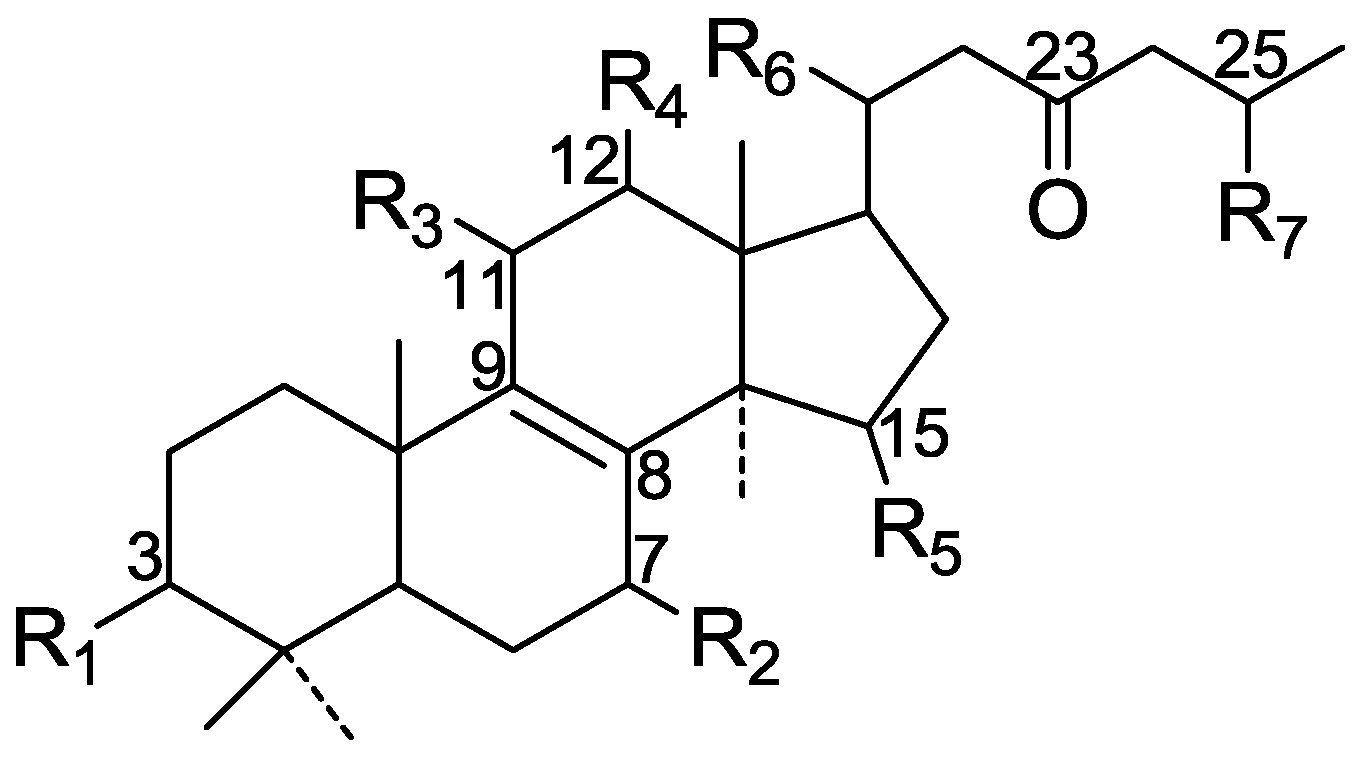
| 38 | Ganoderic acid GS-1 (7β-hydroxy-3,11,15-trioxolanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 39 | Ganoderic acid GS-2 (7β, 15α-dihydroxy-3,11-dioxolanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 40 | Ganoderic acid GS-3 (12β-acetoxy-3β, 7β-dihydroxy-11,15-dioxo-lanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 41 | Ganoderic acid AP2 (12β, 15α-diacetoxy-3β-hydroxy-11-oxolanost-8,24(E)-dien-26-oic acid) | G. applanatum (fruit bodies) | [34] |
| 42 | 23S-Hydroxy-3,7,11,15-tetraoxolanost-8,24E-diene-26-oic acid | G. lucidum (fruit bodies) | [32] |
| 43 | 7-Oxoganoderic acid Z (3β-hydroxy-7-oxo-5α-lanosta-8,24(E)-dien-26-oic acid) | G. lucidum (fruit bodies) | [35] |
| 44 | Ganoderic acid LM2 ((23S) 7β,-dihydroxy-3,11,15-trioxo-5α-lanosta-8,24-dien-26-oic acid) | G. lucidum (fruit bodies) | [36] |
| 45 | Lucialdehyde B ((24E)-3,7-dioxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (fruit bodies) | [25] |
| 46 | Lucialdehyde C ((24E)-3β-hydroxy-7-oxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (fruit bodies) | [25] |
| 47 | Ganoderic acid γ ((23S)-7β, 15α, 23-trihydroxy-3,11-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 48 | Ganoderic acid δ ((23S)-7α, 15α, 23-trihydroxy-3,11-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 49 | Ganoderic acid ε ((23S)-3β, 7β, 23-trihydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 50 | Ganoderic acid ζ ((23S)-3β, 23-dihydroxy-7,11,15-trioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 51 | Ganoderic acid η ((23S)-3β, 7β, 12β, 23-tetrahydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 52 | Ganoderic acid θ ((23S)-3β, 12β, 23-trihydroxy-7,11,15-trioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 53 | Ganoderic acid β (3β, 7β-dihydroxy-11,15-dioxolanosta-8,24(E)-dien-26-oic acid) | G. lucidum (spores) | [38] |
| 54 | Ganolucidic acid E (15α-hydroxy-3,11-dioxo-5α-lanosta-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [39] |
| 55 | Ganoderal B (7α-hydroxy-3-oxo-5α-lanosta-8,24E-dien-26-al) | G. lucidum | [40] |
| 56 | Ganoderic acid Ma (3α, 7α-diacetoxy-15α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 57 | Lucialdehyde D (3,7,11-trioxo-5α-lanosta-8,24-diene-26-al) | G. pfeifferi (fruit bodies) | [42] |
| 58 | Ganoderone A (5α-lanosta-8,24-diene-26-hydroxy-3,7-dione) | G. pfeifferi (fruit bodies) | [42] |
| 59 | ganoderic acid Mi (3α-acetoxy-15α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 60 | 11α-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | G. lucidum | [26] |
| 61 | 11β-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | G. lucidum | [26] |
| 62 | Lucidadiol (5α-lanosta-8,24-dien-3β, 26-dihydroxy-7-one) | G. lucidum | [44] |
| 63 | Lucidal (5α-lanosta-8,24E-dien-3β-hydroxy-7-on-26-al) | G. lucidum | [44] |
| 64 | Ganoderic acid DM (3,7-dioxo-8,24(E)-dien-lanosta-26-oic acid) | G. lucidum (cultured fruit bodies) | [45] |
| 65 | Ganoderic acid V | G. orbiforme | [46] |
| 66 | Ganolucidic acid γa (3β, 7β, 15α, 23-tetrahydroxy-11-oxo-5α-lanosta-8,24-dien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 67 | Ganolucidate F (3β, 15α, 23-trihydroxy-11-oxo-5α-lanosta-8,24-dien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 68 | Lucialdehyde E (7β, 15α-dihydroxy-3,11-dioxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (spores) | [48] |
| 69 | Ganolucidic acid D | G. lucidum (spores) | [37] |
| 70 | Ganoderic acid W | G. lucidum (fruit bodies) | [41] |
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 1 | β-OH | =O | =O | β-O-Ac | =O | α-CH3 | COOBu |
| 2 | =O | β-OH | =O | H | α-OH | α-CH3 | COOBu |
| 3 | β-OH | β-OH | =O | H | =O | α-CH3 | COOBu |
| 4 | β-OH | =O | =O | β-O-Ac | α-OH | α-CH3 | COOH |
| 5 | =O | H | =O | H | α-OH | β-CH3 | COOH |
| 6 | =O | H | =O | H | α-OH | β-CH3 | COOCH3 |
| 7 | β-OH | H | =O | H | α-OH | β-CH3 | COOH |
| 8 | β-OH | H | =O | H | α-OH | β-CH3 | COOCH3 |
| 9 | =O | β-OH | =O | H | α-OH | β-CH3 | COOH |
| 10 | =O | β-OH | =O | H | α-OH | β-CH3 | COOCH3 |
| 11 | β-OH | β-OH | =O | H | =O | β-CH3 | COOH |
| 12 | β-OH | β-OH | =O | H | =O | β-CH3 | COOCH3 |
| 13 | β-OH | β-OH | =O | H | α-OH | β-CH3 | COOH |
| 14 | β-OH | β-OH | =O | H | α-OH | β-CH3 | COOCH3 |
| 15 | =O | β-OH | =O | H | =O | β-CH3 | COOH |
| 16 | =O | β-OH | =O | H | =O | β-CH3 | COOCH3 |
| 17 | β-OH | β-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 18 | β-OH | =O | =O | H | α-OH | α-CH3 | COOCH3 |
| 19 | =O | α-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 20 | β-OH | α-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 21 | O-CHO | β-OH | =O | β-OH | =O | β-CH3 | COOH |
| 22 | =O | α-OH | =O | H | α-OH | α-CH3 | COOH |
| 23 | =O | β-OH | =O | H | =O | α-CH3 | COOH |
| 24 | =O | =O | =O | β-O-COCH3 | =O | α-CH3 | COOEt |
| 25 | β-OH | β-OH | =O | β-O-COCH3 | =O | α-CH3 | COOCH3 |
| 26 | β-OH | =O | =O | =O | =O | α-CH3 | COOH |
| 27 | =O | β-OH | β-OH | H | =O | α-CH3 | COOH |
| 28 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | COOH |
| 29 | =O | =O | =O | β-O-Ac | =O | β-CH3 | COOH |
| 30 | =O | =O | =O | H | =O | β-CH3 | COOH |
| 31 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | COOH |
| 32 | β-OH | =O | =O | H | =O | α-CH3 | COOH |
| 33 | =O | =O | =O | H | α-OH | α-CH3 | COOH |
| 34 | β-OH | β-OH | =O | H | α-OH | α-CH3 | COOH |
| 35 | β-OH | β-OH | =O | β-OH | =O | β-CH3 | COOH |
| 36 | =O | β-OH | =O | β-OH | =O | α-CH3 | COOH |
| 37 | =O | =O | =O | β-OH | =O | α-CH3 | COOH |
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 38 | =O | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 39 | =O | β-OH | =O | H | α-OH | α-CH3 | H | COOH |
| 40 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 41 | β-OH | H | =O | β-O-Ac | α-O-Ac | α-CH3 | H | COOH |
| 42 | =O | =O | =O | H | =O | α-CH3 | β-OH | COOH |
| 43 | β-OH | =O | H | H | H | β-CH3 | H | COOH |
| 44 | =O | OH | =O | H | =O | α-CH3 | OH | COOH |
| 45 | =O | =O | H | H | H | α-CH3 | H | CHO |
| 46 | β-OH | =O | H | H | H | α-CH3 | H | CHO |
| 47 | =O | β-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 48 | =O | α-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 49 | β-OH | β-OH | =O | H | =O | α-CH3 | β-OH | COOH |
| 50 | β-OH | =O | =O | H | =O | α-CH3 | β-OH | COOH |
| 51 | β-OH | β-OH | =O | β-OH | =O | α-CH3 | β-OH | COOH |
| 52 | β-OH | =O | =O | β-OH | =O | α-CH3 | β-OH | COOH |
| 53 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 54 | =O | H | =O | H | α-OH | β-CH3 | H | COOH |
| 55 | =O | α-OH | H | H | H | β-CH3 | H | CHO |
| 56 | α-O-Ac | α-O-Ac | H | H | α-OH | β-CH3 | H | COOH |
| 57 | =O | =O | =O | H | H | α-CH3 | H | CHO |
| 58 | =O | =O | H | H | H | α-CH3 | H | CH2OH |
| 59 | α-O-Ac | α-O-CH3 | H | H | α-OH | β-CH3 | H | COOH |
| 60 | =O | =O | α-OH | H | H | α-CH3 | H | COOH |
| 61 | =O | =O | β-OH | H | H | α-CH3 | H | COOH |
| 62 | β-OH | =O | H | H | H | α-CH3 | H | CH2OH |
| 63 | β-OH | =O | H | H | H | α-CH3 | H | CHO |
| 64 | =O | =O | H | H | H | α-CH3 | H | COOH |
| 65 | =O | α-OH | H | H | α-O-Ac | α-CH3 | H | COOH |
| 66 | β-OH | β-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 67 | β-OH | H | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 68 | =O | β-OH | =O | H | α-OH | α-CH3 | H | CHO |
| 69 | =O | H | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 70 | α-O-Ac | α-OH | H | H | α-O-Ac | β-CH3 | H | COOH |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 71 | Ganoderic acid Mb (3α, 15α, 22-triacetoxy-7α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 72 | Ganoderic acid Mc (3α, 7α, 22-triacetoxy-15α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 73 | Ganoderic acid Md (3α, 22-diacetoxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 74 | Ganoderic acid Mg (3α, 22-diacetoxy-15α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 75 | Ganoderic acid Mh (3α, 22-diacetoxy-7α, 15α-dihydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 76 | Ganoderic acid Mj (22-acetoxy-3α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 77 | 3α, 22β-Diacetoxy-7α-hydroxyl-5α-lanost-8,24E-dien-26-oic acid | G. lucidum (mycelial mat) | [49] |
| 78 | Ganorbiformin B | G. orbiforme | [46] |
| 79 | Ganorbiformin C | G. orbiforme | [46] |
| 80 | Ganorbiformin D | G. orbiforme | [46] |
| 81 | Ganorbiformin E | G. orbiforme | [46] |
| 82 | Ganorbiformin F | G. orbiforme | [46] |
| 83 | Ganoderic acid O ((22S, 24E)-3α, l5α, 22-triacetoxy-7α-hydroxy-5α-lanosta-7,24-dien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 84 | 7-O-Methylganoderic acid O ((22S, 24E)-3α, l5α, 22-triacetoxy-7α-methoxy-5α-lanosta-8,24-dien-26-oic acid) | G. Lucidum (cultured mycelium) | [50] |
| 85 | 12β-Acetoxy-3β-hydroxy-7,11,15,23-tetraoxo-lanost-8,20E-diene-26-oic acid | G. lucidum (fruit bodies) | [32] |
| 86 | 23-Dihydroganoderenic acid D (7β, 23ξ-dihydroxy-3,11,15-trioxolanosta-8,20E(22)-dien-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 87 | Methyl ganoderenate D (7β-hydroxy-3,11,15,23-tetraoxolanosta-8,20E(22)-dien-26-oic acid methyl ester) | G. applanatum (fruit bodies) | [51] |
| 88 | Ganoderenic acid A ((20E)-7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 89 | Ganoderenic acid B ((20E)-3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 90 | Ganoderenic acid C ((20E)-3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 91 | Ganoderenic acid D ((20E)-7β-hydroxy-3,11,15,23-tetraoxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 92 | 12β-Acetoxy-7β-hydroxy-3,11,15,23-tetraoxo-5α-lanosta-8,20-dien-26-oic acid | G. lucidum | [26] |
| 93 | Ganoderenic acid F (3,7,11,15,23-pentaoxo-5α-lanosta-8,20E-dien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
| 94 | Ganoderenic acid G (15α-hydroxy-3,7,11,23-tetraoxo-5α-lanosta-8,20E-dien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
| 95 | Methy ganoderenate H (methyl 3β-hydroxy-7,11,15,23-tetraoxo-5α-lanosta-8,20E-dien-26-oate) | G. applanatum (fruit bodies) | [52] |
| 96 | Methyl ganoderenate I (3β, 15α-dihydroxy-7,11,23-trioxo-5α-lanosta-8,20E-dien-26-oate) | G. applanatum (fruit bodies) | [52] |
| 97 | Ganoderenic acid H | G. lucidum (fruit bodies) | [32] |
| 98 | 12β-Acetoxy-3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanosta-8,20-dien-26-oic acid | G. lucidum | [26] |
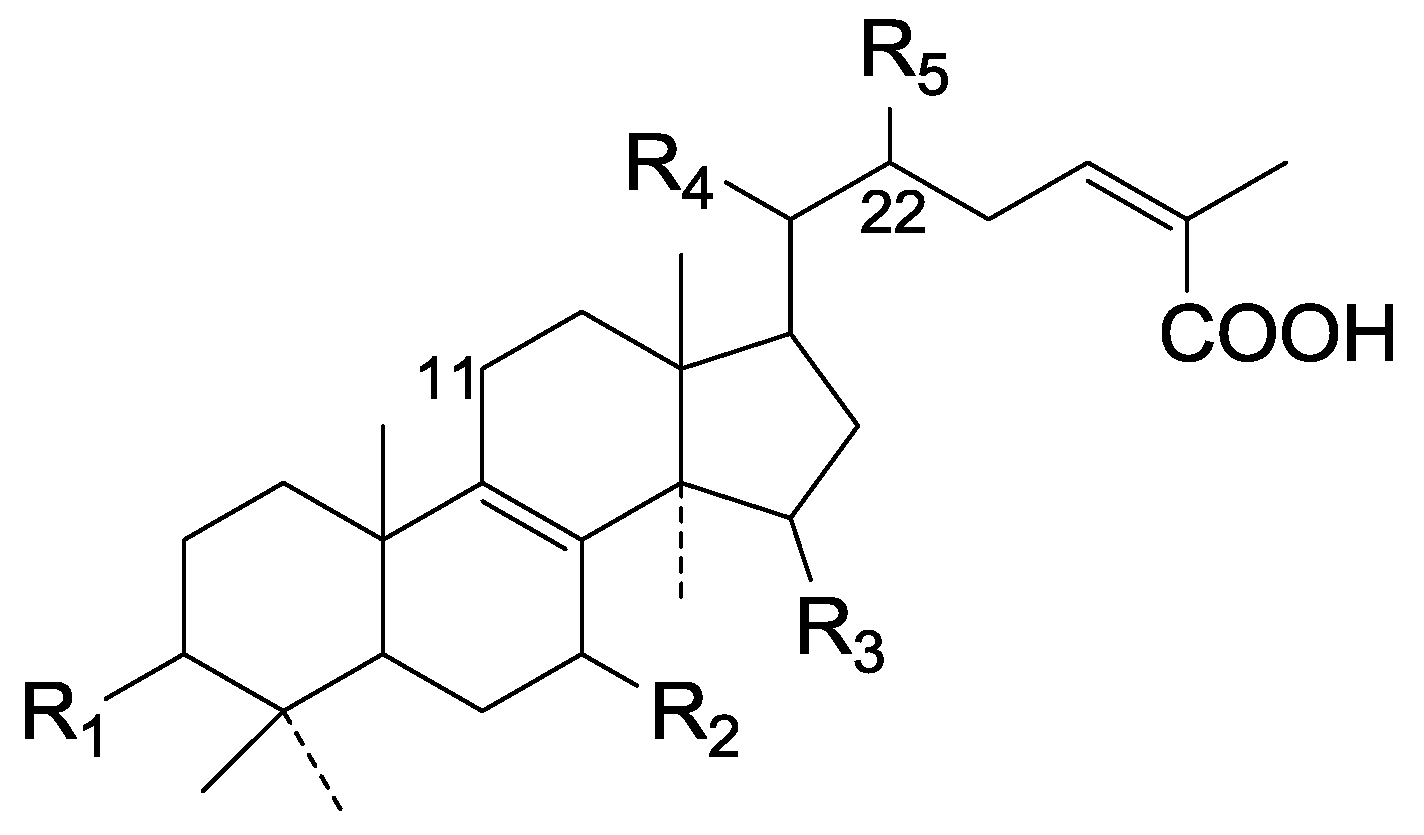
| Cpd | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 71 | α-O-Ac | α-OH | α-O-Ac | β-CH3 | ξ-O-Ac |
| 72 | α-O-Ac | α-O-Ac | α-OH | β-CH3 | ξ-O-Ac |
| 73 | α-O-Ac | α-O-CH3 | H | β-CH3 | ξ-O-Ac |
| 74 | α-O-Ac | α-O-CH3 | α-OH | β-CH3 | ξ-O-Ac |
| 75 | α-O-Ac | α-OH | α-OH | β-CH3 | ξ-O-Ac |
| 76 | α-OH | α-O-CH3 | H | β-CH3 | ξ-O-Ac |
| 77 | α-O-Ac | α-OH | H | α-CH3 | β-O-Ac |
| 78 | β-O-Ac | =O | H | α-CH3 | β-O-Ac |
| 79 | β-OH | =O | H | α-CH3 | β-O-Ac |
| 80 | =O | α-OH | α-O-Ac | α-CH3 | β-O-Ac |
| 81 | =O | α-OH | H | α-CH3 | β-O-Ac |
| 82 | =O | α-O-CH3 | H | α-CH3 | β-O-Ac |
| 83 | α-O-Ac | α-OH | α-O-Ac | α-CH3 | β-O-Ac |
| 84 | α-O-Ac | α-O-CH3 | α-O-Ac | α-CH3 | β-O-Ac |
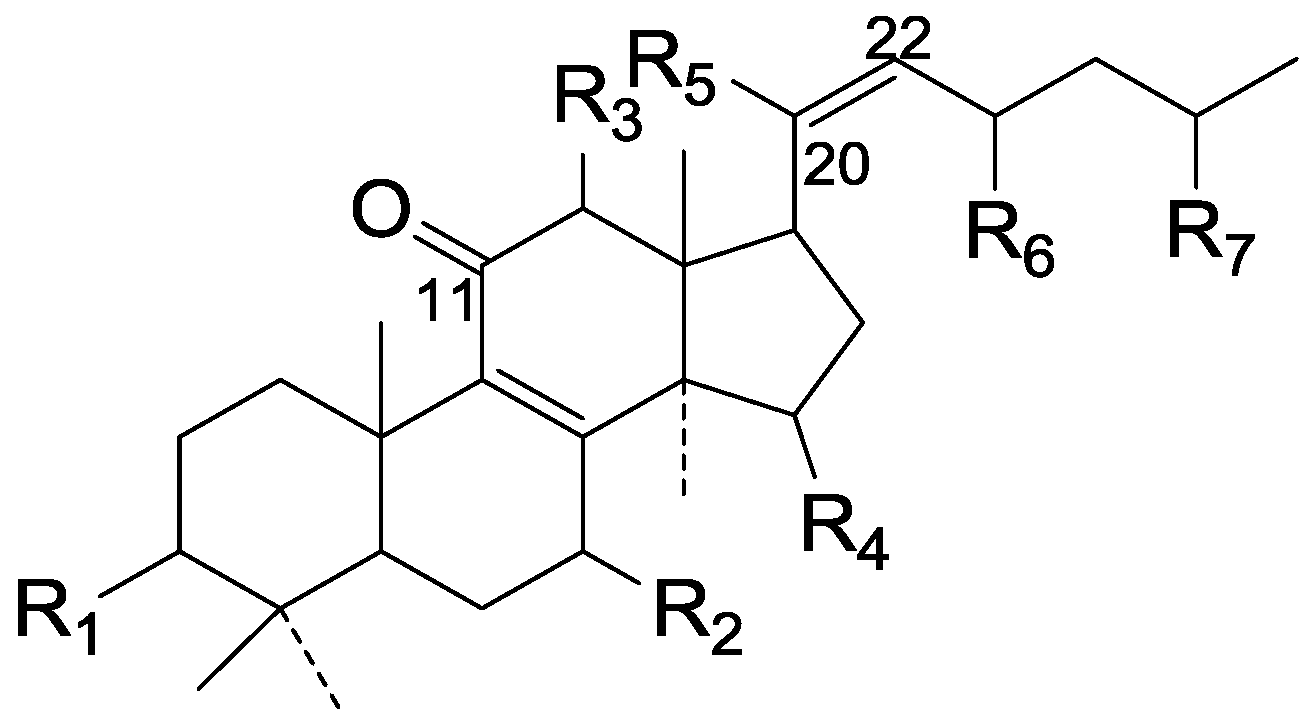
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 85 | β-OH | =O | β-O-Ac | =O | β-CH3 | =O | COOH |
| 86 | =O | β-OH | H | =O | β-CH3 | ξ-OH | ξ-COOH |
| 87 | =O | β-OH | H | =O | β-CH3 | =O | ξ-COOCH3 |
| 88 | =O | β-OH | H | α-OH | β-CH3 | =O | COOH |
| 89 | β-OH | β-OH | H | =O | β-CH3 | =O | COOH |
| 90 | β-OH | β-OH | H | α-OH | β-CH3 | =O | COOH |
| 91 | =O | β-OH | H | =O | β-CH3 | =O | COOH |
| 92 | =O | β-OH | β-O-Ac | =O | β-CH3 | =O | COOH |
| 93 | =O | =O | H | =O | β-CH3 | =O | COOH |
| 94 | =O | =O | H | α-OH | β-CH3 | =O | COOH |
| 95 | β-OH | =O | H | =O | β-CH3 | =O | COCH3 |
| 96 | β-OH | =O | H | α-OH | β-CH3 | =O | COCH3 |
| 97 | β-OH | =O | H | =O | β-CH3 | =O | COOH |
| 98 | β-OH | β-OH | β-O-Ac | =O | α-CH3 | =O | COOH |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 99 | Methyl ganoderate D (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [53,54] |
| 100 | Methyl ganoderate E (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [54,55] |
| 101 | Methyl ganoderate F (methyl 12β-acetoxy-3,7,11,15,23-pentaoxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [56] |
| 102 | Methyl ganoderate H (methyl 3β-hydroxy-12β-acetoxy-7,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [30,56] |
| 103 | Methyl ganoderate G (methyl 3β, 7β, 12β-trihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [20] |
| 104 | Compound C5 | G. lucidum (gill surface) | [30] |
| 105 | Compound C6 | G. lucidum (gill surface) | [30] |
| 106 | Ganoderic acid AP3 (15α, 20ξ-dihydroxy-3,7,11,23-tetraoxolanost-8-en-26-oic acid) | G. applanatum (fruit bodies) | [34] |
| 107 | 23-Dihydroganoderic acid I (3β, 7β, 20,23ξ-tetrahydroxy-11,15-dioxolanosta-8-en-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 108 | 23-Dihydroganoderic acid N (7β, 20,23ξ-trihydroxy-3,11,15-trioxolanosta-8-en-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 109 | 20-Hydroxylganoderic acid G | G. lucidum (fruit bodies) | [57] |
| 110 | Ganoderic acid I | G. lucidum (gills) | [22] |
| 111 | Lucidumol A ((24S)-24,25-dihydroxylanost-8-ene-3,7-dione) | G. lucidum (spores) | [38] |
| 112 | Ganoderiol C (7α-ethoxy-24,25,26-trihydroxy-5α-lanost-8-en-3-one) | G. lucidum (fruit bodies) | [39] |
| 113 | Ganoderiol D (24,25,26-trihydroxy-5α-lanost-8-en-3,7-dione) | G. lucidum (fruit bodies) | [39] |
| 114 | Ganoderiol G (24,25,26-trihydroxy-7α-methoxy-5α-lanost-8-en-3-one) | G. lucidum (fruit bodies) | [39] |
| 115 | Ganoderiol H (3β, 24,25,26-tetrahydroxy-5α-lanost-8-en-7-one) | G. lucidum (fruit bodies) | [39] |
| 116 | Ganoderitriol M ((24S)-lanosta-7-oxo-8-en-3β, 24,25-triol) | G. lucidum (fruit bodies) | [58] |
| 117 | Sinensoic acid (3,26-dihydroxy-5-lanosta-8,24E-dien-21-oic acid) | G. sinense (fruit bodies) | [59] |
| 118 | Tsugarioside B (3α-acetoxy-5α-lanosta-8,24-diene-21-O-β-d-xyloside) | G. tsugae (fruit bodies) | [60] |
| 119 | Tsugaric acid A (3α-acetoxy-5α-lanosta-8,24-dien-21-oic acid) | G. tsugae | [61] |
| 120 | Ganosinoside A (3-oxo-5α-lanosta-8,24-dien-21-oic acid ester β-d-glucoside) | G. sinense (fruit bodies) | [47] |
| 121 | Tsugarioside A (3α-acetoxy-5α-lanosta-8,24-dien-21-oic acid ester β-d-glucoside) | G. tsugae (fruit bodies) | [60] |
| 122 | 3-Oxo-5α-lanosta-8,24-dien-21-oic acid | G. resinaceum (fruit bodies) | [62] |
| 123 | 3β-Hydroxy-5α-lanosta-8,24-dien-21-oic acid | G. tsugae (fruit bodies) | [60] |
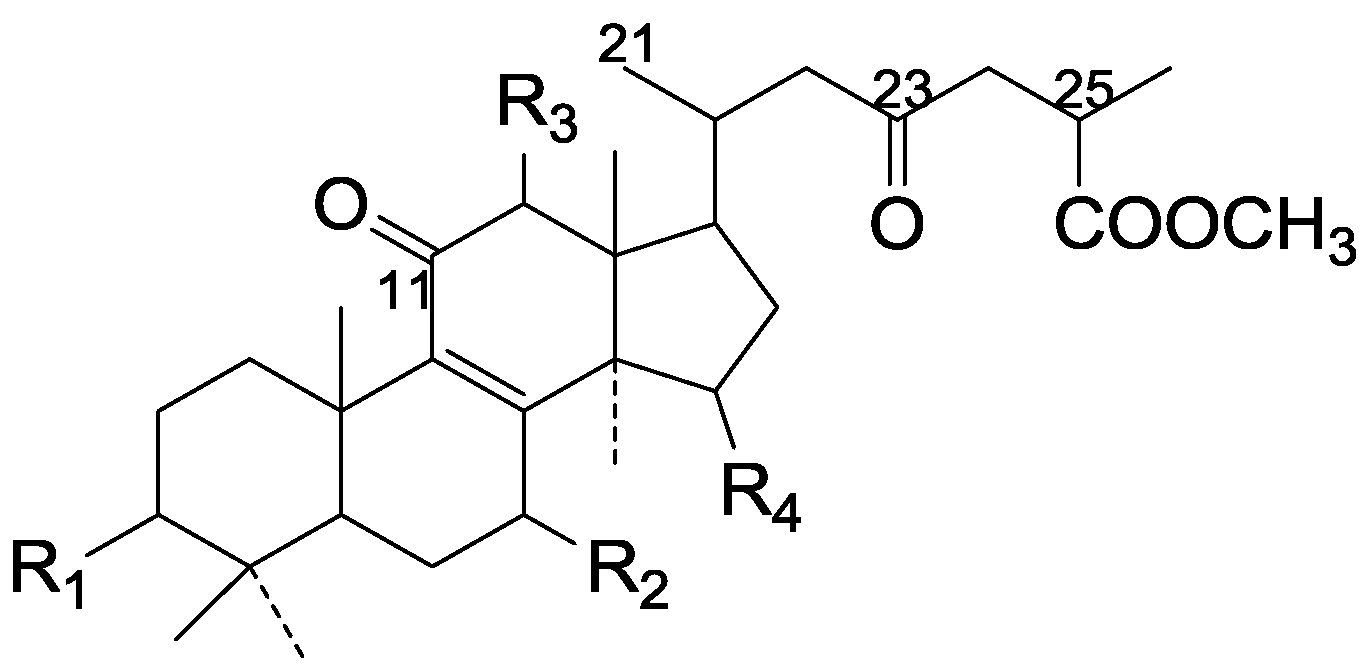
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 99 | β-OH | β-OH | H | α-OH |
| 100 | =O | =O | H | =O |
| 101 | =O | =O | β-O-Ac | =O |
| 102 | β-OH | =O | β-O-Ac | =O |
| 103 | β-OH | β-OH | β-OH | =O |
| 104 | =O | β-OH | OH | =O |
| 105 | β-OH | =O | OH | =O |
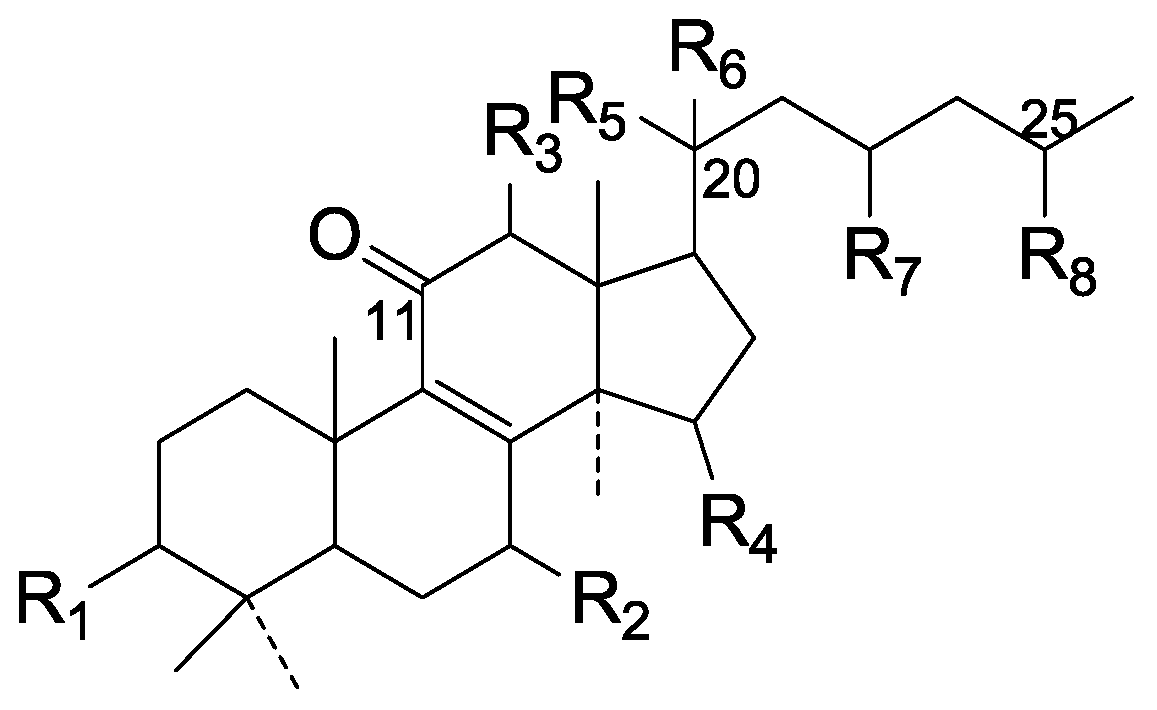
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 106 | =O | =O | H | α-OH | β-CH3 | ξ-OH | =O | COOH |
| 107 | β-OH | β-OH | H | =O | α-CH3 | β-OH | ξ-OH | ξ-COOH |
| 108 | =O | β-OH | H | =O | α-CH3 | β-OH | ξ-OH | ξ-COOH |
| 109 | β-OH | β-OH | β-OH | =O | β-CH3 | β-OH | =O | COOH |
| 110 | β-OH | β-OH | =O | =O | α-CH3 | ξ-OH | =O | COOH |
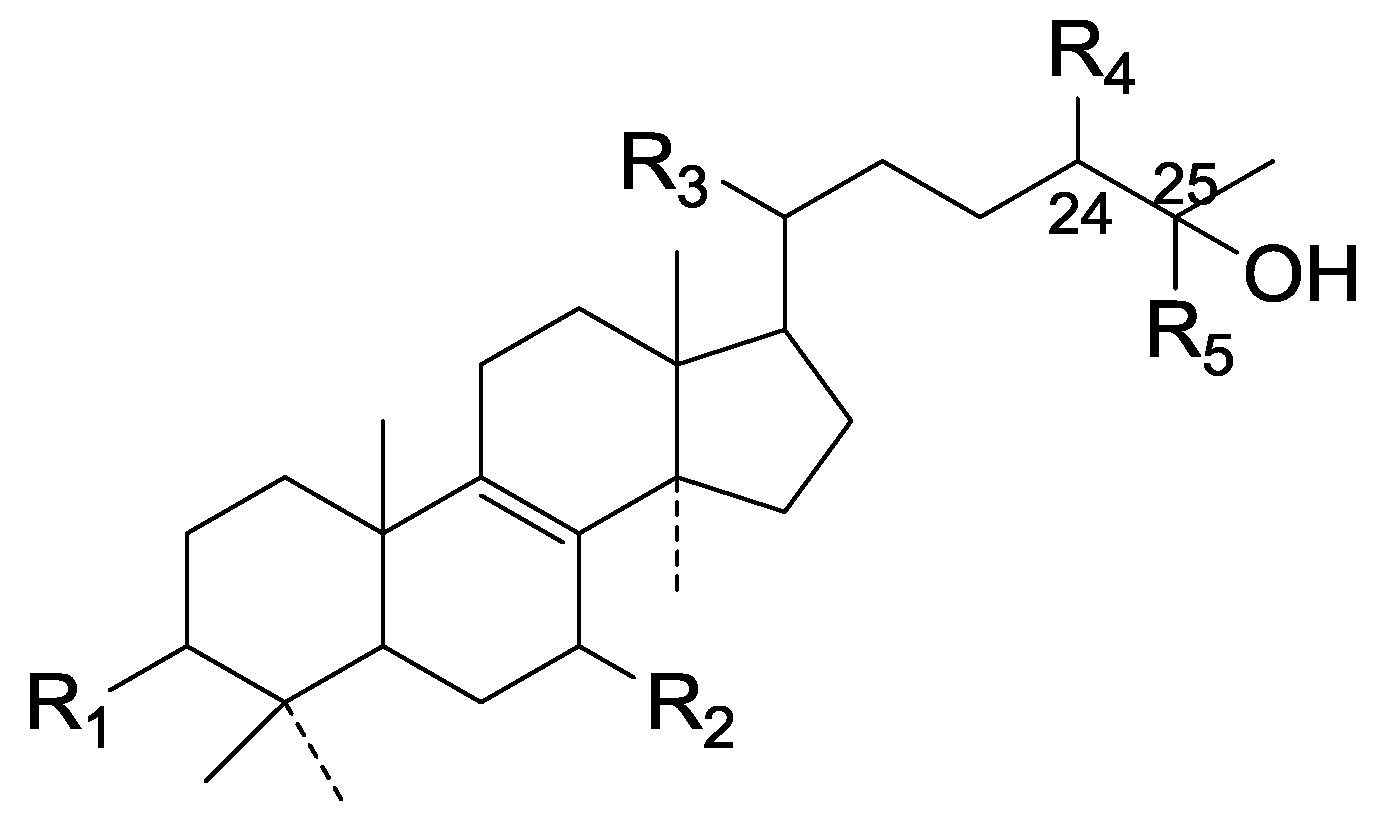
| Cpd | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 111 | =O | =O | α-CH3 | α-OH | CH3 |
| 112 | =O | α-O-Et | β-CH3 | ξ-OH | CH2OH |
| 113 | =O | =O | β-CH3 | ξ-OH | CH2OH |
| 114 | =O | α-O-CH3 | β-CH3 | ξ-OH | CH2OH |
| 115 | β-OH | =O | β-CH3 | ξ-OH | CH2OH |
| 116 | β-OH | =O | α-CH3 | α-OH | CH3 |
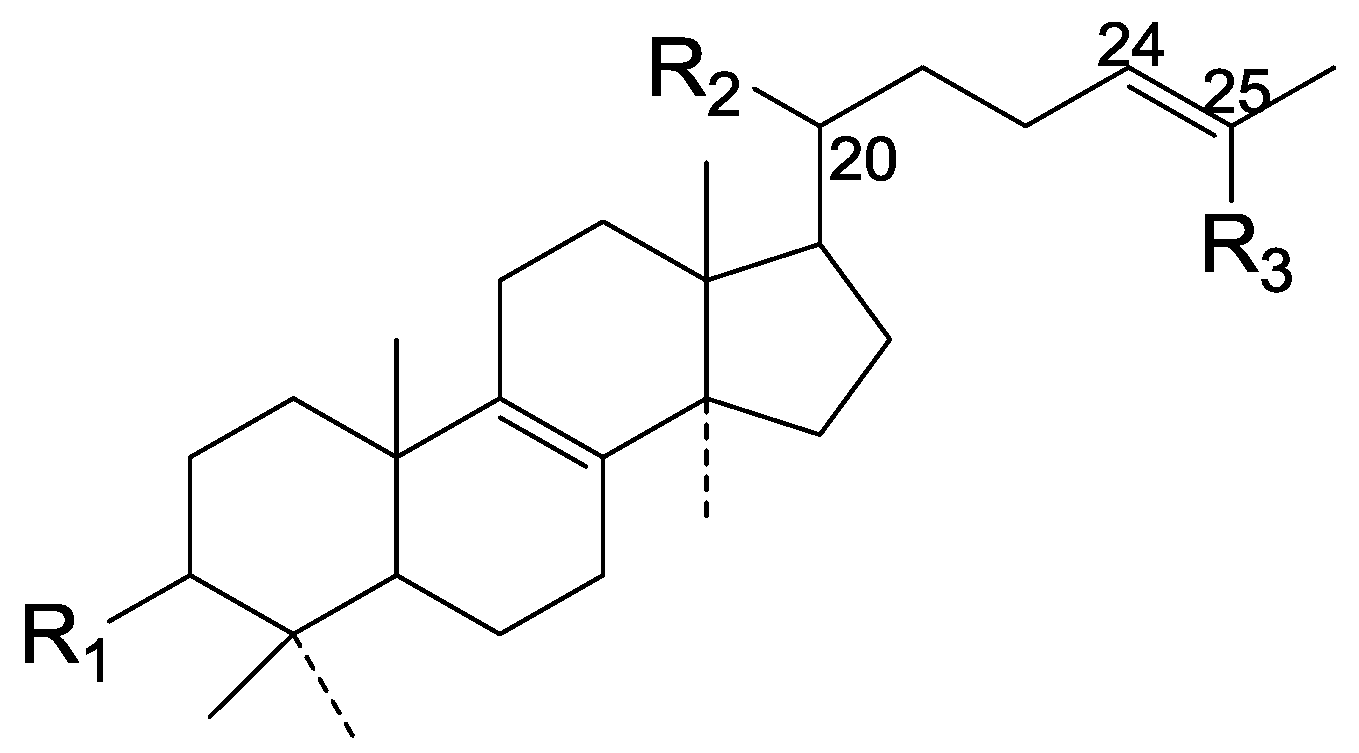
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 117 | β-OH | α-COOH | CH2OH |
| 118 | α-O-Ac | CH2O-β-D-xylosyl | CH3 |
| 119 | α-O-COCH3 | α-COOH | CH3 |
| 120 | =O | α-COO-β-D-glucopyranosyl | CH3 |
| 121 | α-O-Ac | α-COOH | CH3 |
| 122 | =O | α-COOH | CH3 |
| 123 | β-OH | α-COOH | CH3 |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 124 | 3β, 7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | G. lucidum (fruit bodies) | [63] |
| 125 | 3β, 7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid methyl ester | G. lucidum (fruit bodies) | [63] |
| 126 | 12β-Acetoxy-3β, 7β-dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | G. lucidum (fruit bodies) | [63] |
| 127 | Methyl ganoderate I | G. lucidum | [20,22] |
| 128 | Methyl ganoderate AP (methyl 12β, l5α, 20-trihydroxy-3,7,11,23- tetraoxo-5α-lanost-8-en-26-oate) | G. applanatum (fruit bodies) | [52] |
| 129 | Methyl ganoderate N (Methyl 7β, 20-dihydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [64] |
| 130 | Methyl ganoderate M (methyl 7β, 12α-dihydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [64] |
| 131 | Ganoderiol E (3β, 26,27-trihydroxy-5α-lanosta-8,24-dien-7-one) | G. lucidum (fruit bodies) | [39] |
| 132 | Ganoderiol I (15α, 26,27-trihydroxy-5α-lanosta-8,24-dien-3-one) | G. lucidum (fruit bodies) | [39] |
| 133 | Ganoderiol J (26,27-dihydroxy-5α-lanosta-8,24-dien-3,7-dione) | G. sinense (fruit bodies) | [47] |
| 134 | Epoxyganoderiol A (24S, 25S-epoxy-7α, 26-dihydroxy-5α-lanost-8-en-3-one) | G. lucidum | [40] |
| 135 | Ganoderone C (5α-lanosta-8-ene-24,25-epoxy-26-hydroxy-3,7-dione) | G. pfeifferi (fruit bodies) | [42] |
| 136 | 3-O-Acetylganoderic acid B (3β-acetoxy-7β-hydroxy-11,15,23-trioxolanost-8-en-26-oic acid) | G. lucidum (mycelia) | [65] |
| 137 | 3-O-Acetylganoderic acid K (3β-acetyloxy-15α-hydroxy-7,11,23-trioxolanost-8-en-26-oic acid) | G. lucidum (mycelia) | [65] |
| 138 | Ethyl 3-O-acetylganoderate B | G. lucidum (mycelia) | [65] |
| 139 | Ethyl ganoderate J | G. lucidum (mycelia) | [65] |
| 140 | Applanoxidic acid G (15β, 20-dihydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [66] |
| 141 | Applanoxidic acid H (3β, 12α, 20-trihydroxy-7α, 8α-epoxydioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [66] |
| 142 | 8β, 9α-Dihydroganoderic acid J | G. lucidum (fruit bodies) | [57] |
| 143 | Methyl 8β, 9α-dihydroganoderate J | G. lucidum (fruit bodies) | [57] |
| 144 | Ganosporeric acid A (3,7,11,12,15,23-hexaoxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (spores) | [67] |
| 145 | 24ξ-Methyl-5α-lanosta-25-one | G. applanatum (fruit bodies) | [68] |
| 146 | 3α-Carboxyacetoxy-24-methylene-23-oxolanost-8-en-26-oic acid | G. applanatum (fruit bodies) | [69] |
| 147 | 3α-Carboxyacetoxy-24-methyl-23-oxolanost-8-en-26-oic acid | G. applanatum (fruit bodies) | [69] |
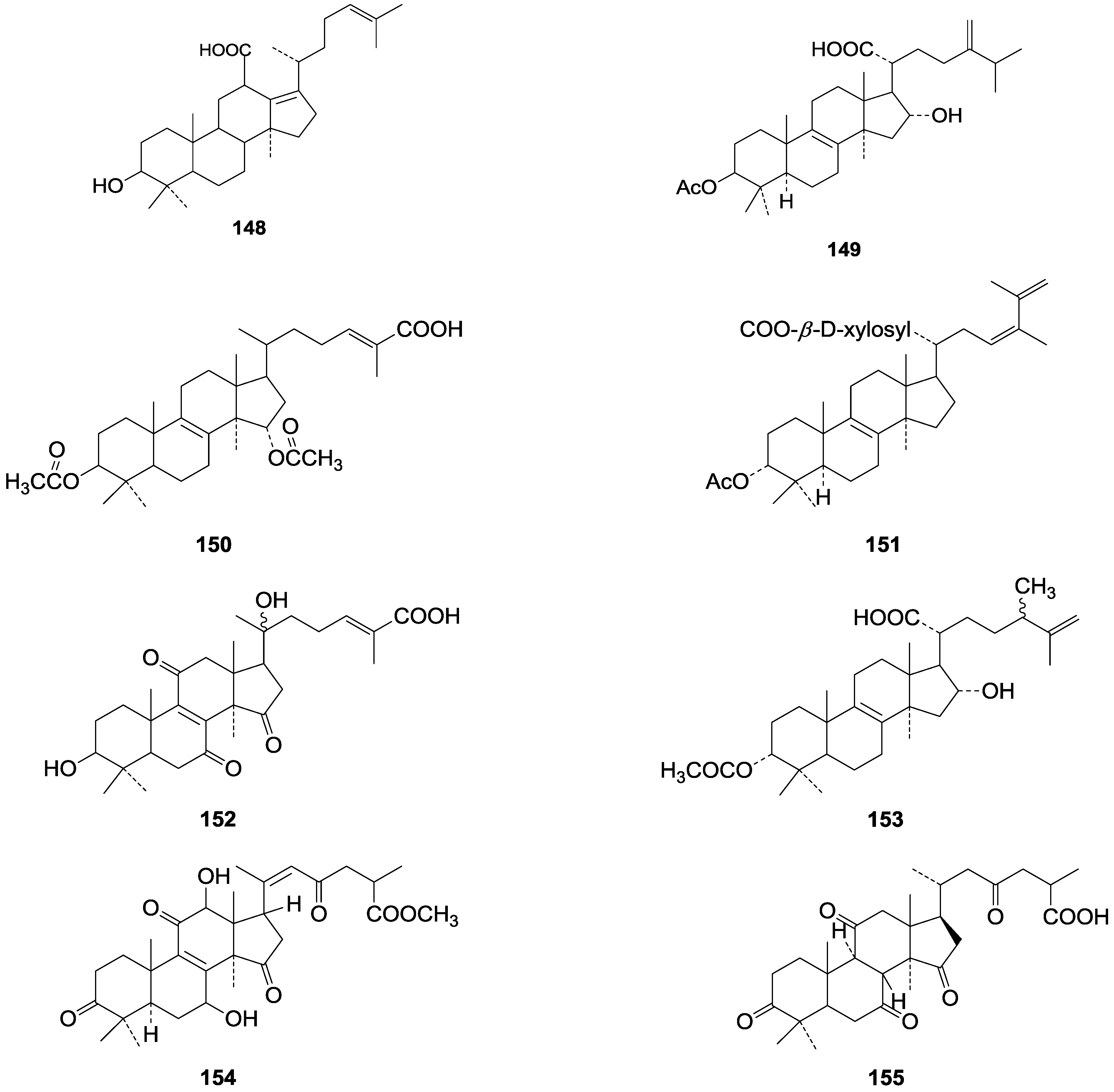
| 148 | Fornicatin C ((3β)-3-hydroxy-18(13→12β)-abeo-lanosta-13(17),24-dien-18-oic acid) | G. fornicatum (fruit bodies) | [70] |
| 149 | 3-Epipachymic acid (3α-acetoxy-16α-hydroxy-24-methylene-5α-lanost-8-en-21-oic acid) | G. resinaceum (fruit bodies) | [62] |
| 150 | 3β, 15α-Diacetoxylanosta-8,24-dien-26-oic acid | G. lucidum (mycelia) | [71] |
| 151 | Tsugaric acid C ((24R,S)-3α-acetoxy-24-hydroxy-5α-lanosta-8,25-dien-21-oic acid) | G. tsugae (fruit bodies) | [60] |
| 152 | Ganoderic acid V1 ((24E)-3β, 20ξ-dihydroxy-7,11,15-trioxo-5α-lanosta-8,24-dien-26-oic acid) | G. lucidum | [72] |
| 153 | Tsugaric acid B (3α-acetoxy-16α-hydroxy-24ξ-methyl-5α-lanosta-8,25-dien-21-oic acid) | G. tsugae | [61] |
| 154 | Methyl ganoderenate E (7β, 12β-dihydroxy-3,11,15,23-tetraoxo-5α-lanosta-8,20E-dien-26-oate) | G. lucidum (fruit bodies) | [64] |
| 155 | 8β, 9α-Dihydroganoderic acid C | G. lucidum (mycelia) | [65] |
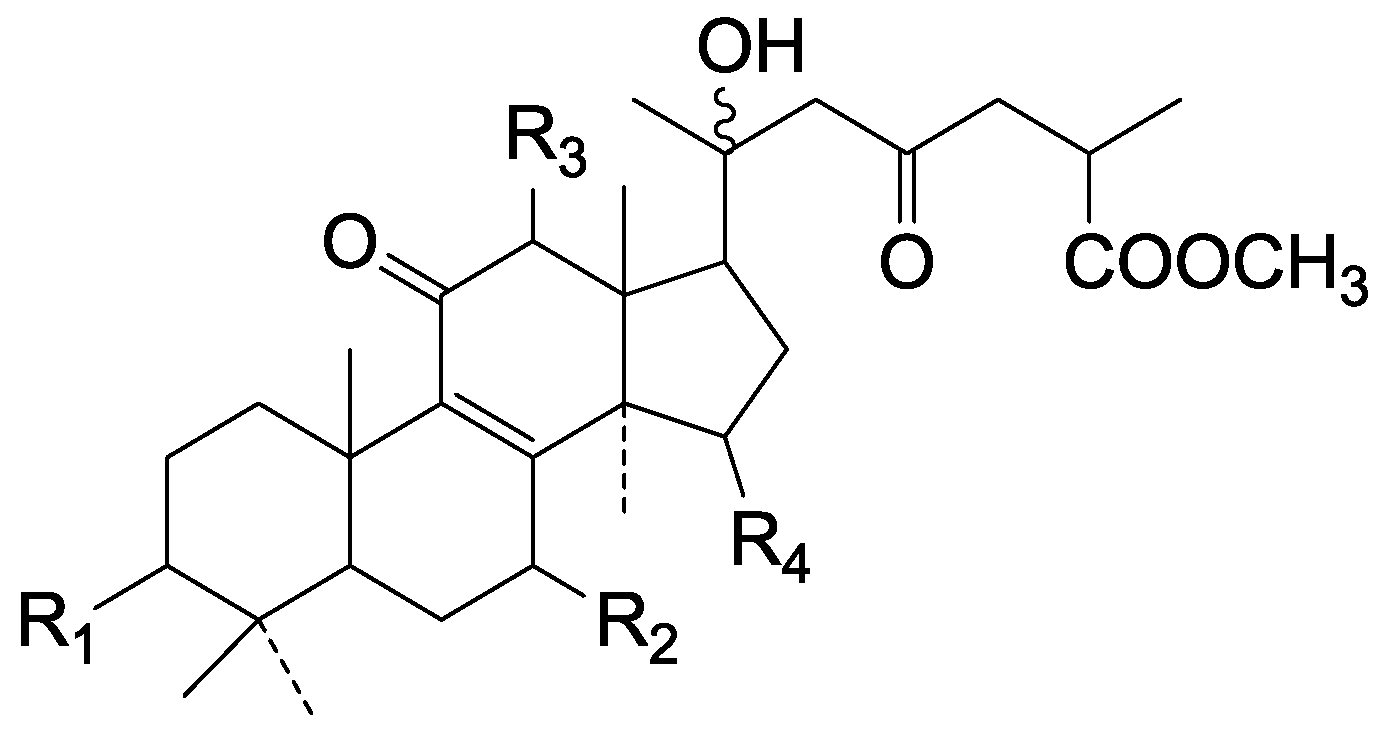
| Cpd | R1 | R2 |
|---|---|---|
| 124 | H | COOH |
| 125 | H | COOCH3 |
| 126 | β-O-Ac | COOH |
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 127 | β-OH | β-OH | H | =O |
| 128 | =O | =O | β-OH | α-OH |
| 129 | =O | β-OH | H | =O |
| 130 | =O | β-OH | α-OH | =O |
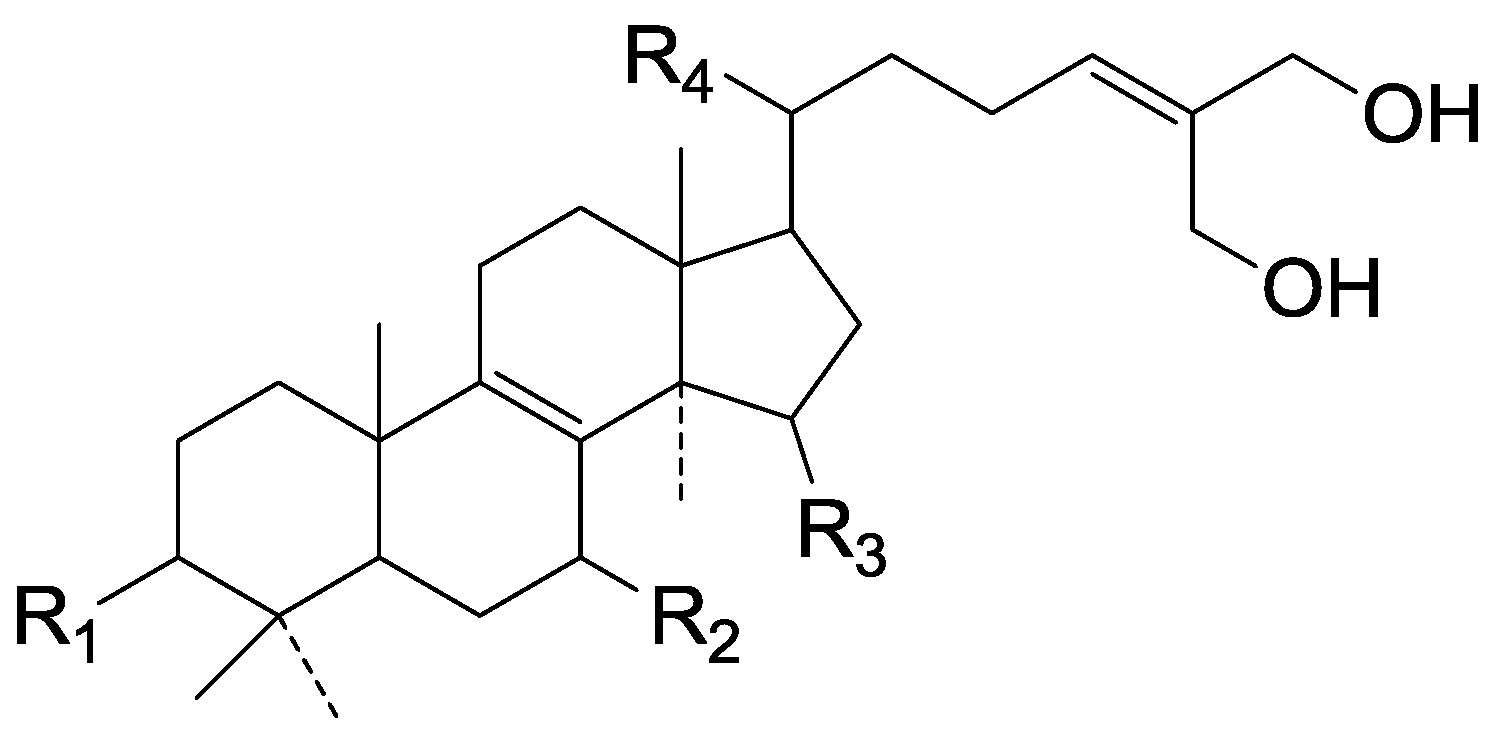
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 131 | β-OH | =O | H | β-CH3 |
| 132 | =O | β-CH3 | α-OH | β-CH3 |
| 133 | =O | =O | H | α-CH3 |
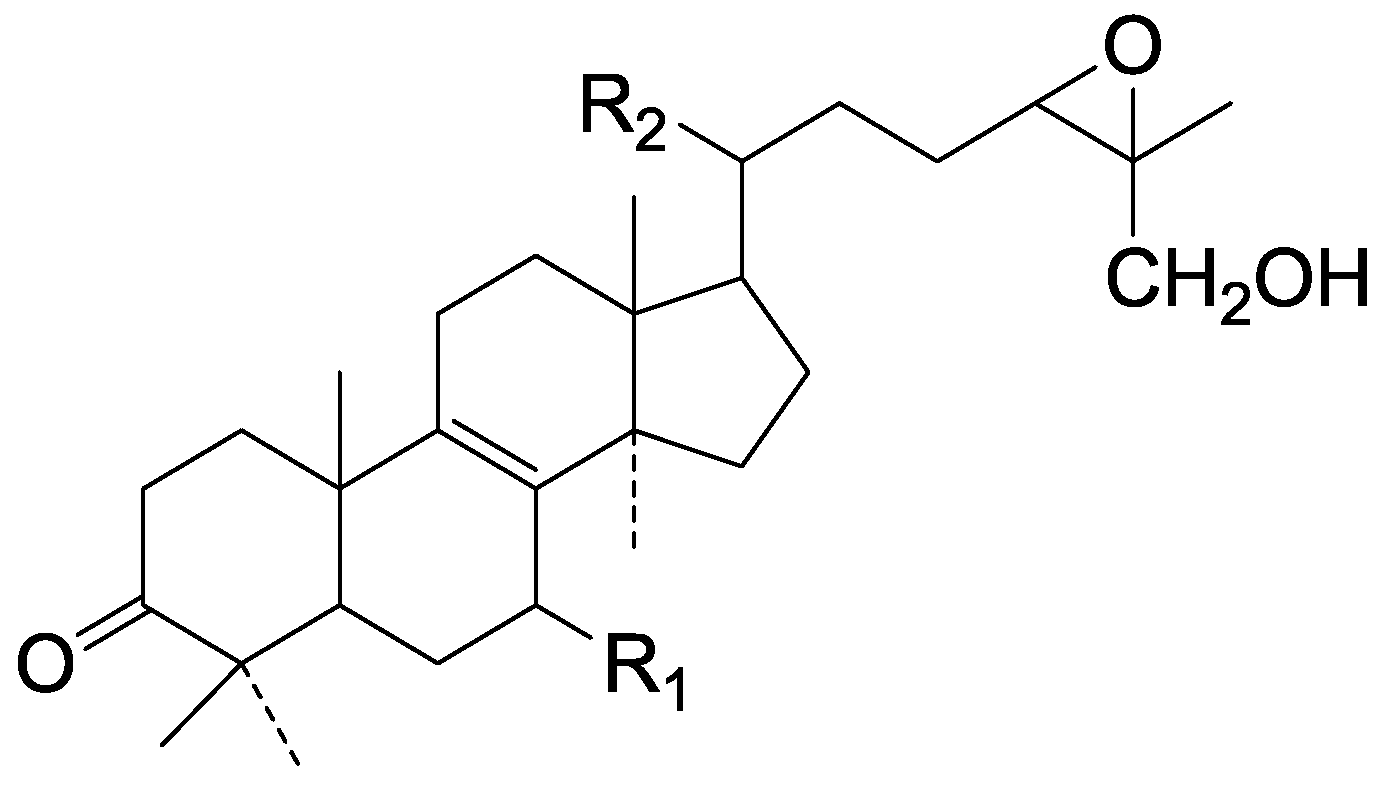
| Cpd | R1 | R2 |
|---|---|---|
| 134 | α-OH | β-CH3 |
| 135 | =O | α-CH3 |
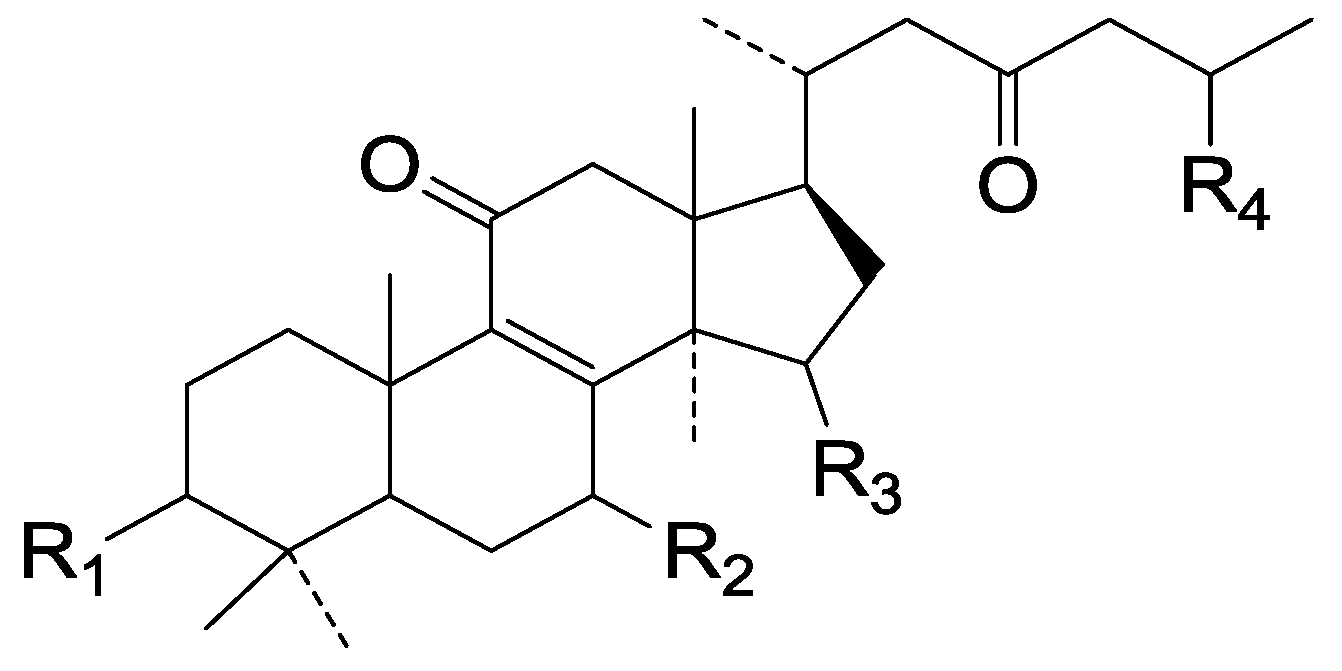
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 136 | β-O-Ac | β-OH | =O | COOH |
| 137 | β-O-Ac | =O | α-OH | COOH |
| 138 | β-O-Ac | β-OH | =O | COOEt |
| 139 | =O | =O | α-OH | COOEt |
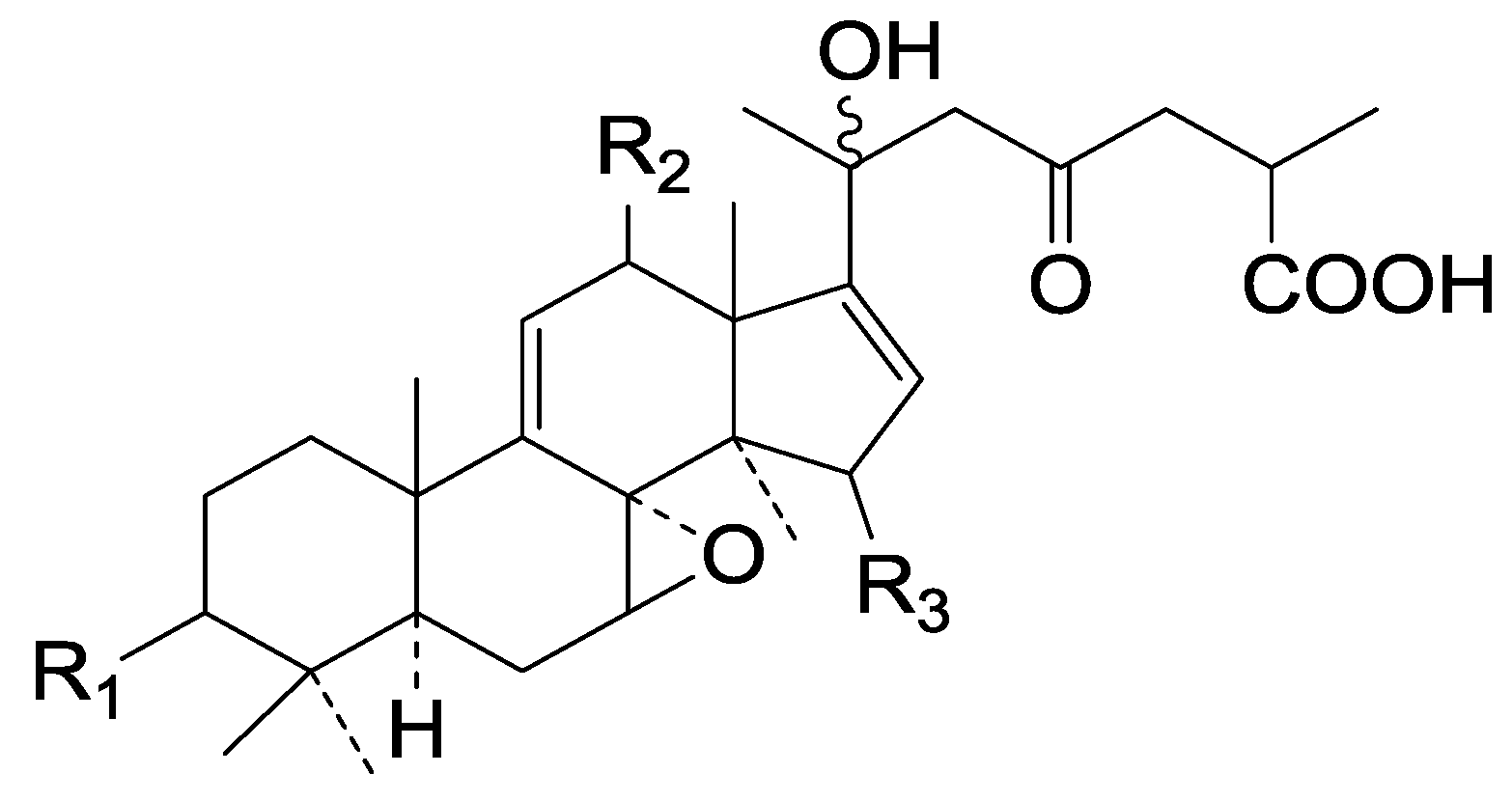
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 140 | =O | =O | β-OH |
| 141 | β-OH | α-OH | =O |
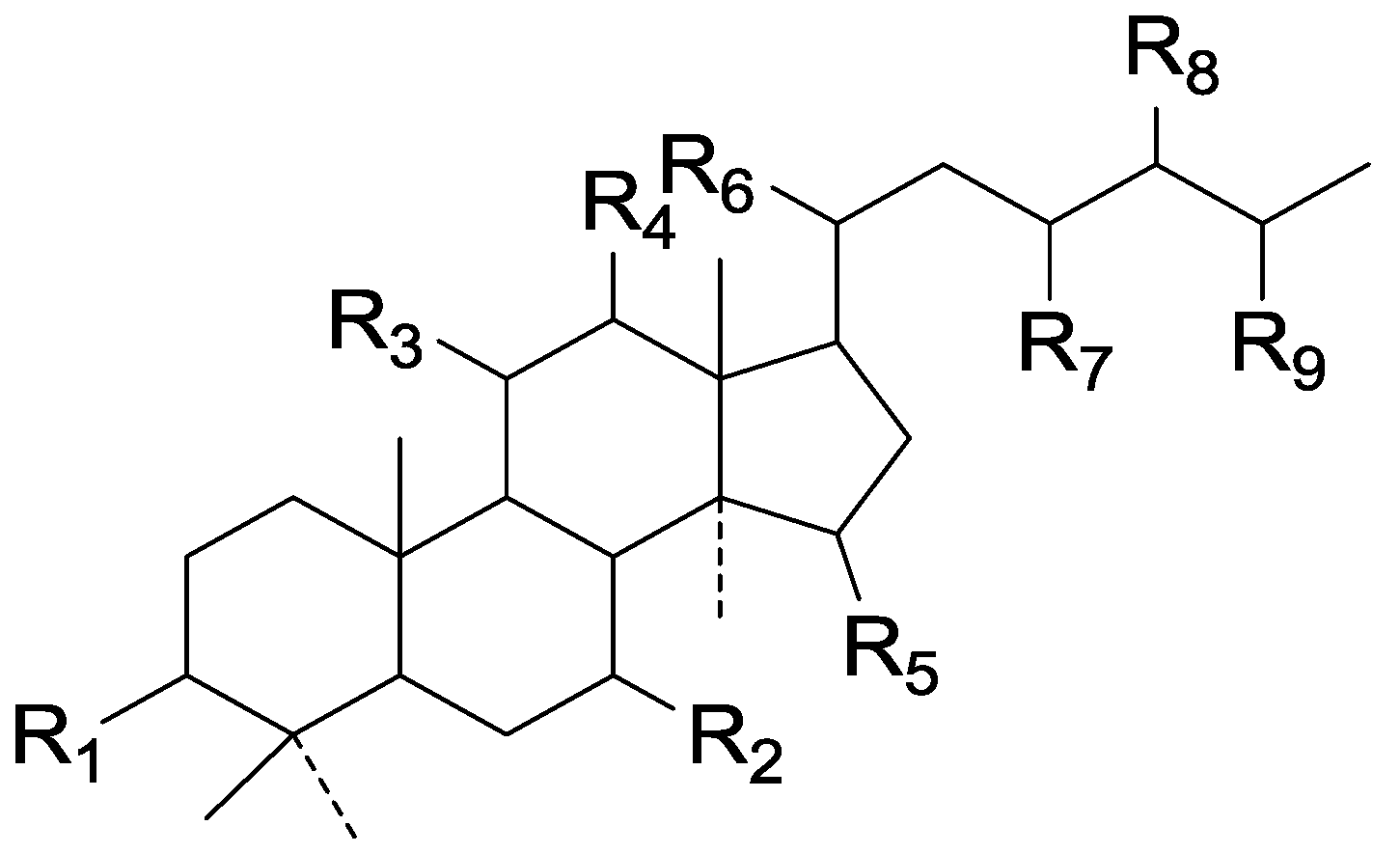
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
|---|---|---|---|---|---|---|---|---|---|
| 142 | =O | =O | =O | H | α-OH | β-CH3 | =O | H | COOH |
| 143 | =O | =O | =O | H | α-OH | β-CH3 | =O | H | COOCH3 |
| 144 | =O | =O | =O | =O | =O | β-CH3 | =O | H | COOH |
| 145 | H | H | H | H | H | α-CH3 | H | ξ-CH3 | =O |
| Cpd | R |
|---|---|
| 146 | CH2 |
| 147 | α-CH3 |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 156 | 15-Hydroxy-ganoderic acid S (15α-hydroxy-3-oxo-5α-lanosta-7,9(11),24(E)-trien-26-oic acid) | G. lucidum (fruit bodies) | [35] |
| 157 | 3α, 16α-Dihydroxylanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
| 158 | 3α, 16α, 26-Trihydroxylanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
| 159 | Ganoderic acid S1 | G. lucidum (fruit bodies) | [73] |
| 160 | Ganoderic acid SZ (3-oxo-lanosta-7,9(11),24(Z)-trien-26-oic acid) | G. lucidum (fruit bodies) | [74] |
| 161 | 5α-Lanosta-7,9(11),24-triene-15α-26-dihydroxy-3-one | G. concinna | [75] |
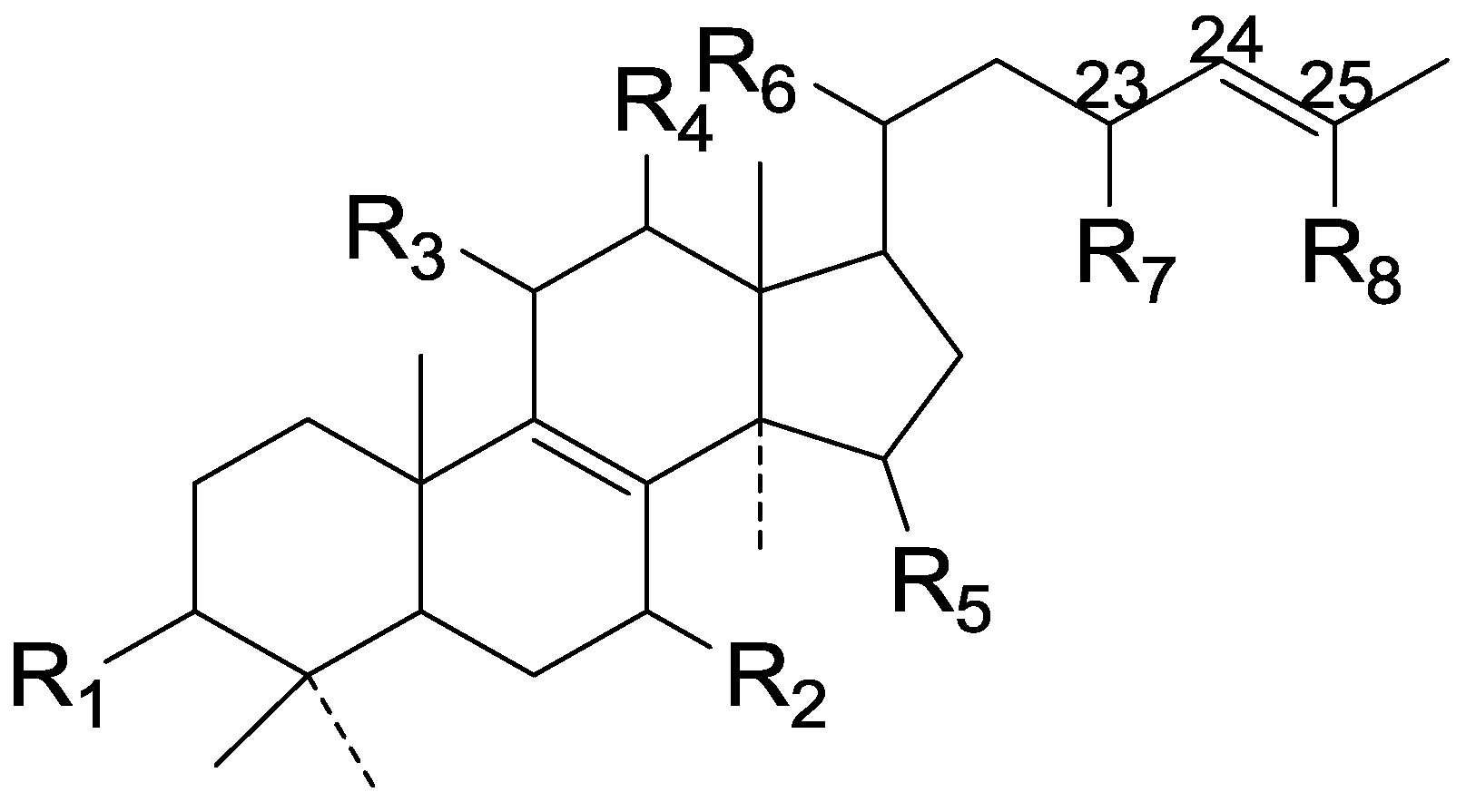
| 162 | Ganoderic acid Me (3α, 15α-diacetoxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (cultured mycelial mat) | [41] |
| 163 | Ganoderic acid Mf (3α-acetoxy-15α-hydroxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (cultured mycelial mat) | [41] |
| 164 | Ganodermenonol (26-hydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (dried fruit bodies) | [76] |
| 165 | Ganodermadiol (5α-lanosta-7,9(11),24-triene-3β, 26-diol) | G. lucidum (dried fruit bodies) | [76] |
| 166 | Ganodermatriol (5α-lanosta-7,9(11),24-triene-3β, 26,27-triol) | G. lucidum (fruit bodies) | [77] |
| 167 | Ganodermic acid S (lanosta-7,9(11),24-trien-3β, 15α-diacetoxy-26-oic acid) | G. lucidum | [78] |
| 168 | Carnosodione (26,27-dihydroxylanosta-7,9(11),24-trien-3,16-dione) | G. carnosum (fruit bodies) | [79] |
| 169 | Canoderol B ((24E)-5α-lanosta-7,9(11),24-trien-3,26-diol) | G. lucidum | [23] |
| 170 | Ganoderic acid Mk (3α, 22-diacetoxy-15α-hydroxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (mycelia mat) | [43] |
| 171 | Ganoderiol B (15α, 26,27-trihydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (fruit bodies) | [77] |
| 172 | Ganoderic acid T ((22S, 24E)-3α, 15α, 22-triacetoxy-5α-lanosta-7,9,(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [80] |
| 173 | Ganoderic acid S ((22S, 24E)-22-acetoxy-3α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [23,80] |
| 174 | Ganoderic acid R ((22S, 24E)-3α, 22-diacetoxy-5α-lanosta-7,9,(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [80] |
| 175 | Ganorbiformin G | G. orbiforme | [46] |
| 176 | Lanosta-7,9(11),24-trien-3β, 15α, 22β-triacetoxy-26-oic acid | G. lucidum | [81] |
| 177 | Lanosta-7,9(11),24-trien-15α-acetoxy-3α-hydroxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 178 | Lanosta-7,9(11),24-trien-3α, l5α-diacetoxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 179 | Lanosta-7,9(11),24-trien-3α, 15α-hydroxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 180 | Lanosta-7,9(11),24-trien-3α-acetoxy-15α, 22β-dihydroxy-26-oic acid | G. lucidum | [81] |
| 181 | Ganodermic acid T-N (3β-hydroxy-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 182 | Ganodermic acid T-O (3β-acetoxy-15α-hydroxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 183 | Ganodermic acid T-Q (3β-oxo-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 184 | Compound 10 | G. orbiforme | [46] |
| 185 | Ganoderic acid P ((22S, 24E)-15α, 22-diacetoxy-3α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 186 | Ganoderic acid Q ((22S, 24E)-3α, 22-diacetoxy-15α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 187 | Ganoderic acid Jc (15α, 23-dihydroxy-3-oxo-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 188 | Ganodermatetraol (3β, 15α, 26,27-tetrahydroxy-5α-lanosta-7,9(11),24-triene) | G. sinense (fruit bodies) | [47] |
| 189 | 5α-Lanosta-7,9(11),24-triene-3β-hydroxy-26-al | G. concinna | [75] |
| 190 | Ganoderiol F (26,27-dihydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (fruit bodies) | [39] |
| 191 | 26,27-Dihydroxy-5α-lanosta-7,9(11),24-triene-3,22-dione | G. lucidum (basidiocarp) | [83] |
| 192 | 26-Hydroxy-5α-lanosta-7,9(11),24-triene-3,22-dione | G. lucidum (basidiocarp) | [83] |
| 193 | Ganodermic acid P1 (lanosta-7,9(11),24-trien-3α, 22β-diacetoxy-15α-hydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 194 | Ganodermic acid P2 (lanosta-7,9(11),24-trien-15α, 22β-diacetoxy-3β-hydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 195 | Lanosta-7,9(11),24-trien-3β, 15α, 22-triacetoxy-26-oic acid | G. amboinense (fruit bodies) | [85] |
| 196 | 16α-Hydroxy-3-oxolanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 197 | Lucialdehyde A ((24E)-3β-hydroxy-5α-lanosta-7,9(11),24-trien-26-al) | G. lucidum (fruit bodies) | [25] |
| 198 | Ganoderiol a triacetate (3β, 24,26-triacetoxy-5α-lanosta-7, 9(11)-dien-25-ol) | G. sinense (fruit bodies) | [86] |
| 199 | Ganoderal A | G. lucidum | [23] |
| 200 | Ganoderol A | G. lucidum | [23] |
| 201 | Lucidumol B ((24S)-lanosta-7,9(11)-diene-3β, 24,25-triol) | G. lucidum (spores) | [38] |
| 202 | Ganodermanontiol (24,25,26-trihydroxy-5α-lanosta-7,9(11)-dien-3-one) | G. lucidum (spores) | [67] |
| 203 | Ganoderiol A (5α-lanosta-7,9(11)-dien-3β, 24,25,26-tetraol) | G. lucidum (fruit bodies) | [77] |
| 204 | Ganodermanondiol | G. lucidum (fruit bodies) | [87] |
| 205 | Ganoderic acid X (3α-hydroxy-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. amboinense | [88] |
| 206 | Ganoderic acid TR | G. lucidum | [89] |
| 207 | Ganodermic acid Ja (lanosta-7,9(11),24-trien-3α, 15α-dihydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 208 | Ganodermic acid Jb (lanosta-7,9(11),24-trien-3β, 15α-dihydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 209 | Ganodermic acid R (lanosta-7,9(11),24-trien-3α, 15α-diacetoxy-26-oic acid) | G. lucidum | [78] |
| 211 | 15α-Hydroxy-3-oxo-5α-lanosta-7,9,24(E)-triene-26-oic acid | G. lucidum | [26] |
| 212 | 15α, 26-Dihydroxy-5α-lanosta-7,9,24(E)-trien-3-one | G. lucidum | [26] |
| 213 | 3β-Hydroxy-5α-lanosta-7,9,24(E)-trien-26-oic acid | G. lucidum | [26] |
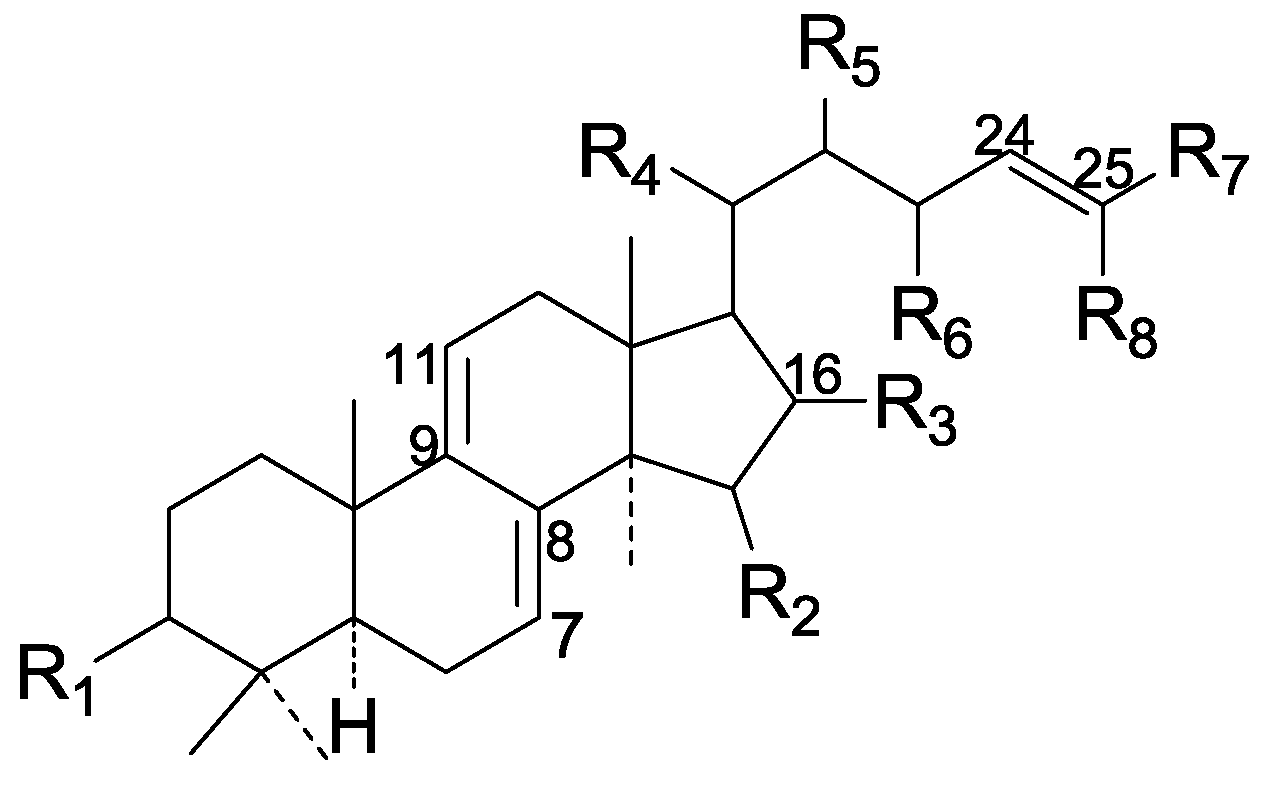
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | |
|---|---|---|---|---|---|---|---|---|---|
| 156 | =O | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 157 | α-OH | H | α-OH | α-COOH | H | H | CH3 | CH3 | |
| 158 | α-OH | H | α-OH | α-COOH | H | H | CH2OH | CH3 | |
| 159 | =O | H | H | α-CH3 | H | H | COOH | CH3 | |
| 160 | =O | H | H | β-CH3 | H | H | COOH | CH3 | |
| 161 | =O | α-OH | H | α-CH3 | H | H | CH2OH | CH3 | |
| 162 | α-O-Ac | α-O-Ac | H | β-CH3 | ξ-H2 | H | COOH | CH3 | |
| 163 | β-O-Ac | α-OH | H | β-CH3 | ξ-H2 | H | COOH | CH3 | |
| 164 | =O | H | H | α-CH3 | H | H | CH2OH | CH3 | |
| 165 | β-OH | H | H | α-CH3 | H | H | CH2OH | CH3 | |
| 166 | β-OH | H | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 167 | β-O-Ac | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 168 | =O | H | O | β-CH3 | H | H | CH2OH | CH2OH | |
| 169 | β-OH | H | H | β-CH3 | H | H | CH2OH | CH3 | |
| 170 | α-O-Ac | α-OH | H | β-CH3 | ξ-O-Ac | H | COOH | CH3 | |
| 171 | =O | α-OH | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 172 | α-O-Ac | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 173 | α-OH | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 174 | α-O-Ac | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 175 | =O | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 176 | β-O-Ac | α-O-Ac | H | β-CH3 | β-O-Ac | H | COOH | CH3 | |
| 177 | α-OH | α-O-Ac | H | β-CH3 | H | =O | COOH | CH3 | |
| 178 | α-O-Ac | α-O-Ac | H | β-CH3 | H | =O | COOH | CH3 | |
| 179 | α-O-Ac | α-OH | H | β-CH3 | H | =O | COOH | CH3 | |
| 180 | α-O-Ac | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 181 | β-OH | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 182 | β-O-Ac | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 183 | =O | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 184 | =O | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 185 | α-OH | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 186 | α-O-Ac | α-OH | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 187 | =O | α-OH | H | α-CH3 | H | OH | COOH | CH3 | |
| 188 | β-OH | α-OH | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 189 | β-OH | H | H | α-CH3 | H | H | CHO | CH3 | |
| 190 | =O | H | H | β-CH3 | H | H | CH2OH | CH2OH | |
| 191 | =O | H | H | α-CH3 | =O | H | CH2OH | CH2OH | |
| 192 | =O | H | H | α-CH3 | =O | H | CH2OH | CH3 | |
| 193 | α-O-Ac | α-OH | H | β-CH3 | O-Ac | H | COOH | CH3 | |
| 194 | β-OH | α-O-Ac | H | β-CH3 | O-Ac | H | COOH | CH3 | |
| 195 | β-O-Ac | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 196 | =O | H | α-OH | α-COOH | H | H | CH3 | CH3 |
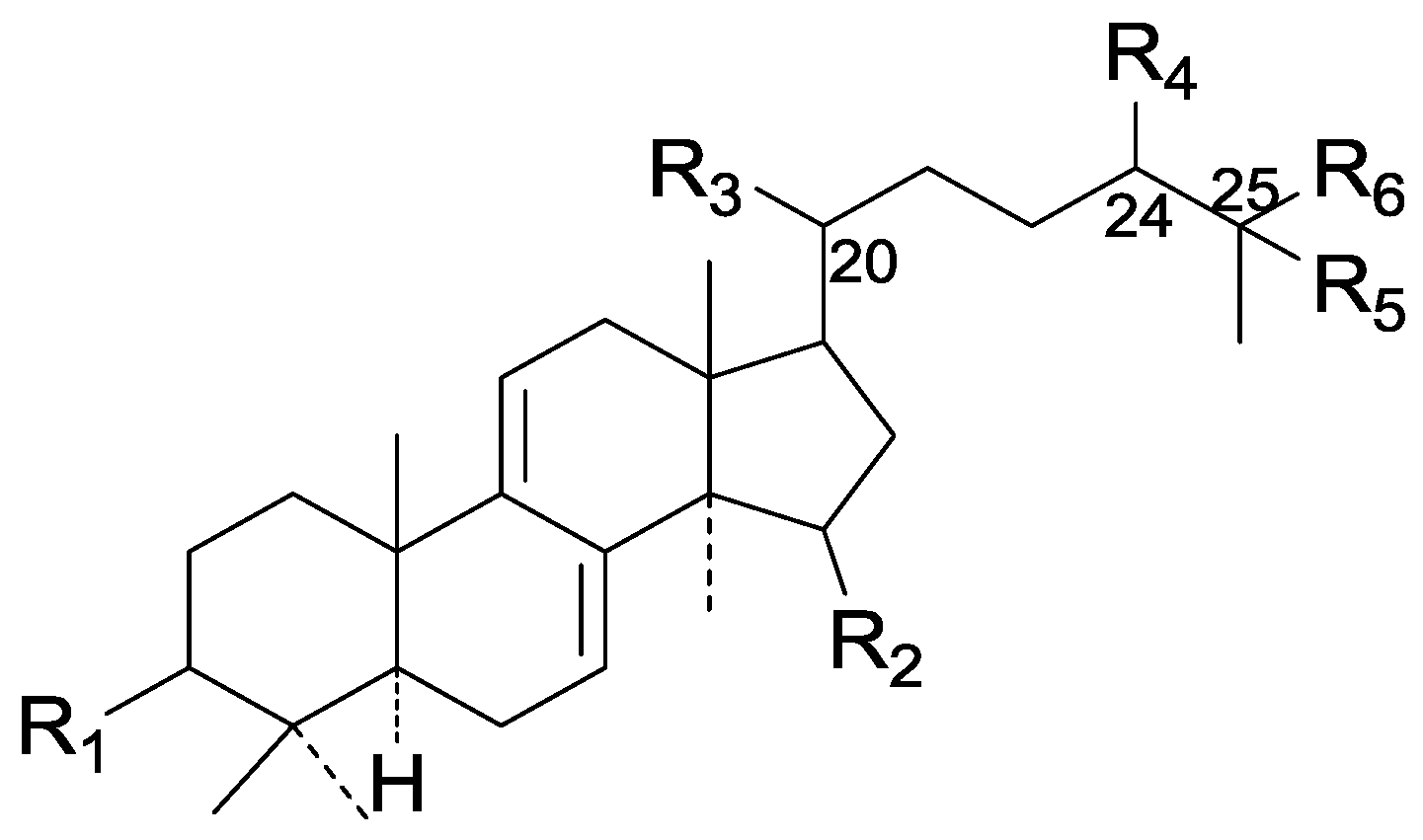
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 197 | β-OH | H | α-CH3 | △24,25 | △24,25 | CHO |
| 198 | β-O-Ac | H | α-CH3 | OAc | OH | CH2-O-Ac |
| 199 | =O | H | β-CH3 | △24,25 | △24,25 | CHO |
| 200 | =O | H | β-CH3 | △24,25 | △24,25 | CH2OH |
| 201 | β-OH | H | α-CH3 | α-OH | OH | CH3 |
| 202 | =O | H | β-CH3 | α-OH | H | CH2OH |
| 203 | β-OH | H | α-CH3 | OH | OH | CH2OH |
| 204 | =O | H | α-CH3 | OH | OH | CH3 |
| 205 | β-OH | α-O-Ac | β-CH3 | H | H | COOH |
| 206 | =O | α-OH | α-CH3 | △24,25 | △24,25 | COOH |
| 207 | α-OH | α-OH | β-CH3 | △24,25 | △24,25 | COOH |
| 208 | β-OH | α-OH | β-CH3 | △24,25 | △24,25 | COOH |
| 209 | α-O-Ac | α-O-Ac | β-CH3 | △24,25 | △24,25 | COOH |
| 210 | =O | H | α-CH3 | OH | OH | CH2OH |
| 211 | =O | α-OH | α-CH3 | △24,25 | △24,25 | COOH |
| 212 | =O | α-OH | α-CH3 | △24,25 | △24,25 | CH2OH |
| 213 | β-OH | H | α-CH3 | △24,25 | △24,25 | COOH |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 214 | 3α, 15α, 22α-Trihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 215 | 3β, 15α, 22β-Trihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 216 | 3α, 15α-Diacetoxy-22α-hydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 217 | 3β, 15α-Diacetoxy-22α-hydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 218 | 22β-Acetoxy-3α, 15α-dihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 219 | 22β-Acetoxy-3β, 15α-dihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 220 | Epoxyganoderiol B (24S, 25S-epoxy-26-hydroxy-5α-lanosta-7,9(11)-diene-3-one) | G. lucidum | [40] |
| 221 | Epoxyganoderiol C (24S, 25S-epoxy-5α-lanosta-7,9(11)-diene-3β, 26-diol) | G. lucidum | [40] |
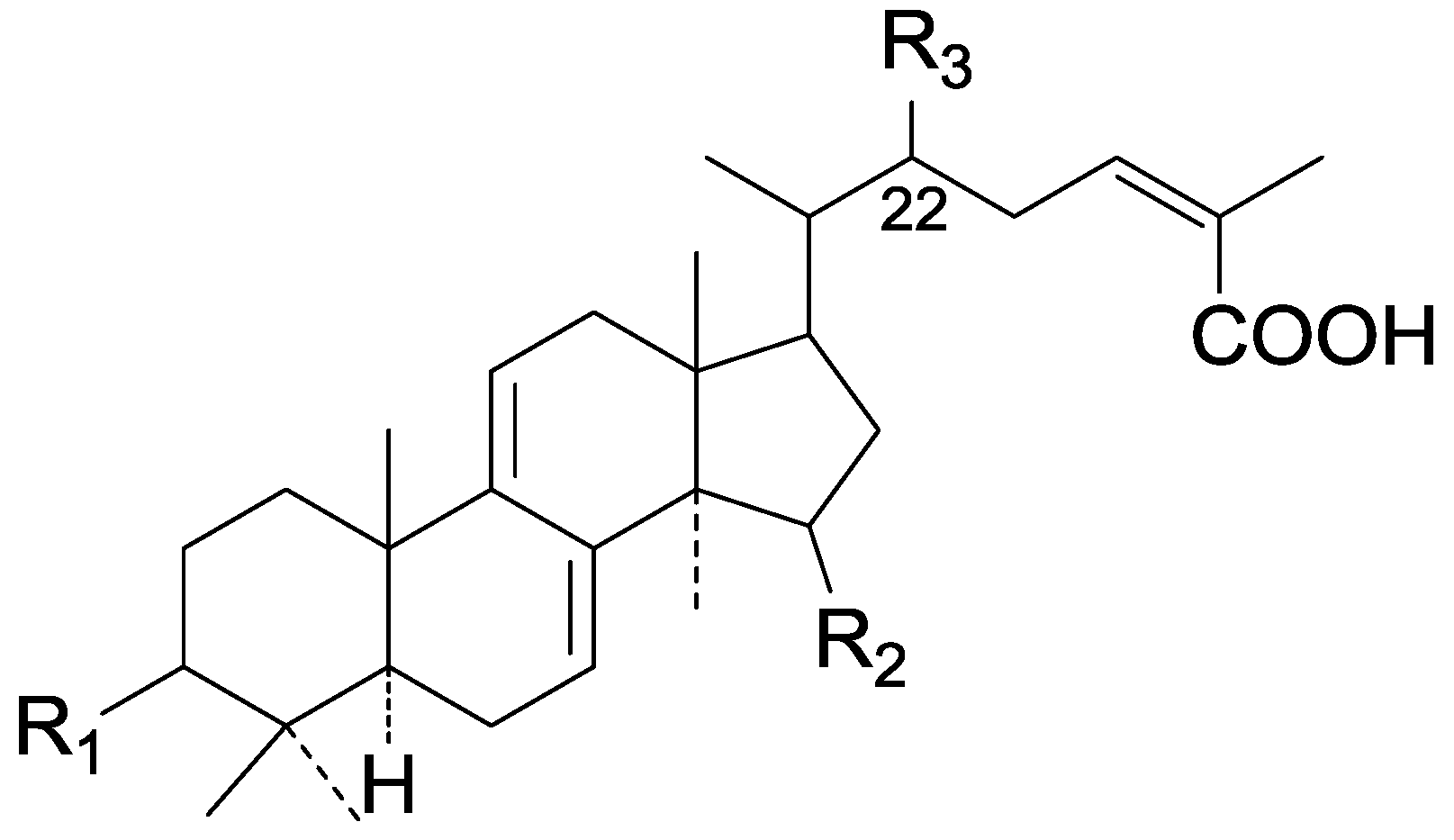
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 214 | α-OH | α-OH | α-OH |
| 215 | β-OH | α-OH | β-OH |
| 216 | α-O-Ac | α-O-Ac | α-OH |
| 217 | β-O-Ac | α-O-Ac | α-OH |
| 218 | α-OH | α-OH | β-O-Ac |
| 219 | β-OH | α-OH | β-O-Ac |
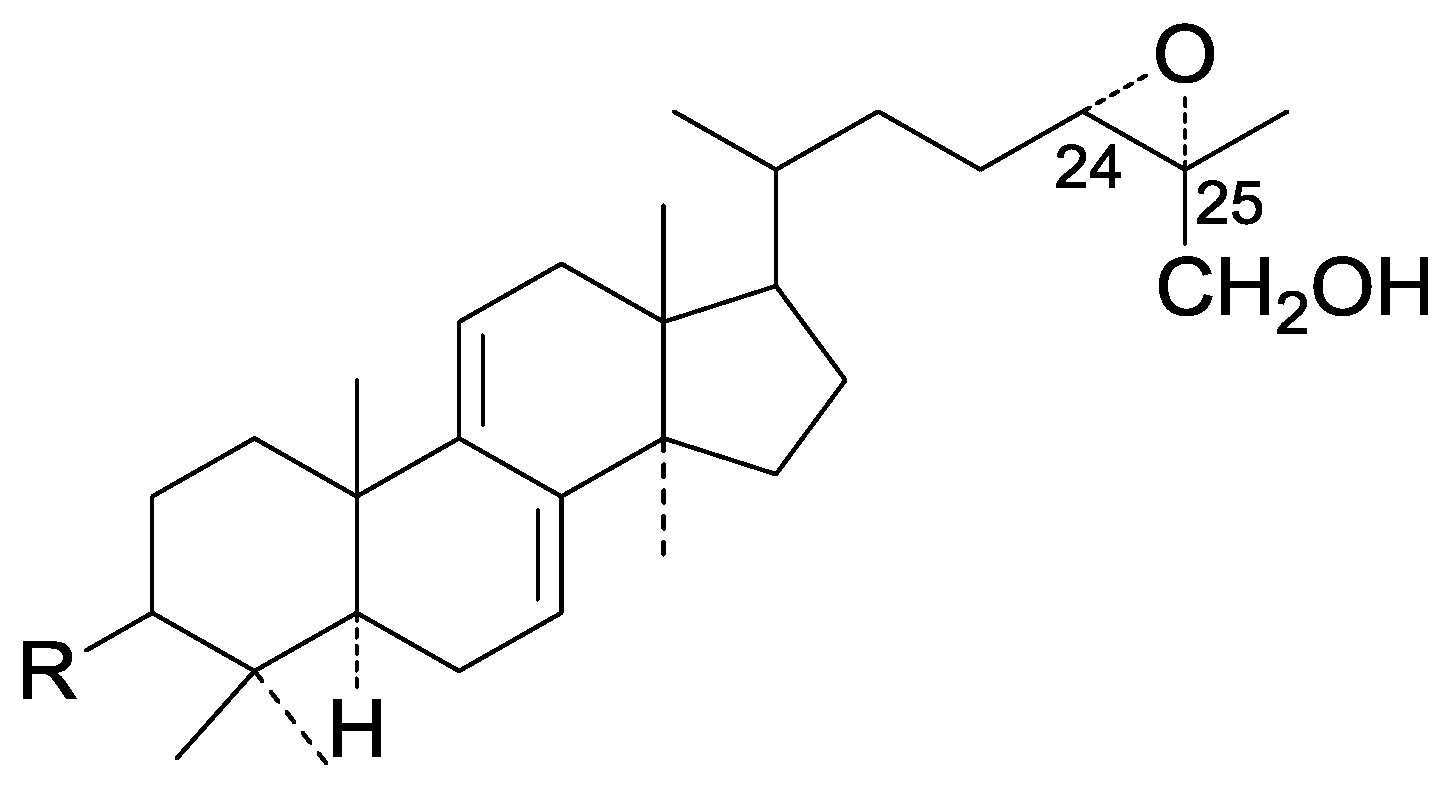
| Cpd | R1 |
|---|---|
| 220 | β-OH |
| 221 | =O |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 222 | Butyl lucidenate N | G. lucidum (fruit bodies) | [17] |
| 223 | Butyl lucidenate A | G. lucidum (fruit bodies) | [17] |
| 224 | 20(21)-Dehydrolucidenic acid N (3β, 7β-dihydroxy-11,15-dioxo-25,26,27- trinorlanosta-8,20-dien-24-oic acid) | G. sinense (fruit bodies) | [33] |
| 225 | 20-Hydroxylucidenic acid A (7β, 20ξ-dihydroxy-3,11,15-trioxo-25,26,27-trinorlanost-8-en-24-oic acid) | G. sinense (fruit bodies) | [33] |
| 226 | Methyl lucidenate D (methyl 12β-acetoxy-3,7,11,15-tetraoxo-5α-lanost-8-en-24-oate) | G. lucidum (fruit bodies) | [53,54] |
| 227 | 20(21)-Dehydrolucidenic acid A (7β-Hydroxy-3,11,15-trioxo-25,26,27-trisnorlanosta-8,20(21)-dien-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 228 | Methyl 20(21)-dehydrolucidenate A (methyl 7β-hydroxy-3,11,15-trioxo-25,26,27-trisnorlanosta-8,20(21)-dien-24-oate) | G. lucidum (fruit bodies) | [90] |
| 229 | Lucidenic acid N (3,7-dihydroxy-4,4,14-trimethyl-11,15-dioxo-5-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [91,92] |
| 230 | Lucidenic acid D (12β-acetoxy-4,4,14α-trimethyl-3,7,11,15-tetraoxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 231 | Methyl lucidenate E | G. lucidum (gills) | [54] |
| 232 | Methyl lucidenate F | G. lucidum (gills) | [23,54] |
| 233 | Ethyl lucidenates A (ethyl 7β-hydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [93] |
| 234 | 3β-Oxo-formyl-7β, 12β-dihydroxy-4,4,14α-trimethyl-5α-chol-11,15-dioxo-8-en(E)-24-oic acid | G. lucidum | [24] |
| 235 | Lucidenic acid A (7β-hydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 236 | Lucidenic acid B (7β, 12-dihydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 237 | Lucidenic acid C (3β, 7β, 12-trihydroxy-4,4,14α-trimethyl-11,15-dioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 238 | 4,4,14α-Trimethyl-3,7-dioxo-5α-chol-8-en-24-oic acid | G. lucidum | [26] |
| 239 | Lucidenic acid P (3β, 7β-dihydroxy-12β-acetoxy-25,26,27-trinor-11,15-dioxo dioxolanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [95] |
| 240 | Methyl lucidenate P | G. lucidum (fruit bodies) | [95] |
| 241 | Methyl lucidenate Q (methyl-7β, 15α-dihydroxy-25,26,27-trinor-3,11-dioxolanost-8-en-24-oate) | G. lucidum (fruit bodies) | [95] |
| 242 | 3β-Hydroxy-4,4,14-trimethyl-7,11,15-trioxochol-8-en-24-oic acid | G. lucidum (fruit bodies) | [28] |
| 243 | Methyl lucidenate D2 | G. lucidum (gill surface) | [30] |
| 244 | Methyl lucidenate E2 | G. lucidum (gill surface) | [30] |
| 248 | Methyl lucidenate N (methyl 3β, 7β-dihydroxy-4,4,14α-trimethyl-11,15-dioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [96] |
| 249 | t-Butyl lucidenate B (t-butyl 7β, 12β-dihydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate) | G.lucidum (fruit bodies) | [96] |
| 250 | Methyl lucidenate A | G. lucidum (fruit bodies) | [93] |
| 251 | Lucidenic acid D2 | G. lucidum (fruit bodies) | [95] |
| 252 | 20-Hydroxylucidenic acid D2 ((20ξ)-12β-acetoxy-20-hydroxy-3,7,11,15-tet-raoxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 253 | 20-Hydroxylucidenic acid F ((20ξ)-20-hydroxy-3,7,11,15-tetraoxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 254 | 20-Hydroxylucidenic acid E2 (12β-acetoxy-3β-hydroxy-7,11,15-trioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 255 | 20-Hydroxylucidenic acid N ((20ξ)-3β, 7β, 20-trihydroxy-11,15-dioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 256 | 20-Hydroxylucidenic acid P ((20ξ)-12β-acetoxy-3β, 7β, 20-trihydroxy-11,15-dioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 257 | Lucidenic acid F | G. lucidum (gills) | [22] |
| 258 | Methyl lucidenate C | G. lucidum | [26] |
| 259 | Lucidenic acid E2 | G. lucidum (fruit bodies) | [95] |
| 260 | Lucideric acid A | G. lucidum | [26] |

| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 222 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOBu |
| 223 | =O | β-OH | =O | H | =O | α-CH3 | H | COOBu |
| 224 | β-OH | β-OH | =O | H | =O | CH2 | △20,21 | COOH |
| 225 | =O | β-OH | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 226 | =O | =O | =O | β-O-Ac | =O | β-CH3 | H | COOCH3 |
| 227 | =O | β-OH | =O | H | =O | CH2 | △20,21 | COOH |
| 228 | =O | β-OH | =O | H | =O | CH2 | △20,21 | COOCH3 |
| 229 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 230 | =O | =O | =O | β-O-Ac | =O | β-CH3 | H | COOH |
| 231 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | H | COOCH3 |
| 232 | =O | =O | =O | H | =O | β-CH3 | H | COOCH3 |
| 233 | =O | OH | =O | H | =O | α-CH3 | H | COOEt |
| 234 | β-O-CHO | β-OH | =O | OH | =O | β-CH3 | H | COOH |
| 235 | =O | β-OH | =O | H | =O | β-CH3 | H | COOH |
| 236 | =O | β-OH | =O | β-OH | =O | β-CH3 | H | COOH |
| 237 | β-OH | β-OH | =O | β-OH | =O | β-CH3 | H | COOH |
| 238 | =O | =O | H | H | H | α-CH3 | H | COOH |
| 239 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 240 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOCH3 |
| 241 | =O | β-OH | =O | H | α-OH | α-CH3 | H | COOCH3 |
| 242 | β-OH | =O | =O | H | =O | α-CH3 | H | COOH |
| 243 | =O | =O | =O | O-Ac | =O | β-CH3 | H | COOCH3 |
| 244 | β-OH | =O | =O | O-Ac | =O | β-CH3 | H | COOCH3 |
| 245 | =O | =O | =O | α-OH | =O | β-CH3 | H | COOCH3 |
| 246 | β-OH | =O | =O | β-OH | =O | β-CH3 | H | COOCH3 |
| 247 | β-OH | α-OH | =O | H | α-OH | β-CH3 | H | COOCH3 |
| 248 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 249 | =O | β-OH | =O | β-OH | =O | α-CH3 | H | COOBu |
| 250 | =O | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 251 | =O | =O | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 252 | =O | =O | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 253 | =O | =O | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 254 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 255 | β-OH | β-OH | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 256 | β-OH | β-OH | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 257 | =O | =O | =O | H | =O | α-CH3 | H | COOH |
| 258 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 259 | β-OH | =O | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 260 | =O | β-OH | =O | H | =O | α-CH3 | H | COOH |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 261 | 4,4,14α-Trimethyl-5α-chol-7,9(11)-dien-3-oxo-24-oic acid | G. lucidum (dried fruit bodies) | [73] |
| 262 | Ganoderic acid Jd (15α-hydroxy-3-oxo-5α-lano-sta-7,9(11)-dien-24-oic acid) | G. sinense (fruit bodies) | [47] |
| 263 | Methyl lucidenate H (methyl 3β, 7β-dihydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-11,15-dioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 264 | Methyl lucidenate I (3β-hydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-7,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 265 | Methyl lucidenate J (3β, 12β-dihydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-7,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 266 | Methyl lucidenate Ha | G. sinense (fruit bodies) | [47] |
| 267 | Colossolactone I ((22S)-3-β-hydroxylanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 268 | Colossolactone II ((22S)-1,3-β-dihydroxylanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 269 | Colossolactone D | G. colossum (fruit bodies) | [98] |
| 270 | Colossolactone E | G. colossum (fruit bodies) | [98] |
| 271 | Colossolactone F | G. colossum (fruit bodies) | [98] |
| 272 | Colossolactone G | G. colossum (fruit bodies) | [98] |
| 273 | Ganosporelactone A | G. lucidum (spores) | [99] |
| 274 | Ganosporelactone B | G. lucidum (spores) | [99] |
| 275 | Ganosinensin B (ganodermanontriol 24-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)-2-oxo-ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9-dienate) | G. sinense (fruit bodies) | [100] |
| 276 | Ganosinensin C (ganodermanontriol 24-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9- dien-ate) | G. sinense (fruit bodies) | [100] |
| 277 | Ganodermacetal (methyl 7β, 15α-isopropylide-nedioxy-3β-hydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. amboinense (fruit bodies) | [85] |
| 278 | Methyl ganoderate A acetonide (methyl 7β, 15α-isopropylidenedioxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [16] |
| 279 | Applanoxidic acid A (15α-hydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [101] |
| 280 | Applanoxidic acid B (3β-hydroxy-7α, 8α-epoxy-12,15,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [101] |
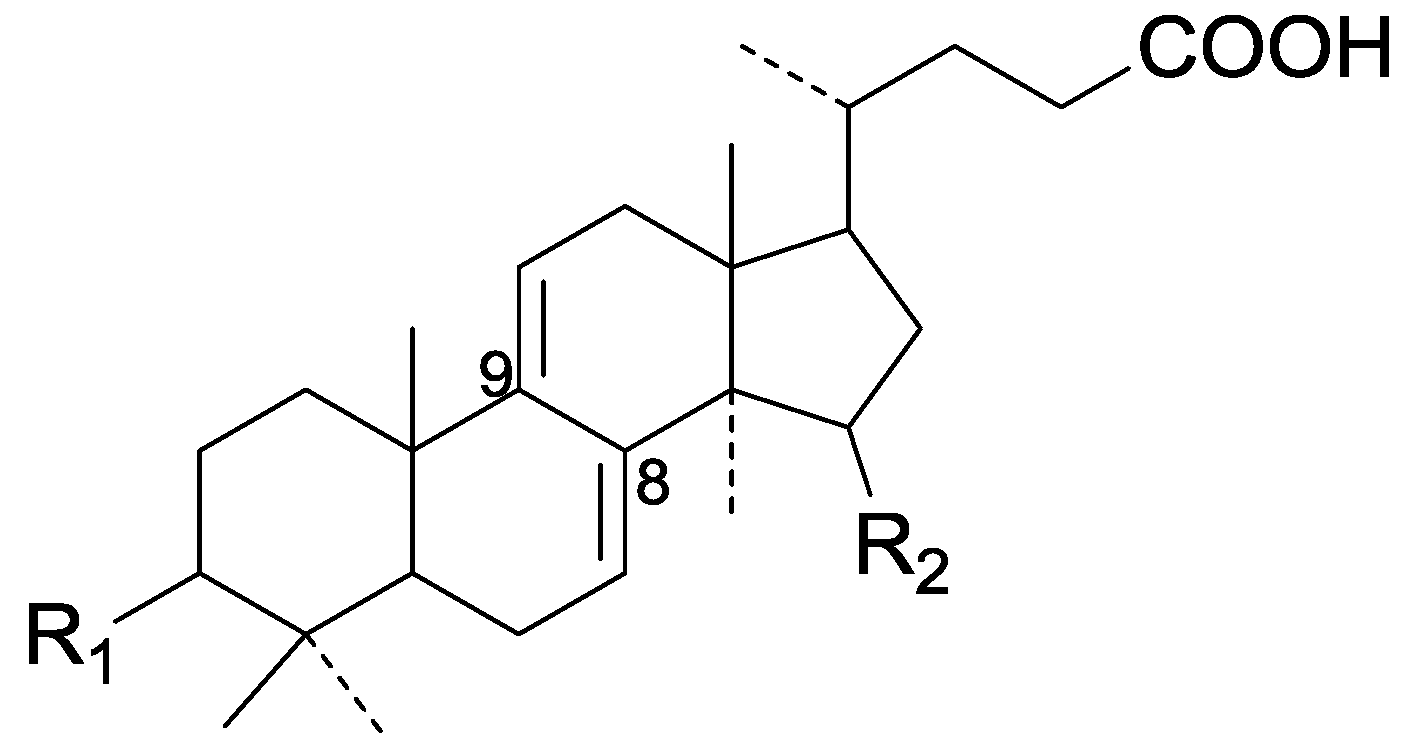
| Cpd | R1 | R2 |
|---|---|---|
| 261 | =O | H |
| 262 | =O | α-OH |
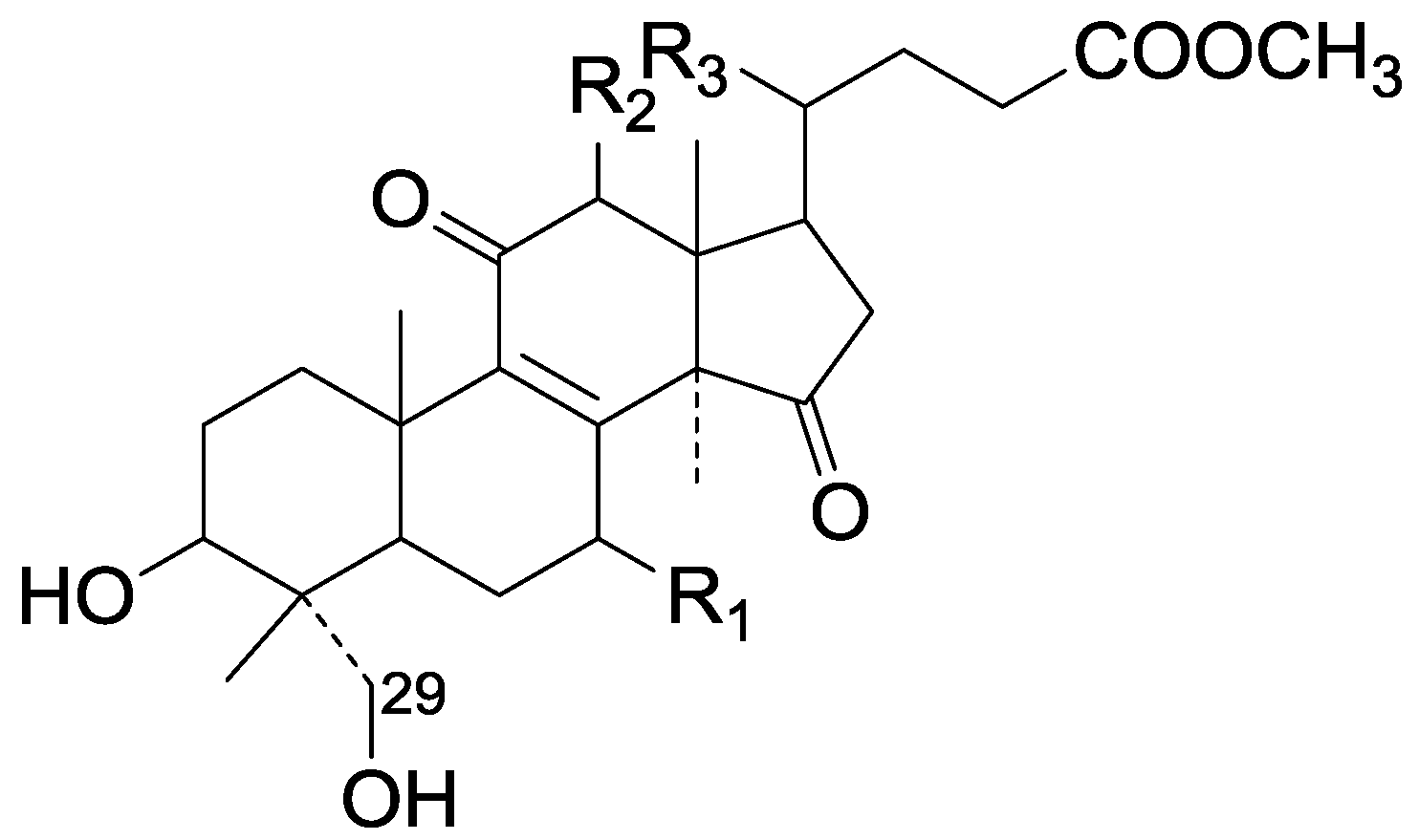
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 263 | β-OH | H | β-CH3 |
| 264 | =O | H | β-CH3 |
| 265 | =O | β-OH | β-CH3 |
| 266 | β-OH | H | α-CH3 |
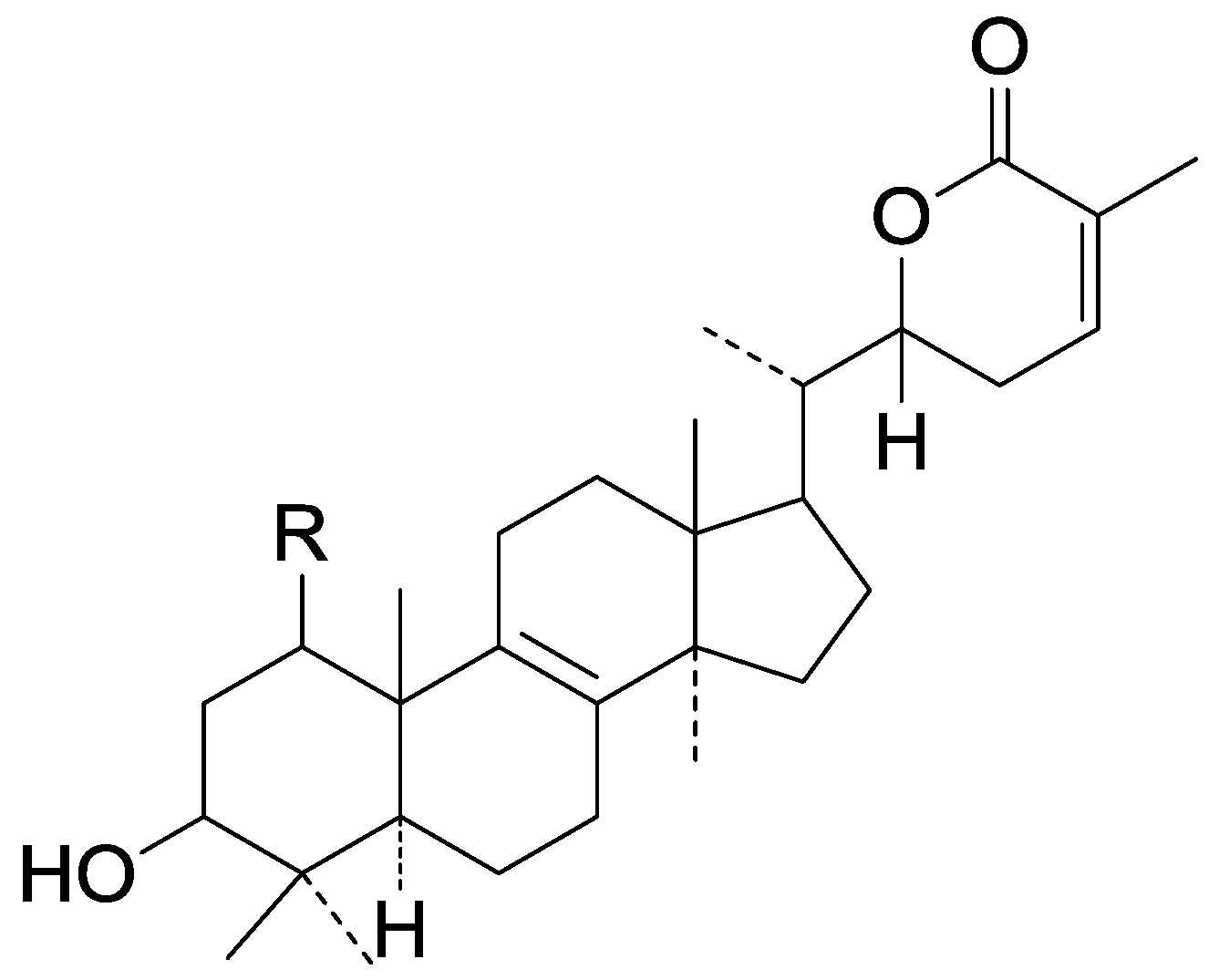
| Cpd | R |
|---|---|
| 267 | H |
| 268 | β-OH |
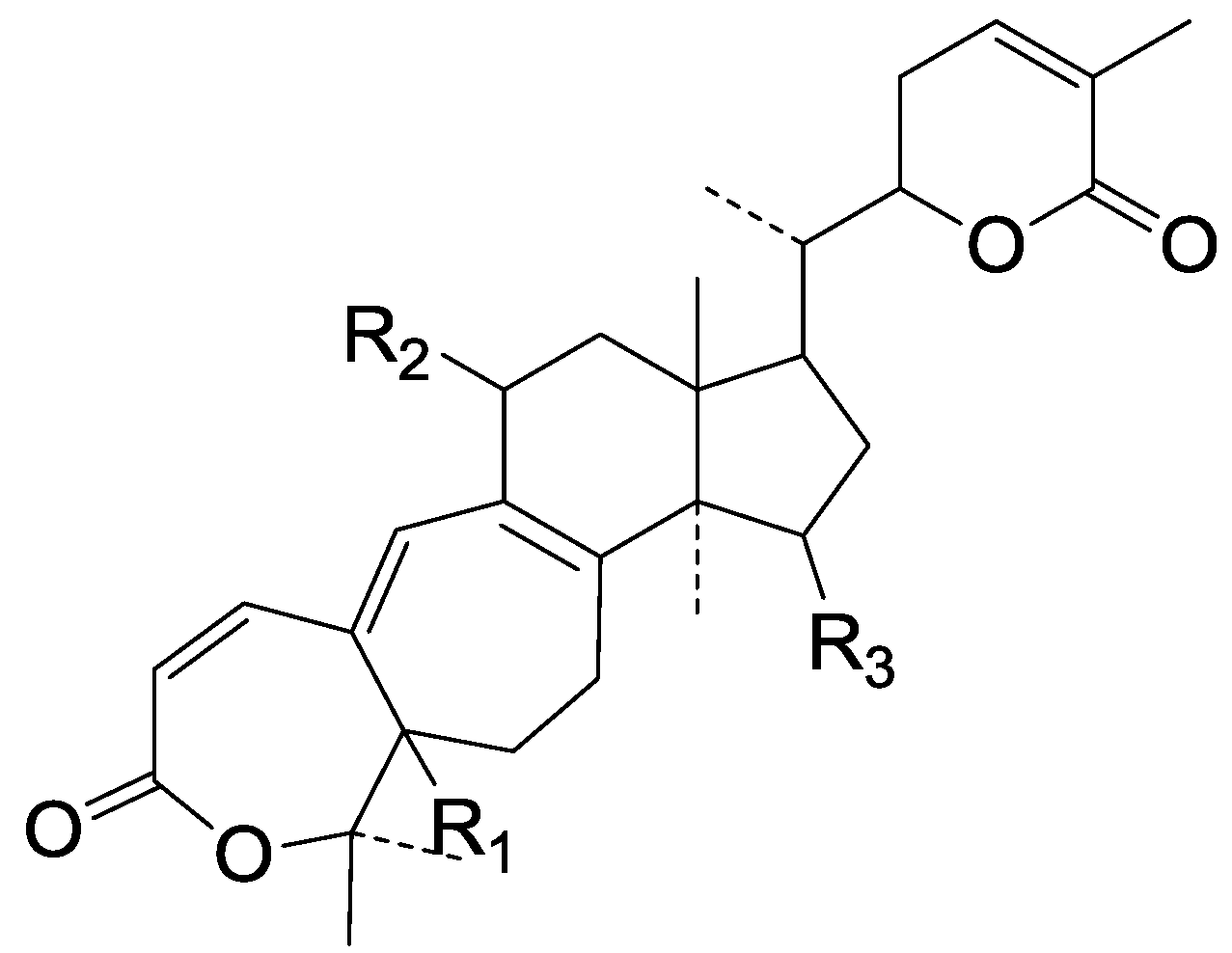
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 269 | α-H | β-H | β-OH |
| 270 | α-H | β-H | β-O-Ac |
| 271 | α-H | β-OH | β-O-Ac |
| 272 | ξ-OH | H | β-O-COCH3 |
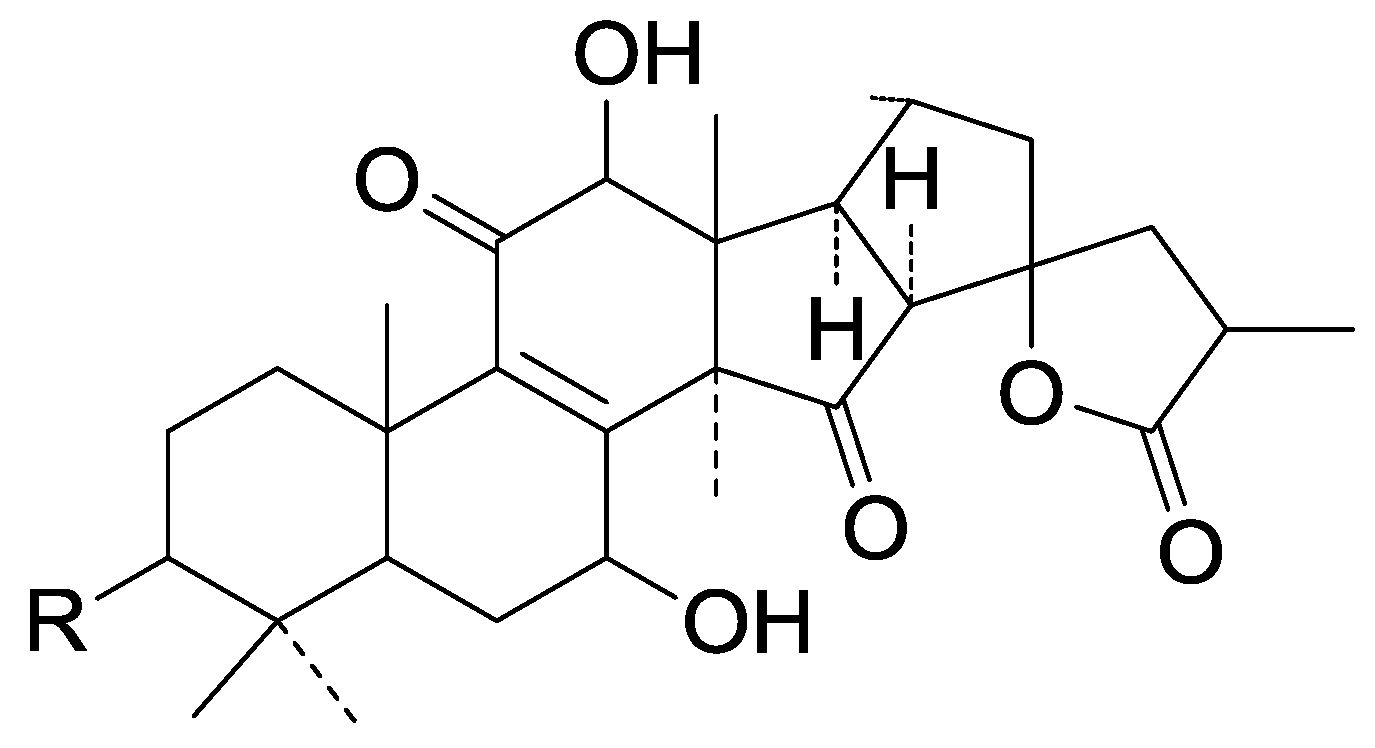
| Cpd | R |
|---|---|
| 273 | =O |
| 274 | β-OH |
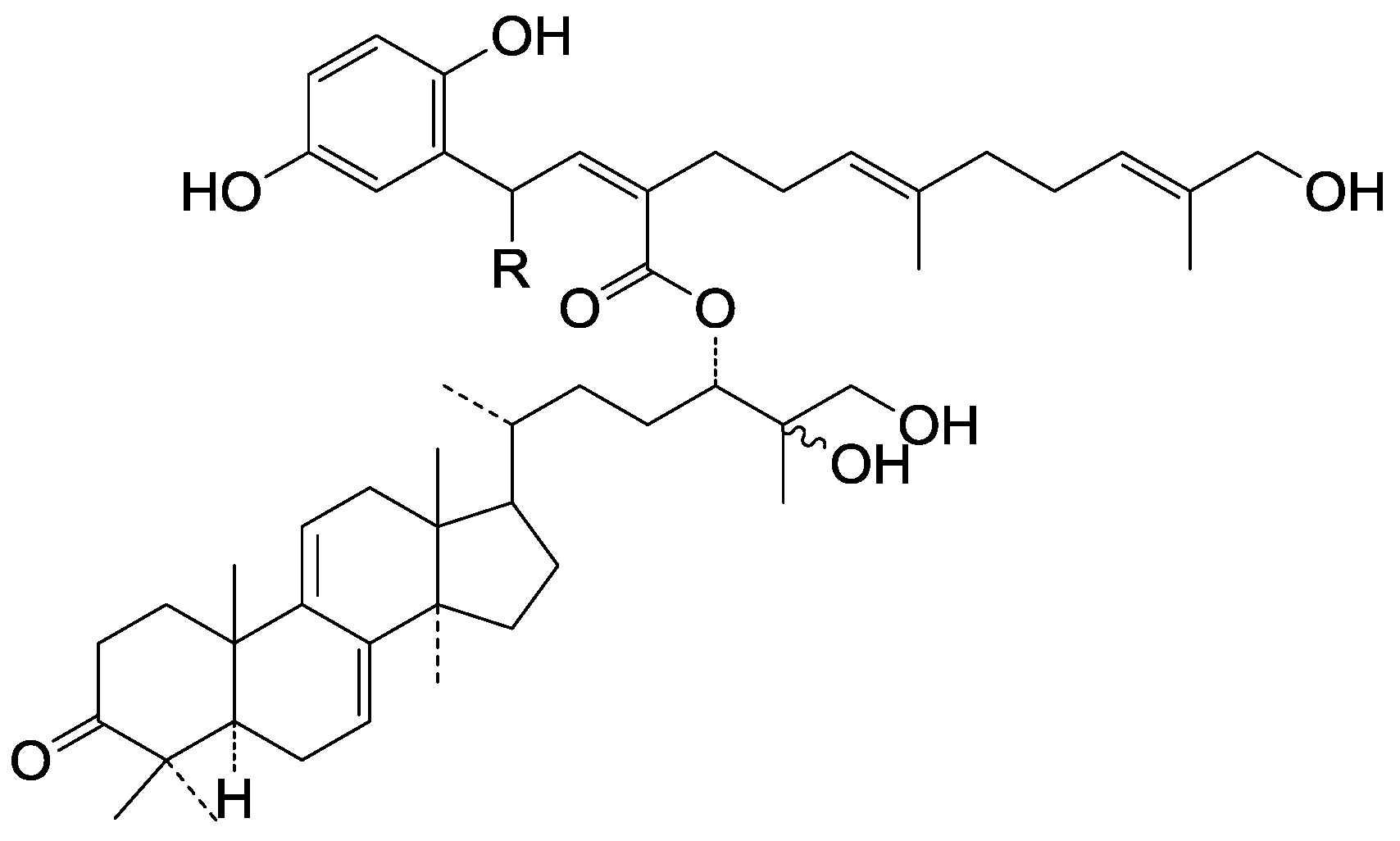
| Cpd | R |
|---|---|
| 275 | =O |
| 276 | H |
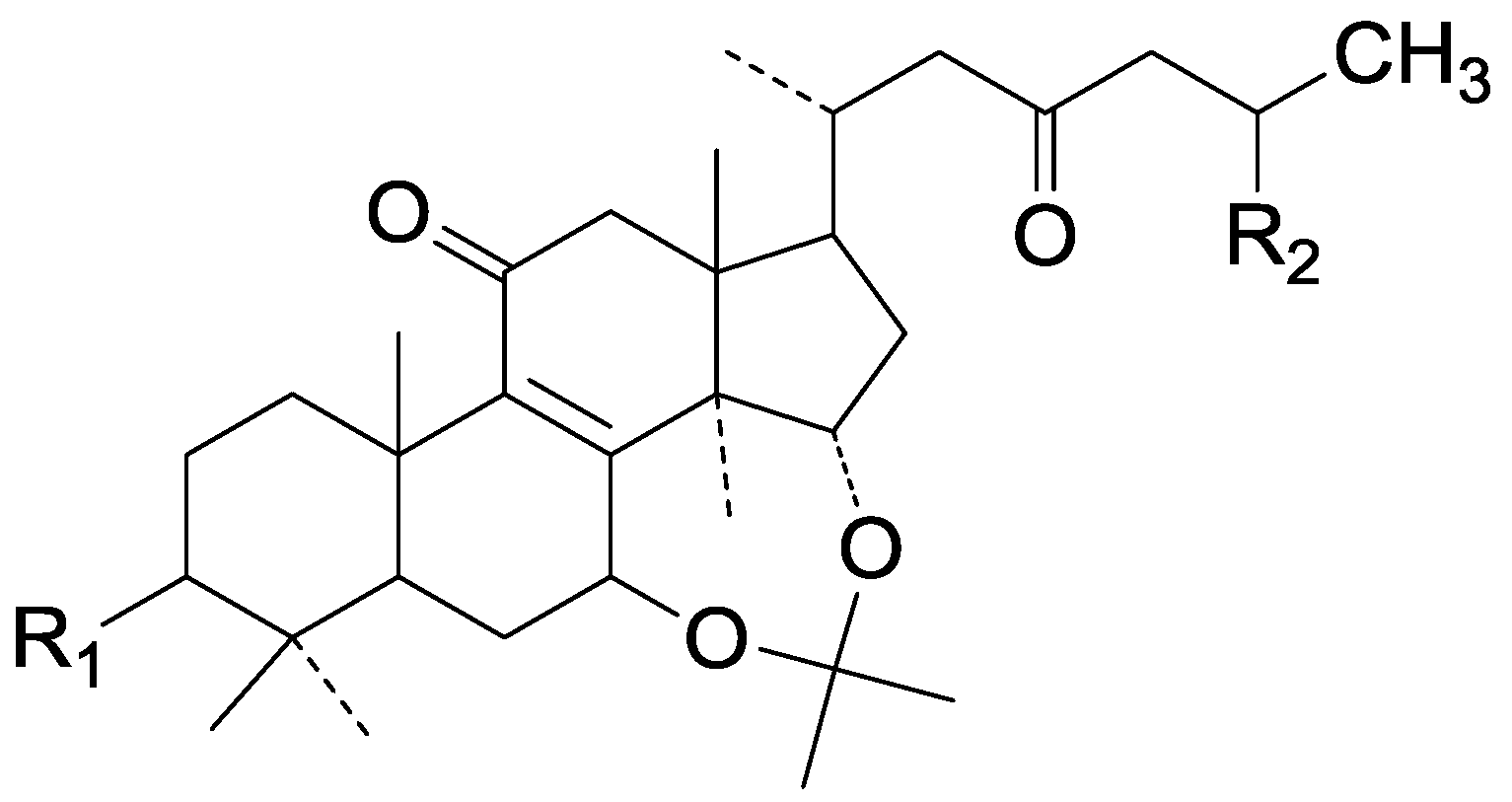
| Cpd | R1 | R2 |
|---|---|---|
| 277 | β-OH | COOH |
| 278 | =O | COOCH3 |
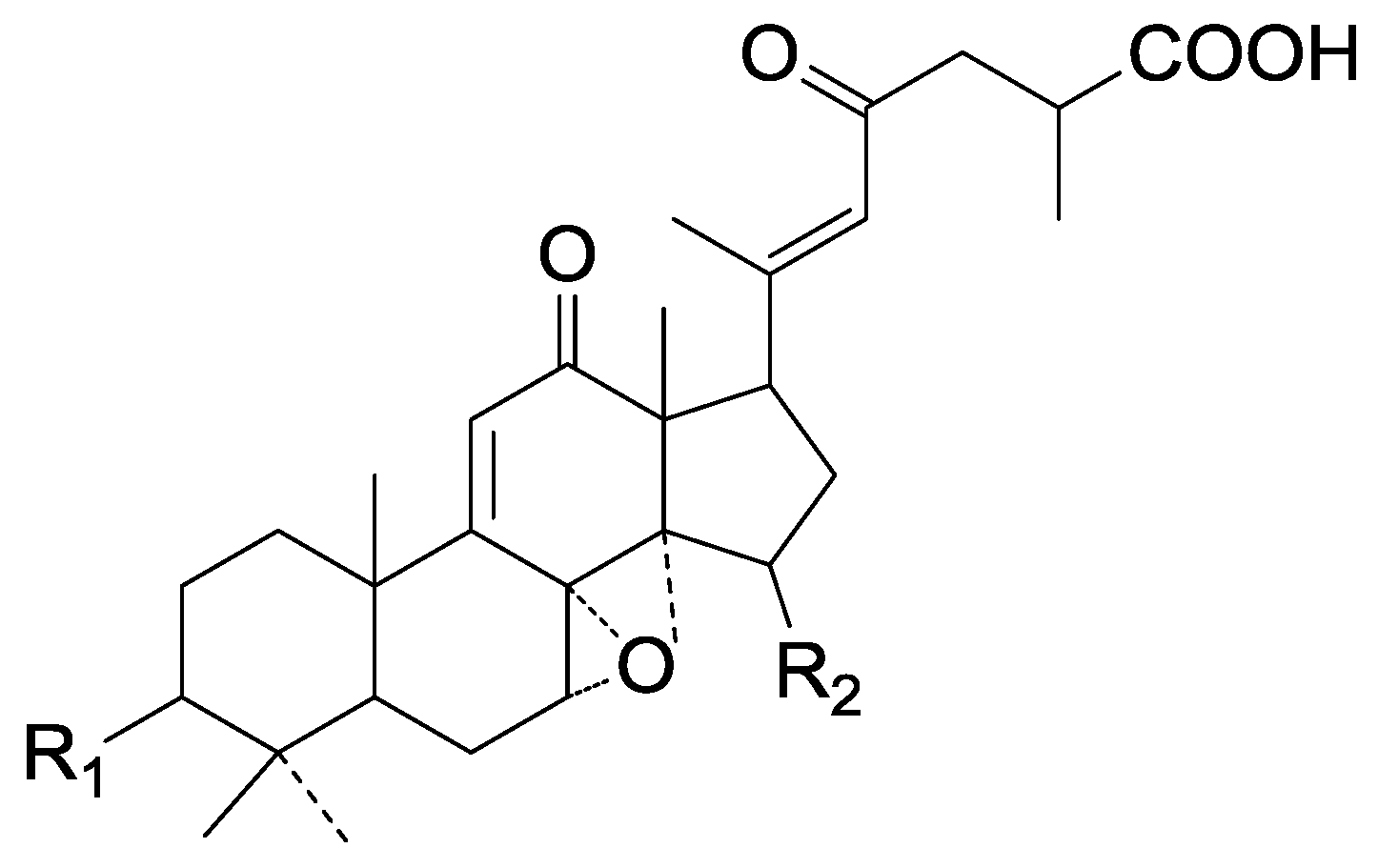
| Cpd | R1 | R2 |
|---|---|---|
| 279 | =O | α-OH |
| 280 | β-OH | =O |
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 281 | Applanoxidic acid C (20-hydroxy-7α, 8α-epoxy-3,12,15,23-tetraoxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [101] |
| 282 | Applanoxidic acid D (3β, 20-dihydroxy-7α, 8α-epoxy-12,15,23-trioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [101] |
| 283 | Lanosta-7,9(11),24-trien-3-one15,26-dihydroxy | G. zonatum Murill. | [27] |
| 284 | Lanosta-7,9(11),24-trien-26-oic,3-hydroxy | G. zonatum Murill. | [27] |
| 285 | Ganoderic acid Y ((24E)-3-ol-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. zonatum Murill. | [27] |
| 286 | Applanoxidic acid E (15β-hydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [66] |
| 287 | Applanoxidic acid F (7α, 8α-epoxy-3,12,15,23-tetraoxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [66] |
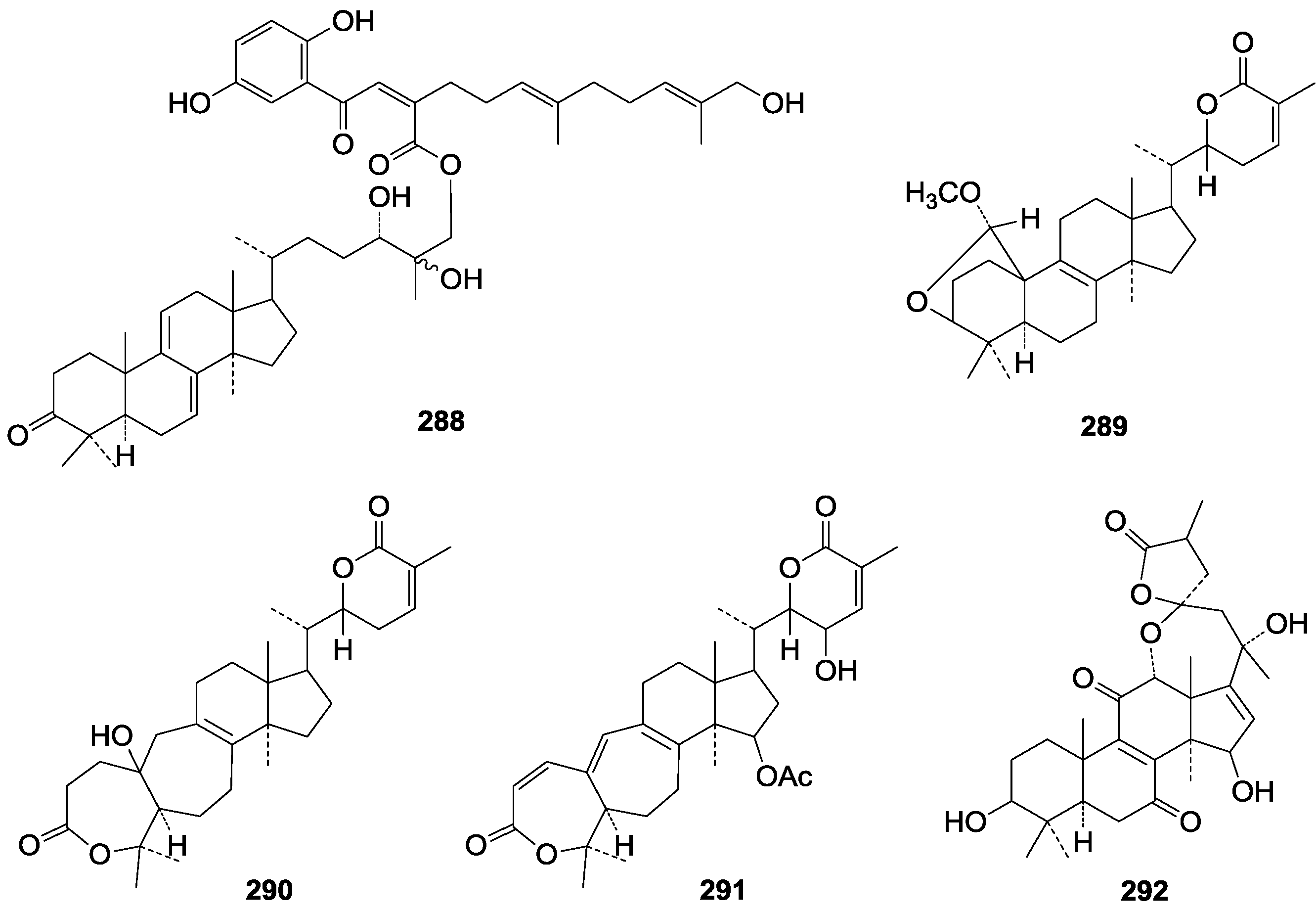
| 288 | Ganosinensin A (ganodermanontriol 26-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)-2-oxo-ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9-dienate}) | G. sinense (fruit bodies) | [100] |
| 289 | Colossolactone III ((22S)-3β, 19-epoxy-lanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 290 | Colossolactone IV ((22S)-A,B-dihomo-19-nor-4-oxalanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 291 | Colossolactone VIII ((22S, 23R)-A,B-dihomo-19-nor-15-β-acetoxy-23-hydroxy-4-oxa-3-oxolanosta-1,8,19,24-tetraen-26,22-olide) | G. colossum | [102] |
| 292 | Austrolactone ((23S, 25S)-12α, 23-epoxy-3β, 15β, 20α-trihydroxy-7,11-dioxo-5α-lanosta-8,16-dien-23,26-olide) | G. australe | [103] |
| 293 | Ganolactone B (3β, 7β-dihydroxy-11,15-dioxolanosta-8-en-24→20 lactone) | G. sinense (fruit bodies) | [86] |
| 294 | Ganolactone (7β-hydroxy-3,11,15-trioxo-lanosta-8-en-24→20s lactone) | G. lucidum (fruit bodies) | [104] |
| 295 | Colossolactone B | G. colossum (fruit bodies) | [98] |
| 296 | Colossolactone C | G. colossum (fruit bodies) | [98] |
| 297 | 3α-(3-Hydroxy-5-methoxy-3-methyl-1,5-dioxopentyloxy)-24-methylene-5α-lanost-8-en-21-oic acid | G. resinaceum (fruit bodies) | [62] |
| 298 | Colossolactone A | G. colossum (fruit bodies) | [98] |
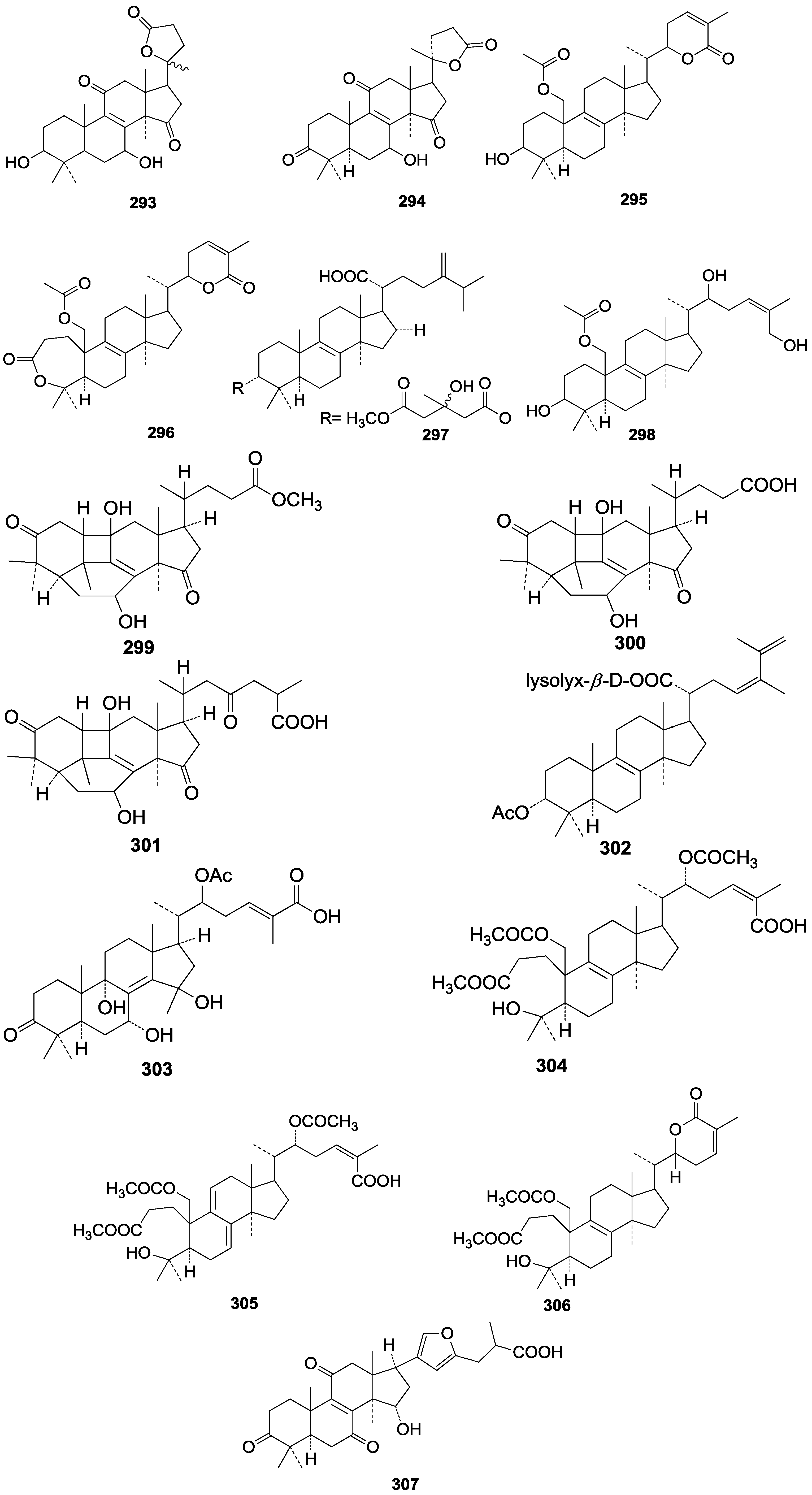
| 299 | Methyl ganosinensate A | G. sinense (fruit bodies) | [56] |
| 300 | Ganosinensic acid A | G. sinense (fruit bodies) | [56] |
| 301 | Ganosinensic acid B | G. sinense (fruit bodies) | [56] |
| 302 | Tsugarioside C (3α-acetoxy-(Z)-24-methyl-5α-lanosta-8,23,25-trien-21-oic acid ester β-D-xyloside) | G. tsugae (fruit bodies) | [60] |
| 303 | Ganorbiformin A | G. colossum (fruit bodies) | [46] |
| 304 | Colossolactone V ((22R)-3,4-seco-19,22-diacetoxy-4-hydroxylanosta-8,24(Z)-dien-3,26-dioic acid 3-methyl-ester) | G. colossum (fruit bodies) | [102] |
| 305 | Colossolactone VI ((22R)-3,4-seco-19,22-diacetoxy-4-hydroxylanosta-7,9(11),24(Z)-trien-3,26-dioic acid 3-methyl ester) | G. colossum (fruit bodies) | [102] |
| 306 | Colossolactone VII ((22S)-3,4-seco-19-acetoxy-4-hydroxylanosta-8,24-dien-26,22-olide 3-methyl ester) | G. colossum (fruit bodies) | [102] |
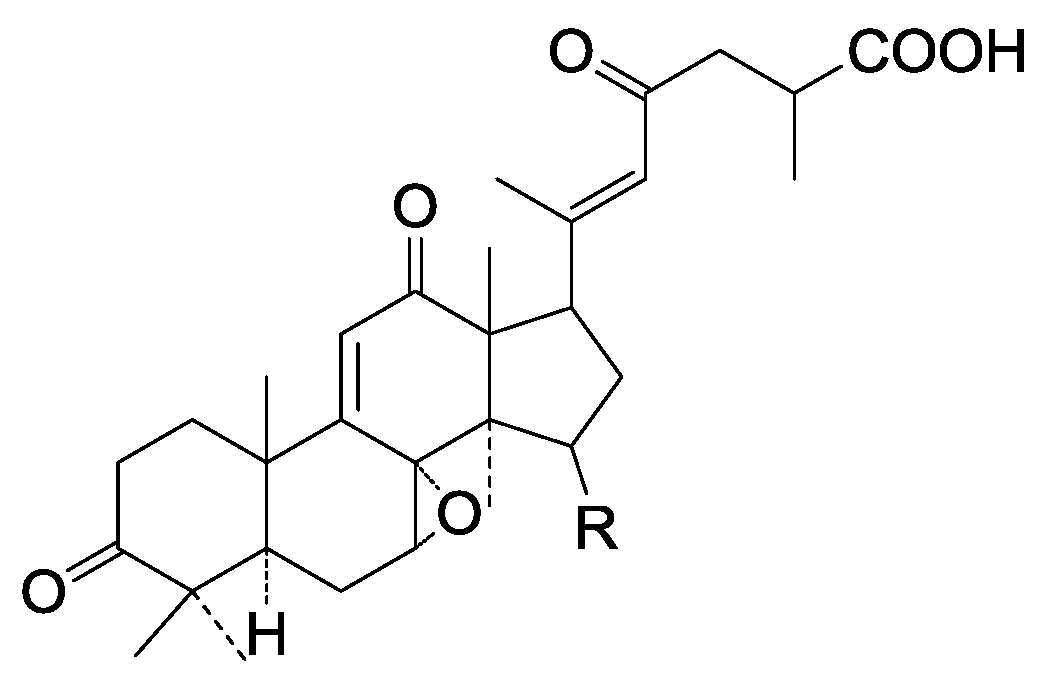
| 307 | Furanoganoderic acid (21,23-epoxy-15α-hydroxy-3,7,1l-trioxo-5α-lanosta-8,20(21),22-trien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
| 308 | Fornicatin B (7β-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-4(28),8-dien-3,24-dioic acid) | G. fornicatum (fruit bodies) | [105] |
| 309 | Fornicatin G (7β-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-4(28),8-dien-24-oic-3-acetyl ester) | G. cochlear (sporophore) | [106] |
| 310 | Fornicatin A (4, 7β-epoxy-28-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-8-en-3,24-dioic acid) | G. fornicatum (fruit bodies) | [105] |
| 311 | Fornicatin H (4, 7β-epoxy-28-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlansta-8-en-3,24-diester) | G. cochlear (sporophore) | [106] |
| 312 | Australic acid ((20Z, 23R, 25R)-15α-acetyl-7α, 8α-epoxy-12-oxo-3,4-seco-5α-lanosta-4(28),9,20(22)-trien-23,26-olid-3-oic acid) | G. australe | [103] |
| 313 | Lucidone A | G. tsugae | [107] |
| 314 | Lucidenol | G. tsugae | [107] |
| 315 | Ganosineniol A | G. sinense (fruit bodies) | [47] |
| 316 | 8α, 9α-Epoxy-4,4,14α-trimethyl-3,7,11,15,20-pentaoxo-5α-pregnane | G. concinna | [75] |
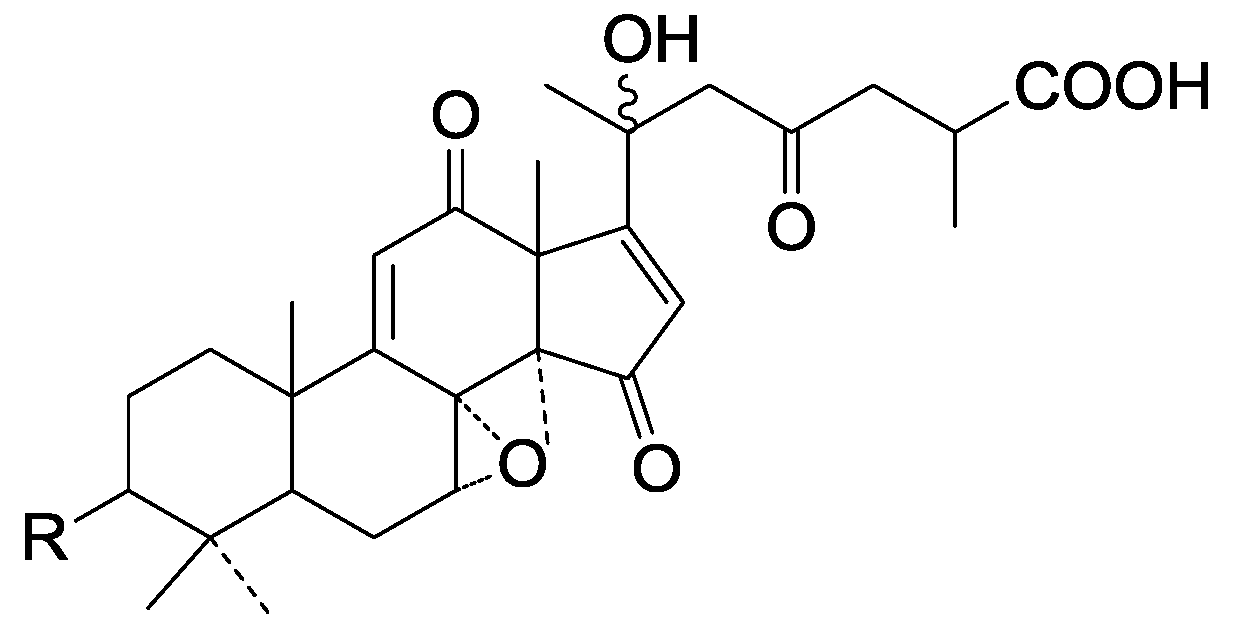
| Cpd | R |
|---|---|
| 281 | =O |
| 282 | β-OH |

| Cpd | R | R2 |
|---|---|---|
| 283 | α-OH | COOH |
| 284 | =O | CH2OH |
| 285 | β-OH | COOH |
| Cpd | R |
|---|---|
| 286 | α-OH |
| 287 | =O |
| Cpd | R |
|---|---|
| 308 | COOH |
| 309 | COOCH2CH3 |
| Cpd | R1 | R2 |
|---|---|---|
| 310 | COOH | COOH |
| 311 | COOCH3 | COOCH3 |

| Cpd | R1 | R2 |
|---|---|---|
| 313 | =O | =O |
| 314 | =O | OH |
| 315 | α-OH | CH2OH |
3. 13C-NMR Data of Ganoderma Triterpenes
| NO. | 1b) | 2b) | 3b) | 4b) | 6b) | 8b) | 9b) | 10b) | 11b) | 12b) | 13b) | 14b) | 15b) | 16b) | 17b) | 18b) | 19b) | 20b) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 33.4 | 35.7 | 35.0 | 33.1 | 35.1 | 34.4 | 35.7 | 35.6 | 34.8 | 34.8 | 35.0 | 34.7 | 35.7 | 35.7 | 34.6 | 34.3 | 34.8 | 34.2 |
| C2 | 27.5 | 34.5 | 27.9 | 27.2 | 34.2 | 28.0 | 34.4 | 34.4 | 27.6 | 28.3 | 28.0 | 28.2 | 34.3 | 34.3 | 27.8 | 27.7 | 34.1 | 27.3 |
| C3 | 77.5 | 217.1 | 78.5 | 77.3 | 217.7 | 78.7 | 208.4 | 208.7 | 78.3 | 78.5 | 77.5 | 78.2 | 217.5 | 217.5 | 78.2 | 77.5 | 217.8 | 78.5 |
| C4 | 40.6 | 46.8 | 39.0 | 39.0 | 47.0 | 39.0 | 47.0 | 46.7 | 38.8 | 39.0 | 38.9 | 38.6 | 46.8 | 46.8 | 38.6 | 40.2 | 47.2 | 39.1 |
| C5 | 51.5 | 49.0 | 49.3 | 51.2 | 51.7 | 51.8 | 49.2 | 48.8 | 49.2 | 45.7 | 49.5 | 49.1 | 49.0 | 49.0 | 49.1 | 49.8 | 45.2 | 47.7 |
| C6 | 36.8 | 29.3 | 26.7 | 36.6 | 18.7 | 17.4 | 29.2 | 29.1 | 26.6 | 27.8 | 27.6 | 27.8 | 27.7 | 27.8 | 28.2 | 36.5 | 27.9 | 28.0 |
| C7 | 199.1 | 69.1 | 67.1 | 199.0 | 29.6 | 30.4 | 69.1 | 68.9 | 66.9 | 67.0 | 69.5 | 69.5 | 66.4 | 66.3 | 69.5 | 205.3 | 66.7 | 68.0 |
| C8 | 151.9 | 159.1 | 157.1 | 145.6 | 163.2 | 162.9 | 159.6 | 159.5 | 156.8 | 155.0 | 159.6 | 158.0 | 157.8 | 157.8 | 158.1 | 154.6 | 159.3 | 158.8 |
| C9 | 145.9 | 140.5 | 142.9 | 151.7 | 138.6 | 140.0 | 140.6 | 140.1 | 142.7 | 142.9 | 142.2 | 142.0 | 141.3 | 141.3 | 141.9 | 149.8 | 140.0 | 141.6 |
| C10 | 39.3 | 38.2 | 38.8 | 40.3 | 37.1 | 37.8 | 46.8 | 46.8 | 38.6 | 45.4 | 38.9 | 38.6 | 38.3 | 38.3 | 38.5 | 38.9 | 38.0 | 38.6 |
| C11 | 194.2 | 199.6 | 198.0 | 193.9 | 198.1 | 198.3 | 200.0 | 199.7 | 197.9 | 200.0 | 201.2 | 199.8 | 197.6 | 197.6 | 199.9 | 201.3 | 199.1 | 199.4 |
| C12 | 79.3 | 51.9 | 50.5 | 79.1 | 51.7 | 52.1 | 51.9 | 51.8 | 50.3 | 51.0 | 52.3 | 52.0 | 50.2 | 50.2 | 51.9 | 52.3 | 51.8 | 52.2 |
| C13 | 48.1 | 47.0 | 45.5 | 47.9 | 46.8 | 47.2 | 38.2 | 38.0 | 45.4 | 38.8 | 47.4 | 47.1 | 45.0 | 45.0 | 47.1 | 48.0 | 46.4 | 46.1 |
| C14 | 58.7 | 54.1 | 59.6 | 58.5 | 53.6 | 53.5 | 54.2 | 54.1 | 59.4 | 58.5 | 54.4 | 54.0 | 59.4 | 59.4 | 54.0 | 52.8 | 53.4 | 53.5 |
| C15 | 205.9 | 72.7 | 217.7 | 66.2 | 72.9 | 73.0 | 72.6 | 72.4 | 217.5 | 207.5 | 72.4 | 72.4 | 216.6 | 216.4 | 72.5 | 72.1 | 72.4 | 72.3 |
| C16 | 38.1 | 36.7 | 41.1 | 38.0 | 38.6 | 38.7 | 36.2 | 36.3 | 40.9 | 41.0 | 35.9 | 36.2 | 41.0 | 41.0 | 36.1 | 36.3 | 37.8 | 37.8 |
| C17 | 44.9 | 48.4 | 45.8 | 44.6 | 48.7 | 48.7 | 48.3 | 48.2 | 45.6 | 49.2 | 48.5 | 48.1 | 45.7 | 45.8 | 48.1 | 48.2 | 49.0 | 49.0 |
| C18 | 12.3 | 17.5 | 17.6 | 12.1 | 17.2 | 17.1 | 17.4 | 17.3 | 17.4 | 17.2 | 17.2 | 17.1 | 17.7 | 17.7 | 17.1 | 17.4 | 17.5 | 17.3 |
| C19 | 18.1 | 19.5 | 18.7 | 17.9 | 19.0 | 19.0 | 19.6 | 19.4 | 18.5 | 17.6 | 19.6 | 19.5 | 18.2 | 18.2 | 19.6 | 17.6 | 17.5 | 17.3 |
| C20 | 29.6 | 32.8 | 32.1 | 29.4 | 32.6 | 32.5 | 32.8 | 32.8 | 32.0 | 32.1 | 33.0 | 32.7 | 32.0 | 32.0 | 32.7 | 32.4 | 32.5 | 32.5 |
| C21 | 21.8 | 19.7 | 19.8 | 21.5 | 19.4 | 19.4 | 19.4 | 19.7 | 19.6 | 19.8 | 19.7 | 19.6 | 19.6 | 19.7 | 19.6 | 19.5 | 19.3 | 19.3 |
| C22 | 48.6 | 49.9 | 49.4 | 48.5 | 49.6 | 49.7 | 49.8 | 49.7 | 49.0 | 49.3 | 50.0 | 49.8 | 49.0 | 49.1 | 49.7 | 49.5 | 49.6 | 49.6 |
| C23 | 207.7 | 208.6 | 207.9 | 206.1 | 208.3 | 208.3 | 217.3 | 217.4 | 207.6 | 215.9 | 210.0 | 208.5 | 207.5 | 207.6 | 208.7 | 208.2 | 208.3 | 208.3 |
| C24 | 46.8 | 46.9 | 46.8 | 46.6 | 46.8 | 46.8 | 46.7 | 46.8 | 46.6 | 46.9 | 46.9 | 46.7 | 46.6 | 46.8 | 46.7 | 46.8 | 46.9 | 46.9 |
| C25 | 34.9 | 35.0 | 35.1 | 35.1 | 34.7 | 34.6 | 34.8 | 34.8 | 34.6 | 34.9 | 35.0 | 34.7 | 34.5 | 34.7 | 34.6 | 34.7 | 34.7 | 34.7 |
| C26 | 175.9 | 176.0 | 175.9 | 181.0 | 176.2 | 176.1 | 27.4 | 27.5 | 180.3 | 26.8 | 178.5 | 176.2 | 180.3 | 176.1 | 176.3 | 176.1 | 176.2 | 176.4 |
| C27 | 17.3 | 17.3 | 17.3 | 17.1 | 17.1 | 17.1 | 180.1 | 176.3 | 16.9 | 176.2 | 17.2 | 17.1 | 16.9 | 17.1 | 17.1 | 17.1 | 17.1 | 17.1 |
| C28 | 28.1 | 27.5 | 28.4 | 27.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C29 | 15.7 | 20.9 | 15.6 | 15.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C30 | 21.5 | 19.8 | 24.6 | 21.2 | 27.8 | 28.3 | 17.0 | 17.2 | 28.2 | 15.5 | 28.3 | 28.2 | 27.0 | 27.0 | 28.2 | 27.8 | 27.6 | 28.2 |
| OCOCH3 | Bu1' | Bu1' | CH3CO | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | |
| 170.4 | 64.8 | 64.8 | 170.2 | 20.6 | 15.7 | 20.8 | 20.8 | 15.5 | 24.5 | 15.9 | 15.7 | 20.8 | 20.8 | 15.7 | 15.4 | 20.5 | 15.8 | |
| OCOCH3 | Bu 2' | Bu 2' | CH3CO | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | |
| 21.0 | 30.9 | 30.9 | 20.9 | 18.8 | 18.8 | 19.8 | 19.7 | 24.4 | 18.7 | 19.5 | 19.4 | 24.7 | 24.7 | 19.4 | 20.3 | 21.1 | 21.1 | |
| N-BU1' | Bu 3' | Bu3' | OCH3 | OCH3 | COOCH3 | COOCH3 | COOCH3 | COOCH3 | OCH3 | OCH3 | OCH3 | OCH3 | ||||||
| 64.8 | 19.3 | 19.3 | 51.9 | 51.8 | 52.0 | 51.7 | 51.9 | 51.9 | 51.9 | 51.9 | 51.9 | 51.9 | ||||||
| NO. | 21b) | 24b) | 25b) | 26b) | 27c) | 29b) | 30b) | 35c) | 38b) | 39b) | 40b) | 41b) | 42d) | 43b) | 44a) | 45b) | 46b) | 47c) |
| C1 | 34.5 | 33.9 | 34.4 | 34.5 | 35.4 | 37.4 | 37.2 | 35.2 | 35.6 | 35.7 | 34.4 | 33.8 | 34.0 | 34.8 | 36.0 | 35.4 | 34.8 | 35.2 |
| C2 | 24.0 | 33.6 | 27.4 | 28.4 | 34.7 | 34.1 | 34.6 | 27.6 | 34.4 | 34.5 | 27.4 | 27.5 | 33.5 | 27.4 | 34.5 | 34.3 | 28.8 | 33.9 |
| C3 | 79.9 | 215.1 | 78.2 | 78.4 | 221.0 | 214.9 | 215.4 | 78.0 | 216.8 | 218.0 | 78.2 | 78.5 | 215.5 | 78.0 | 215.9 | 214.6 | 78.0 | 218.6 |
| C4 | 38.0 | 46.8 | 38.6 | 40.7 | 47.2 | 46.9 | 47.0 | 39.1 | 46.2 | 47.0 | 38.5 | 38.8 | 38.7 | 39.0 | 47.0 | 47.2 | 39.8 | 46.4 |
| C5 | 49.2 | 50.8 | 49.1 | 53.0 | 45.7 | 51.0 | 50.9 | 49.8 | 48.8 | 49.0 | 49.1 | 51.3 | 49.7 | 49.9 | 48.7 | 50.4 | 50.7 | 48.3 |
| C6 | 26.6 | 37.4 | 36.7 | 37.8 | 28.1 | 33.7 | 33.8 | 27.4 | 27.6 | 29.2 | 26.6 | 17.3 | 36.8 | 36.6 | 28.8 | 37.1 | 36.7 | 28.1 |
| C7 | 66.1 | 198.6 | 66.2 | 201.1 | 67.1 | 198.6 | 199.4 | 67.0 | 66.3 | 69.2 | 66.2 | 29.3 | 199.6 | 199.1 | 66.0 | 198.0 | 199.0 | 68.2 |
| C8 | 157.4 | 149.8 | 155.8 | 151.9 | 161.8 | 149.9 | 149.8 | 157.3 | 157.8 | 159.2 | 155.9 | 161.5 | 146.1 | 138.9 | 159.7 | 139.8 | 138.9 | 160.4 |
| C9 | 141.6 | 145.9 | 142.9 | 153.6 | 140.2 | 146.1 | 146.8 | 143.1 | 141.2 | 140.6 | 142.0 | 140.1 | 149.0 | 164.9 | 140.9 | 162.6 | 164.7 | 139.6 |
| C10 | 37.5 | 39.2 | 38.5 | 42.4 | 38.4 | 39.4 | 39.3 | 38.9 | 38.2 | 38.2 | 38.5 | 37.5 | 46.4 | 39.8 | 38.4 | 39.4 | 38.9 | 37.6 |
| C11 | 199.3 | 194.1 | 192.0 | 195.0 | 72.0 | 194.1 | 199.4 | 200.3 | 197.7 | 200.1 | 192.1 | 191.6 | 199.8 | 23.7 | 198.2 | 23.8 | 23.6 | 200.3 |
| C12 | 77.9 | 78.9 | 79.5 | 199.8 | 52.4 | 79.0 | 48.9 | 78.4 | 50.1 | 52.0 | 79.8 | 80.1 | 48.5 | 30.2 | 50.9 | 30.1 | 30.2 | 51.5 |
| C13 | 51.9 | 47.6 | 49.6 | 62.2 | 47.6 | 47.7 | 43.9 | 52.4 | 44.9 | 46.9 | 49.9 | 51.5 | 56.8 | 45.0 | 45.2 | 45.0 | 45.0 | 46.3 |
| C14 | 60.3 | 58.7 | 60.6 | 61.3 | 54.1 | 58.7 | 57.2 | 60.9 | 59.3 | 54.1 | 60.5 | 53.9 | 43.5 | 47.8 | 59.0 | 47.8 | 49.0 | 53.7 |
| C15 | 216.8 | 205.6 | 216.2 | 207.5 | 201.0 | 205.5 | 207.0 | 217.8 | 218.1 | 72.9 | 216.4 | 74.6 | 207.8 | 32.0 | 217.0 | 28.7 | 27.5 | 71.6 |
| C16 | 38.3 | 37.8 | 37.9 | 40.8 | 35.3 | 37.8 | 39.8 | 38.6 | 41.2 | 36.7 | 37.3 | 33.6 | 39.9 | 28.8 | 42.0 | 31.8 | 32.0 | 35.6 |
| C17 | 45.7 | 44.3 | 45.2 | 40.7 | 49.5 | 44.5 | 44.3 | 46.6 | 46.7 | 48.7 | 46.1 | 48.6 | 44.4 | 49.0 | 46.8 | 49.0 | 49.9 | 48.5 |
| C18 | 12.0 | 12.0 | 12.0 | 13.5 | 17.8 | 12.1 | 16.0 | 12.3 | 17.7 | 17.4 | 13.4 | 12.3 | 15.4 | 15.8 | 17.8 | 15.9 | 15.3 | 16.6 |
| C19 | 18.8 | 18.7 | 18.6 | 18.6 | 17.9 | 18.7 | 18.6 | 19.1 | 18.2 | 19.6 | 18.6 | 19.0 | 18.4 | 18.4 | 18.2 | 17.9 | 18.4 | 18.9 |
| C20 | 28.7 | 29.4 | 28.2 | 33.8 | 33.2 | 29.5 | 32.1 | 29.1 | 35.5 | 36.2 | 35.5 | 34.1 | 32.9 | 36.2 | 33.9 | 36.2 | 36.3 | 33.1 |
| C21 | 21.3 | 21.6 | 21.9 | 20.4 | 19.5 | 21.6 | 19.8 | 21.8 | 18.2 | 18.5 | 20.8 | 19.7 | 19.7 | 18.6 | 19.8 | 18.6 | 18.6 | 19.0 |
| C22 | 48.3 | 48.4 | 47.9 | 50.2 | 50.0 | 48.4 | 48.8 | 48.7 | 34.5 | 34.8 | 33.1 | 33.7 | 42.8 | 34.8 | 43.8 | 34.7 | 34.8 | 42.9 |
| C23 | 208.1 | 207.6 | 207.4 | 211.1 | 210.7 | 207.3 | 207.6 | 210.3 | 25.6 | 25.9 | 26.5 | 26.3 | 65.4 | 25.9 | 66.5 | 26.0 | 26.0 | 65.9 |
| C24 | 46.1 | 46.6 | 46.6 | 48.0 | 47.2 | 46.4 | 46.5 | 46.9 | 144.1 | 145.3 | 143.2 | 144.2 | 144.4 | 145.6 | 145.0 | 155.2 | 155.3 | 143.2 |
| C25 | 34.2 | 34.7 | 34.6 | 36.6 | 37.4 | 34.6 | 34.6 | 35.4 | 127.0 | 127.2 | 127.1 | 127.2 | 126.9 | 126.6 | 128.8 | 139.2 | 139.2 | 128.4 |
| C26 | 180.5 | 175.6 | 176.1 | 180.7 | 179.0 | 180.8 | 180.9 | 178.8 | 171.2 | 172.0 | 171.0 | 172.7 | 169.0 | 172.4 | 170.7 | 195.3 | 195.3 | 170.2 |
| C27 | 16.9 | 17.1 | 17.1 | 18.0 | 17.4 | 16.9 | 16.9 | 17.3 | 12.1 | 12.3 | 12.1 | 12.0 | 12.7 | 12.0 | 13.4 | 9.2 | 9.2 | 12.3 |
| C28 | 27.9 | 27.6 | 28.0 | 28.7 | 27.8 | -- | -- | -- | 24.7 | 19.7 | 24.1 | 28.2 | 27.0 | 25.0 | 27.0 | 25.4 | 27.5 | 27.0 |
| C29 | 16.4 | 20.3 | 15.4 | 16.6 | 20.7 | -- | -- | -- | 26.9 | 27.6 | 28.1 | 15.7 | 19.9 | 27.5 | 20.8 | 21.4 | 15.8 | 20.1 |
| C30 | 23.0 | 20.7 | 24.0 | 24.9 | 21.4 | 27.6 | 27.6 | 28.4 | 20.7 | 20.9 | 15.4 | 19.8 | 20.0 | 15.3 | 25.1 | 24.9 | 25.0 | 19.0 |
| C31 | CH3CO | CH3CO | C31 | C31 | C31 | CH3CO | 12-COCH3 | |||||||||||
| 161.0 | 170.2 | 170.4 | 20.4 | 20.3 | 15.8 | 170.1 | 170.5 | |||||||||||
| CH3CO | CH3CO | C32 | C32 | C32 | CH3CO | 12-COCH3 | ||||||||||||
| 20.9 | 20.9 | 20.8 | 21.0 | 23.5 | 20.7 | 21.0 | ||||||||||||
| OCH2CH3 | OCH3 | OCOCH3 | 15-COCH3 | |||||||||||||||
| 60.7 | 51.9 | 170.2 | 170.6 | |||||||||||||||
| NO | 48c) | 49c) | 50c) | 51c) | 52c) | 53b) | 56b) | 57b) | 58b) | 60b) | 61b) | 62b) | 63b) | 64b) | 65b) | 66a) | 67a) | 68a) |
| C1 | 34.9 | 35.7 | 33.6 | 34.4 | 33.2 | 34.7 | 30.1 | 35.0 | 35.3 | 34.8 | 34.5 | 34.7 | 34.6 | 35.4 | 35.2 | 35.5 | 35.2 | 35.2 |
| C2 | 34.3 | 28.0 | 26.9 | 27.1 | 26.7 | 27.6 | 23.3 | 34.1 | 34.3 | 34.6 | 34.4 | 27.3 | 27.3 | 34.4 | 34.3 | 29.0 | 28.9 | 34.1 |
| C3 | 219.5 | 78.7 | 77.2 | 78.0 | 76.8 | 78.3 | 77.3 | 215.5 | 214.6 | 214.4 | 214.9 | 77.8 | 77.8 | 214.6 | 217.2 | 77.6 | 77.9 | 218.4 |
| C4 | 46.6 | 39.7 | 39.0 | 38.4 | 40.0 | 38.8 | 36.2 | 46.9 | 47.2 | 47.5 | 47.4 | 38.8 | 38.8 | 47.2 | 46.6 | 39.3 | 39.7 | 45.9 |
| C5 | 45.3 | 50.0 | 50.8 | 49.0 | 51.2 | 49.1 | 40.5 | 49.8 | 50.4 | 50.8 | 51.6 | 49.7 | 49.8 | 49.0 | 44.8 | 49.9 | 52.4 | 48.6 |
| C6 | 27.7 | 27.6 | 36.2 | 26.5 | 36.4 | 26.6 | 26.1 | 37.1 | 37.1 | 37.5 | 37.2 | 36.5 | 36.5 | 37.2 | 28.4 | 28.8 | 30.9 | 28.8 |
| C7 | 66.6 | 67.7 | 199.9 | 66.2 | 199.5 | 66.8 | 70.0 | 201.3 | 198.1 | 199.4 | 199.8 | 199.0 | 198.9 | 198.0 | 66.2 | 69.4 | 17.9 | 68.6 |
| C8 | 160.9 | 158.4 | 148.6 | 156.6 | 146.2 | 156.9 | 131.1 | 151.5 | 139.6 | 142.1 | 139.7 | 138.9 | 138.8 | 139.6 | 134.8 | 160.5 | 164.8 | 159.8 |
| C9 | 139.7 | 143.9 | 151.8 | 142.0 | 151.0 | 142.7 | 145.1 | 149.8 | 162.7 | 158.7 | 160.6 | 164.8 | 164.6 | 162.8 | 140.1 | 141.3 | 139.8 | 140.3 |
| C10 | 37.9 | 39.4 | 40.4 | 38.1 | 38..8 | 38.6 | 38.3 | 38.8 | 39.4 | 40.1 | 39.7 | 39.7 | 39.7 | 39.4 | 38.1 | 39.1 | 38.3 | 37.5 |
| C11 | 200.1 | 200.1 | 200.1 | 199.5 | 201.4 | 198.0 | 21.4 | 201.8 | 23.8 | 65.8 | 67.2 | 23.6 | 23.5 | 23.8 | 20.8 | 200.1 | 198.6 | 199.8 |
| C12 | 52.0 | 51.2 | 49.6 | 78.2 | 77.6 | 50.3 | 31.2 | 51.2 | 30.1 | 44.6 | 42.7 | 30.0 | 30.1 | 30.2 | 31.2 | 52.9 | 52.9 | 51.6 |
| C13 | 47.0 | 46.4 | 44.2 | 51.7 | 49.5 | 45.3 | 45.8 | 46.7 | 44.9 | 47.6 | 43.4 | 44.8 | 44.9 | 45.0 | 45.3 | 47.6 | 47.4 | 46.2 |
| C14 | 53.4 | 60.3 | 57.0 | 60.1 | 57.5 | 59.3 | 52.1 | 48.7 | 47.8 | 48.1 | 48.9 | 47.7 | 47.7 | 47.8 | 51.2 | 54.7 | 54.0 | 53.7 |
| C15 | 71.7 | 218.5 | 209.0 | 217.4 | 207.6 | 217.9 | 72.4 | 31.9 | 31.9 | 32.6 | 31.9 | 31.9 | 31.9 | 28.7 | 76.4 | 72.4 | 72.1 | 72.1 |
| C16 | 37.5 | 42.0 | 45.6 | 36.8 | 36.7 | 41.1 | 39.7 | 27.43 | 28.7 | 27.9 | 28.7 | 28.7 | 28.7 | 31.9 | 36.4 | 37.5 | 39.6 | 36.2 |
| C17 | 49.7 | 47.2 | 42.7 | 46.2 | 45.5 | 46.1 | 48.8 | 49.1 | 49.0 | 49.7 | 48.1 | 48.9 | 48.9 | 50.5 | 49.3 | 49.5 | 49.9 | 47.5 |
| C18 | 17.3 | 17.6 | 15.9 | 11.9 | 10.6 | 17.4 | 16.3 | 16.9 | 15.9 | 16.9 | 17.3 | 15.7 | 15.7 | 15.9 | 16.6 | 17.4 | 17.0 | 17.0 |
| C19 | 17.5 | 18.8 | 17.6 | 18.6 | 17.6 | 18.4 | 17.7 | 17.9 | 17.9 | 19.2 | 19.5 | 18.2 | 18.2 | 17.9 | 17.3 | 19.9 | 19.4 | 19.3 |
| C20 | 33.5 | 34.0 | 33.1 | 28.5 | 29.4 | 35.5 | 36.2 | 36.1 | 36.2 | 36.0 | 36.2 | 36.1 | 36.1 | 36.2 | 36.2 | 34.6 | 34.4 | 35.9 |
| C21 | 19.2 | 20.0 | 19.3 | 22.0 | 21.3 | 18.2 | 18.4 | 18.3 | 18.7 | 18.4 | 18.6 | 18.6 | 18.5 | 18.4 | 18.2 | 20.2 | 19.9 | 18.4 |
| C22 | 43.3 | 43.7 | 40.4 | 41.3 | 41.8 | 37.4 | 34.7 | 34.5 | 35.9 | 34.6 | 34.6 | 35.8 | 34.7 | 34.7 | 34.6 | 44.5 | 44.4 | 34.4 |
| C23 | 66.4 | 66.8 | 65.9 | 67.0 | 66.5 | 25.6 | 25.8 | 25.92 | 24.5 | 25.8 | 25.8 | 24.4 | 25.9 | 25.9 | 25.9 | 67.0 | 66.9 | 26.0 |
| C24 | 142.4 | 144.2 | 142.8 | 142.7 | 142.2 | 143.9 | 145.2 | 154.6 | 126.8 | 145.3 | 145.3 | 126.8 | 155.1 | 145.5 | 144.9 | 145.4 | 145.4 | 156.3 |
| C25 | 130.2 | 129.6 | 128.8 | 129.3 | 129.0 | 127.5 | 126.8 | 139.4 | 134.4 | 126.8 | 126.7 | 134.3 | 139.1 | 126.5 | 126.7 | 128.7 | 128.7 | 138.6 |
| C26 | 172.0 | 171.2 | 170.8 | 170.8 | 175.0 | 172.3 | 172.4 | 195.2 | 69.0 | 172.7 | 172.7 | 69.0 | 195.3 | 172.5 | 171.2 | 170.8 | 170.8 | 194.4 |
| C27 | 13.1 | 13.1 | 12.6 | 12.8 | 12.6 | 12.1 | 12.0 | 9.2 | 13.6 | 11.9 | 11.9 | 13.5 | 9.0 | 12.0 | 12.1 | 13.5 | 13.5 | 9.2 |
| C28 | 27.5 | 28.6 | 27.5 | 27.9 | 27.4 | 28.1 | 27.2 | 27.44 | 25.4 | 25.1 | 25.1 | 24.9 | 24.9 | 25.0 | 26.5 | 28.8 | 28.9 | 20.8 |
| C29 | 20.5 | 16.1 | 15.3 | 15.2 | 15.2 | 15.4 | 22.7 | 20.3 | 21.4 | 21.6 | 21.8 | 27.3 | 27.3 | 25.9 | 21.2 | 16.7 | 16.7 | 27.5 |
| C30 | 21.0 | 24.9 | 21.6 | 22.9 | 20.1 | 24.4 | 17.9 | 25.87 | 24.9 | 25.3 | 24.9 | 15.2 | 15.2 | 21.4 | 20.1 | 20.2 | 19.8 | 19.1 |
| COCH3 | ||||||||||||||||||
| 171.9 | ||||||||||||||||||
| COCH3 | ||||||||||||||||||
| 21.9 | ||||||||||||||||||
| NO. | 69c) | 70b) | 71b) | 72b) | 73b) | 74b) | 75b) | 77b) | 78b) | 79b) | 80b) | 81b) | 82b) | 83h) | 84b) | 85b) | 86b) | 87b) |
| C1 | 34.9 | 30.3 | 30.3 | 30.1 | 30.2 | 30.3 | 30.3 | 30.3 | 34.5 | 34.8 | 35.2 | 35.3 | 35.3 | 31.3 | 30.0 | 33.3 | 35.9 | 35.6 |
| C2 | 34.0 | 23.3 | 23.3 | 23.3 | 23.4 | 23.2 | 23.3 | 23.3 | 23.8 | 27.4 | 34.2 | 34.3 | 34.3 | 24.3 | 23.3 | 27.3 | 34.5 | 34.2 |
| C3 | 219.6 | 77.5 | 77.5 | 77.3 | 77.6 | 77.3 | 77.5 | 77.6 | 79.6 | 77.9 | 217.2 | 217.4 | 217.4 | 77.7 | 77.2 | 77.4 | 216.7 | 216.4 |
| C4 | 46.8 | 36.3 | 36.3 | 36.2 | 36.4 | 36.5 | 36.4 | 36.3 | 37.8 | 38.9 | 46.6 | 46.7 | 46.7 | 37.2 | 36.3 | 39.1 | 47.0 | 46.7 |
| C5 | 51.3 | 40.1 | 40.0 | 40.5 | 40.4 | 40.1 | 40.5 | 40.2 | 49.9 | 49.8 | 44.7 | 45.0 | 45.0 | 40.8 | 39.6 | 51.2 | 49.2 | 48.8 |
| C6 | 18.4 | 27.4 | 27.4 | 26.0 | 22.4 | 21.3 | 28.1 | 28.9 | 36.4 | 36.6 | 28.4 | 30.0 | 23.3 | 28.5 | 21.3 | 36.5 | 27.9 | 27.6 |
| C7 | 29.3 | 66.5 | 66.5 | 69.9 | 76.5 | 76.3 | 66.8 | 67.0 | 198.6 | 198.9 | 66.1 | 66.7 | 76.1 | 67.1 | 75.9 | 198.5 | 66.5 | 66.2 |
| C8 | 165.1 | 133.9 | 133.8 | 131.1 | 134.3 | 132.8 | 134.5 | 135.6 | 138.9 | 138.8 | 134.6 | 136.4 | 135.3 | 141.7 | 142.9 | 145.8 | 157.8 | 157.3 |
| C9 | 137.9 | 141.8 | 141.8 | 145.0 | 141.3 | 143.6 | 141.7 | 141.3 | 164.4 | 164.6 | 140.1 | 139.4 | 139.5 | 135.1 | 132.7 | 151.9 | 141.4 | 141.2 |
| C10 | 36.8 | 38.4 | 38.4 | 38.3 | 38.1 | 38.4 | 38.1 | 38.2 | 39.6 | 39.8 | 38.1 | 37.9 | 37.8 | 39.2 | 38.7 | 40.5 | 38.6 | 38.3 |
| C11 | 199.1 | 20.7 | 20.7 | 21.2 | 21.0 | 20.9 | 20.6 | 21.0 | 23.6 | 23.6 | 20.7 | 21.3 | 21.0 | 21.5 | 21.1 | 193.5 | 197.5 | 196.8 |
| C12 | 51.6 | 31.2 | 31.2 | 31.2 | 31.1 | 31.4 | 31.7 | 31.0 | 30.1 | 30.1 | 31.2 | 31.0 | 31.1 | 32.1 | 30.7 | 78.5 | 49.2 | 48.9 |
| C13 | 46.4 | 45.5 | 45.3 | 45.6 | 45.0 | 46.2 | 45.7 | 45.0 | 44.9 | 44.9 | 45.1 | 45.0 | 44.9 | 46.0 | 45.6 | 57.8 | 45.9 | 45.9 |
| C14 | 53.3 | 51.2 | 51.3 | 52.1 | 50.0 | 52.8 | 52.4 | 49.7 | 47.8 | 47.8 | 51.2 | 49.7 | 49.9 | 52.4 | 51.7 | 48.7 | 58.8 | 58.6 |
| C15 | 72.0 | 76.5 | 76.0 | 72.2 | 30.2 | 72.0 | 72.4 | 29.8 | 31.9 | 31.9 | 75.9 | 29.9 | 30.1 | 76.0 | 75.2 | 204.6 | 217.3 | 216.6 |
| C16 | 38.1 | 36.6 | 36.3 | 39.3 | 27.9 | 37.3 | 38.1 | 27.9 | 28.5 | 28.5 | 36.1 | 27.9 | 27.8 | 37.3 | 37.1 | 37.6 | 38.1 | 37.8 |
| C17 | 49.0 | 49.3 | 45.9 | 45.5 | 47.2 | 46.4 | 46.4 | 47.1 | 45.6 | 45.7 | 45.9 | 47.1 | 47.2 | 46.9 | 45.2 | 48.9 | 48.3 | 49.7 |
| C18 | 16.7 | 16.5 | 16.3 | 16.2 | 15.9 | 16.6 | 16.4 | 15.9 | 15.6 | 15.6 | 16.4 | 16.0 | 16.0 | 17.0 | 16.1 | 13.3 | 19.2 | 19.0 |
| C19 | 18.6 | 17.5 | 17.5 | 17.7 | 17.5 | 17.2 | 17.3 | 17.3 | 18.5 | 18.4 | 17.3 | 17.3 | 17.4 | 18.0 | 17.6 | 17.8 | 18.4 | 18.1 |
| C20 | 33.1 | 36.3 | 39.9 | 39.5 | 39.8 | 39.3 | 39.5 | 39.7 | 39.5 | 39.5 | 39.9 | 39.7 | 39.7 | 41.0 | 39.9 | 154.7 | 138.5 | 153.3 |
| C21 | 18.8 | 18.2 | 12.7 | 12.9 | 12.9 | 13.0 | 12.9 | 12.8 | 13.1 | 13.1 | 12.6 | 12.8 | 12.8 | 13.6 | 12.7 | 21.1 | 18.3 | 21.0 |
| C22 | 42.9 | 34.7 | 74.4 | 74.6 | 74.8 | 74.9 | 74.7 | 74.7 | 74.8 | 74.8 | 74.3 | 74.7 | 74.7 | 74.8 | 74.3 | 126.0 | 126.9 | 124.7 |
| C23 | 66.0 | 25.9 | 31.9 | 31.7 | 31.9 | 31.9 | 31.9 | 31.8 | 31.8 | 31.8 | 31.9 | 31.8 | 31.8 | 32.8 | 31.8 | 197.8 | 74.5 | 197.9 |
| C24 | 142.4 | 145.0 | 138.9 | 139.1 | 139.6 | 139.1 | 139.1 | 139.4 | 139.4 | 139.4 | 138.8 | 139.6 | 139.5 | 140.0 | 139.1 | 47.5 | 37.2 | 47.7 |
| C25 | 129.5 | 126.8 | 129.5 | 129.3 | 129.1 | 129.3 | 129.3 | 129.2 | 129.0 | 129.0 | 129.5 | 129.2 | 129.0 | 130.2 | 129.3 | 34.4 | 34.5 | 34.8 |
| C26 | 171.5 | 172.2 | 172.0 | 171.7 | 172.1 | 172.0 | 171.4 | 172.4 | 171.3 | 171.2 | 171.9 | 172.0 | 171.7 | 172.9 | 172.2 | 180.2 | 179.8 | 176.3 |
| C27 | 12.7 | 12.0 | 12.3 | 12.3 | 12.3 | 12.3 | 12.4 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 13.1 | 12.3 | 17.0 | 16.0 | 17.2 |
| C28 | 27.5 | 27.4 | 27.4 | 27.2 | 27.3 | 27.3 | 27.4 | 27.4 | 27.4 | 27.4 | 26.5 | 26.5 | 26.5 | -- | -- | 27.9 | 27.3 | 27.0 |
| C29 | 20.2 | 21.9 | 22.0 | 21.9 | 22.2 | 22.0 | 21.9 | 22.0 | 16.3 | 15.3 | 21.2 | 21.3 | 21.3 | -- | -- | 15.5 | 21.0 | 20.8 |
| C30 | 18.7 | 20.2 | 20.2 | 18.0 | 25.6 | 18.5 | 19.2 | 26.3 | 25.1 | 25.0 | 26.1 | 26.1 | 25.4 | 28.2 | 27.3 | 21.3 | 24.8 | 24.7 |
| OCOCH3 | COCH3 | COCH3 | COCH3 | COCH3 | COCH3 | CO | 3-OC- | 22-OC- | 15-OC- | 22-OC- | 7-OCH3 | C31 | C31 | C31 | OCH3 | |||
| 170.9 | 170.9 | 171.9 | 171.1 | 170.9 | 170.9 | 170.9 | OCH3 | OCH3 | OCH3 | OCH3 | 55.8 | 22.6 | 22.2 | 170.3 | 51.9 | |||
| OCOCH3 | COCH3 | COCH3 | COCH3 | COCH3 | COCH3 | CO | 170.8 | 170.6 | 170.5 | 170.7 | C32 | C32 | C32 | |||||
| 21.4 | 21.4 | 21.7 | 21.6 | 21.5 | 21.4 | 170.7 | 21.1 | 19.0 | 20.5 | |||||||||
| NO. | 88c) | 89c) | 90b) | 91b) | 92b) | 93h) | 94h) | 96h) | 99b) | 100b) | 101b) | 102b) | 103b) | 104b) | 105b) | 106b) | 107b) | 108b) |
| C1 | 35.5 | 34.8 | 34.8 | 35.6 | 35.1 | 34.9 | 35.2 | 34.5 | 35.7 | 37.3 | 37.5 | 36.6 | 34.6 | 35.2 | 33.4 | 35.0 | 35.1 | 35.9 |
| C2 | 34.3 | 27.7 | 27.8 | 34.2 | 34.1 | 34.7 | 33.9 | 27.9 | 34.3 | 34.7 | 34.1 | 27.3 | 27.6 | 34.3 | 27.4 | 33.9 | 28.0 | 34.5 |
| C3 | 217.4 | 78.3 | 78.1 | 216.5 | 215.9 | 213.3 | 213.0 | 77.0 | 217.5 | 217.2 | 214.8 | 77.3 | 78.3 | 216.8 | 77.5 | 215.4 | 78.6 | 216.8 |
| C4 | 46.7 | 39.0 | 38.8 | 46.8 | 46.8 | 46.8 | 46.3 | 40.4 | 46.8 | 43.9 | 46.9 | 40.4 | 38.6 | 47.0 | 40.3 | 46.4 | 39.1 | 47.0 |
| C5 | 48.8 | 49.2 | 50.0 | 48.9 | 49.2 | 48.5 | 49.0 | 50.1 | 49.0 | 50.9 | 51.0 | 51.4 | 49.2 | 49.5 | 51.5 | 49.0 | 49.4 | 49.2 |
| C6 | 29.0 | 26.7 | 27.6 | 27.7 | 27.6 | 36.1 | 36.8 | 36.5 | 27.8 | 33.8 | 33.7 | 33.2 | 26.9 | 27.8 | 36.8 | 36.8 | 26.9 | 27.9 |
| C7 | 68.9 | 66.9 | 69.4 | 66.3 | 65.8 | 198.4 | 204.5 | 205.0 | 66.3 | 199.3 | 198.7 | 198.7 | 66.2 | 65.8 | 198.8 | 204.6 | 67.1 | 66.5 |
| C8 | 159.1 | 156.6 | 158.8 | 157.4 | 156.7 | 146.9 | 150.3 | 149.6 | 157.8 | 149.7 | 149.9 | 151.6 | 157.4 | 158.3 | 150.5 | 150.4 | 156.9 | 157.9 |
| C9 | 140.3 | 142.8 | 142.0 | 141.4 | 141.6 | 149.5 | 152.6 | 154.6 | 141.3 | 146.8 | 146.1 | 145.7 | 141.9 | 140.5 | 147.1 | 152.3 | 142.7 | 141.3 |
| C10 | 38.1 | 38.7 | 38.8 | 38.4 | 38.2 | 39.5 | 39.2 | 38.8 | 38.3 | 39.4 | 39.3 | 39.1 | 38.4 | 37.9 | 39.2 | 39.1 | 38.9 | 38.5 |
| C11 | 199.1 | 198.0 | 200.0 | 197.8 | 191.8 | 198.1 | 199.8 | 200.1 | 197.6 | 199.3 | 194.1 | 193.9 | 199.3 | 199.5 | 201.6 | 201.2 | 198.0 | 197.8 |
| C12 | 50.5 | 49.1 | 50.8 | 48.9 | 78.5 | 47.8 | 50.5 | 51.0 | 50.2 | 48.9 | 79.0 | 79.1 | 77.9 | 78.1 | 77.5 | 52.1 | 51.1 | 50.9 |
| C13 | 48.1 | 46.3 | 49.3 | 46.0 | 50.3 | 44.8 | 48.6 | 49.2 | 45.0 | 47.0 | 47.7 | 47.9 | 51.9 | 51.7 | 49.8 | 47.8 | 46.0 | 45.7 |
| C14 | 53.4 | 58.7 | 53.5 | 58.7 | 59.9 | 56.8 | 52.4 | 52.5 | 59.4 | 57.2 | 58.6 | 58.4 | 60.3 | 60.4 | 57.9 | 52.8 | 59.8 | 59.8 |
| C15 | 72.7 | 216.4 | 72.5 | 216.4 | 215.1 | 204.9 | 72.9 | 72.9 | 216.4 | 206.8 | 205.4 | 205.5 | 216.8 | 215.5 | 206.0 | 71.8 | 217.9 | 218.0 |
| C16 | 31.8 | 37.7 | 31.6 | 37.9 | 38.4 | 37.1 | 32.3 | 32.5 | 41.0 | 39.8 | 37.8 | 37.8 | 38.4 | 38.5 | 37.9 | 30.2 | 36.2 | 36.4 |
| C17 | 52.2 | 49.7 | 52.4 | 49.7 | 50.1 | 50.8 | 52.0 | 52.1 | 45.8 | 44.5 | 44.5 | 44.7 | 45.8 | 45.8 | 45.3 | 50.9 | 50.2 | 50.4 |
| C18 | 19.0 | 18.8 | 18.8 | 19.0 | 14.4 | 17.5 | 16.7 | 15.5 | 17.7 | 16.1 | 12.1 | 12.1 | 12.0 | 12.1 | 10.9 | 18.9 | 19.1 | 19.4 |
| C19 | 19.9 | 18.4 | 19.7 | 18.1 | 18.1 | 18.4 | 17.4 | 17.3 | 18.2 | 18.6 | 18.7 | 17.9 | 18.8 | 18.3 | 18.1 | 17.5 | 18.6 | 18.4 |
| C20 | 157.0 | 153.8 | 157.3 | 153.6 | 154.1 | 153.6 | 155.6 | 155.7 | 32.0 | 32.0 | 29.4 | 29.3 | 28.7 | 28.7 | 29.5 | 73.3 | 73.4 | 73.3 |
| C21 | 21.3 | 21.0 | 21.3 | 20.9 | 20.3 | 21.5 | 21.1 | 21.1 | 19.7 | 19.8 | 21.6 | 21.6 | 21.4 | 21.4 | 21.3 | 26.6 | 26.4 | 26.3 |
| C22 | 124.3 | 124.7 | 124.5 | 124.7 | 126.1 | 124.6 | 124.5 | 124.6 | 49.1 | 49.1 | 48.5 | 48.4 | 48.4 | 48.5 | 48.9 | 52.5 | 48.5 | 48.5 |
| C23 | 198.6 | 197.2 | 199.6 | 196.9 | 197.8 | 197.2 | 197.4 | 197.3 | 207.6 | 207.6 | 207.4 | 207.4 | 208.1 | 208.1 | 208.1 | 211.2 | 74.8 | 74.8 |
| C24 | 47.5 | 47.6 | 48.1 | 47.5 | 47.5 | 47.6 | 47.6 | 47.9 | 46.8 | 46.7 | 46.7 | 46.6 | 46.4 | 46.4 | 46.6 | 47.7 | 36.8 | 36.8 |
| C25 | 35.1 | 34.8 | 34.8 | 34.8 | 34.4 | 35.1 | 35.2 | 35.2 | 34.7 | 34.6 | 34.7 | 34.6 | 34.6 | 34.7 | 34.7 | 34.5 | 33.8 | 33.7 |
| C26 | 180.2 | 180.0 | 179.0 | 180.6 | 180.0 | 181.2 | 181.2 | 175.9 | 176.1 | 176.1 | 176.0 | 176.0 | 176.1 | 176.1 | 176.1 | 177.8 | 178.8 | 178.8 |
| C27 | 17.1 | 17.1 | 17.3 | 17.0 | 17.0 | 17.0 | 17.0 | 17.3 | 17.1 | 17.1 | 17.1 | 17.0 | 17.1 | 17.1 | 17.1 | 17.0 | 16.1 | 16.1 |
| C28 | -- | -- | -- | -- | 26.6 | 27.2 | 27.0 | 27.7 | -- | -- | -- | -- | -- | -- | -- | 27.2 | 28.4 | 27.2 |
| C29 | -- | -- | -- | -- | 21.0 | 20.3 | 20.3 | 18.7 | -- | -- | -- | -- | -- | -- | -- | 20.2 | 15.7 | 21.0 |
| C30 | 27.5 | 28.2 | 28.2 | 27.0 | 24.0 | 20.9 | 20.7 | 20.7 | 27.0 | 27.6 | 27.6 | 27.9 | 28.1 | 26.3 | 28.0 | 20.6 | 25.1 | 25.3 |
| C31 | C31 | C31 | C31 | CH3CO | CO- | C31 | C31 | C31 | C31 | C31 | C31 | C31 | ||||||
| 20.7 | 15.5 | 15.8 | 20.8 | 170.6 | OCH3 | 20.8 | 20.9 | 20.8 | 15.5 | 15.4 | 21.3 | 15.6 | ||||||
| C32 | C32 | C32 | C32 | CH3CO | 51.4 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | ||||||
| 19.4 | 24.4 | 19.5 | 24.7 | 20.6 | 24.7 | 20.3 | 20.4 | 21.2 | 23.1 | 23.3 | 20.3 | |||||||
| NO. | 109f) | 111b) | 113b) | 115a) | 116b) | 117a) | 118b) | 119b) | 120b) | 124b) | 125b) | 126b) | 127b) | 128h) | 129b) | 133a) | 134b) | 135b) |
| C1 | 35.9 | 35.3 | 35.4 | 35.2 | 34.9 | 35.9 | 30.2 | 30.4 | 36.1 | 34.6 | 34.6 | 34.2 | 34.9 | 34.5 | 35.7 | 35.2 | 35.5 | 35.4 |
| C2 | 28.4 | 34.4 | 34.4 | 28.9 | 27.5 | 28.5 | 23.3 | 23.4 | 34.7 | 27.5 | 27.7 | 27.3 | 27.8 | 33.9 | 34.3 | 34.3 | 34.4 | 34.3 |
| C3 | 79.2 | 214.8 | 214.6 | 77.1 | 78.0 | 77.8 | 78.1 | 78.0 | 216.3 | 78.2 | 78.2 | 78.2 | 78.4 | 208.9 | 217.8 | 214.9 | 217.3 | 214.6 |
| C4 | 39.9 | 47.2 | 47.3 | 40.1 | 38.8 | 39.3 | 36.7 | 36.8 | 47.3 | 38.6 | 38.7 | 38.5 | 38.9 | 46.2 | 45.3 | 47.1 | 46.7 | 47.2 |
| C5 | 50.5 | 50.3 | 49.1 | 49.6 | 49.9 | 50.7 | 45.3 | 45.3 | 51.3 | 49.3 | 49.3 | 49.2 | 49.2 | 49.1 | 49.4 | 50.2 | 50.7 | 50.4 |
| C6 | 28.4 | 37.1 | 37.2 | 37.2 | 36.5 | 18.5 | 18.0 | 18.0 | 19.6 | 26.0 | 26.1 | 26.0 | 26.7 | 36.8 | 27.7 | 37.0 | 28.2 | 37.1 |
| C7 | 67.7 | 198.2 | 198.1 | 198.7 | 198.8 | 26.6 | 26.0 | 26.0 | 26.5 | 67.1 | 67.1 | 66.6 | 66.9 | 203.5 | 66.3 | 198.2 | 66.8 | 198.0 |
| C8 | 157.3 | 139.5 | 139.6 | 138.9 | 138.8 | 134.1 | 134.0 | 133.8 | 135.0 | 158.0 | 158.1 | 158.3 | 156.6 | 151.1 | 157.6 | 139.4 | 136.7 | 139.5 |
| C9 | 144.2 | 162.8 | 162.8 | 164.9 | 164.6 | 134.1 | 134.6 | 134.6 | 133.9 | 142.2 | 142.1 | 142.6 | 142.3 | 152.6 | 141.0 | 163.0 | 139.6 | 162.6 |
| C10 | 39.8 | 39.4 | 39.5 | 39.5 | 39.7 | 37.2 | 36.9 | 36.9 | 37.1 | 39.1 | 39.2 | 38.7 | 38.7 | 38.9 | 38.3 | 39.3 | 38.0 | 39.4 |
| C11 | 199.4 | 23.8 | 23.9 | 23.8 | 23.7 | 21.0 | 21.0 | 20.8 | 21.4 | 197.4 | 197.3 | 192.2 | 197.8 | 200.9 | 197.6 | 23.8 | 21.1 | 23.8 |
| C12 | 78.7 | 30.1 | 30.2 | 30.5 | 30.2 | 30.6 | 30.8 | 30.8 | 29.0 | 44.3 | 44.4 | 75.8 | 50.7 | 78.5 | 50.5 | 30.0 | 31.0 | 30.1 |
| C13 | 53.3 | 44.9 | 45.0 | 45.2 | 45.0 | 44.7 | 44.3 | 44.3 | 45.0 | 51.4 | 51.4 | 54.0 | 45.7 | 54.4 | 46.8 | 44.8 | 45.1 | 44.9 |
| C14 | 62.7 | 47.4 | 47.8 | 48.2 | 47.8 | 49.6 | 49.9 | 49.6 | 49.9 | 58.3 | 58.3 | 59.4 | 59.7 | 55.0 | 59.7 | 47.7 | 49.7 | 47.8 |
| C15 | 217.4 | 28.6 | 28.7 | 28.3 | 32.0 | 27.3 | 27.5 | 27.0 | 31.0 | 210.9 | 210.8 | 209.9 | 217.7 | 72.1 | 216.8 | 31.8 | 30.0 | 31.9 |
| C16 | 38.6 | 31.8 | 31.9 | 32.7 | 28.7 | 29.2 | 29.7 | 29.0 | 27.3 | 122.4 | 122.3 | 122.5 | 36.1 | 33.3 | 36.3 | 28.6 | 29.9 | 28.7 |
| C17 | 53.9 | 49.0 | 50.5 | 50.6 | 49.0 | 47.5 | 40.6 | 47.2 | 47.6 | 187.6 | 187.6 | 187.1 | 49.3 | 55.3 | 49.0 | 48.8 | 45.1 | 49.0 |
| C18 | 13.8 | 15.9 | 16.0 | 16.1 | 15.9 | 16.1 | 16.1 | 16.0 | 16.5 | 30.9 | 30.9 | 26.3 | 19.0 | 13.1 | 19.3 | 15.8 | 16.2 | 15.9 |
| C19 | 19.5 | 24.9 | 17.9 | 18.3 | 18.4 | 19.2 | 19.0 | 18.9 | 18.6 | 18.5 | 18.5 | 18.4 | 18.4 | 17.3 | 18.1 | 17.8 | 17.2 | 17.9 |
| C20 | 73.8 | 36.6 | 36.6 | 37.3 | 36.7 | 48.9 | 44.9 | 47.5 | 48.5 | 28.6 | 28.6 | 29.1 | 73.0 | 72.9 | 72.9 | 36.0 | 36.4 | 36.2 |
| C21 | 28.3 | 18.9 | 18.9 | 19.3 | 19.0 | 178.4 | 70.2 | 182.0 | 175.8 | 19.4 | 19.4 | 18.5 | 26.7 | 26.4 | 26.7 | 18.5 | 18.5 | 18.7 |
| C22 | 52.3 | 33.4 | 33.6 | 34.5 | 33.5 | 33.0 | 30.7 | 32.5 | 33.4 | 47.6 | 47.6 | 47.4 | 52.7 | 51.2 | 52.7 | 36.0 | 32.8 | 32.6 |
| C23 | 211.5 | 28.6 | 28.8 | 29.1 | 28.6 | 26.1 | 24.7 | 25.9 | 26.4 | 205.8 | 205.9 | 206.1 | 210.4 | 208.9 | 210.4 | 24.2 | 25.3 | 25.0 |
| C24 | 49.4 | 79.5 | 79.1 | 77.1 | 79.6 | 124.0 | 124.8 | 123.6 | 124.7 | 46.1 | 46.3 | 46.0 | 47.7 | 48.3 | 47.7 | 131.4 | 60.6 | 60.3 |
| C25 | 36.2 | 73.2 | 73.9 | 74.8 | 73.2 | 136.7 | 131.4 | 132.2 | 131.9 | 34.4 | 34.6 | 34.2 | 34.5 | 35.0 | 34.5 | 136.7 | 60.7 | 60.7 |
| C26 | 180.2 | 23.2 | 67.7 | 69.3 | 23.3 | 67.8 | 17.7 | 17.6 | 25.8 | 179.9 | 176.0 | 180.3 | 175.9 | 176.3 | 175.9 | 67.4 | 65.4 | 65.5 |
| C27 | 17.8 | 26.5 | 20.9 | 20.1 | 26.6 | 13.7 | 25.7 | 25.7 | 17.8 | 16.9 | 17.1 | 16.7 | 17.0 | 17.2 | 17.0 | 59.8 | 14.2 | 14.2 |
| C28 | 28.9 | 25.3 | 25.4 | 27.9 | 25.0 | -- | 27.6 | 27.6 | 26.4 | 28.2 | 28.2 | 28.0 | -- | 27.9 | 27.0 | 25.2 | 26.5 | 25.4 |
| C29 | 16.4 | 21.4 | 21.4 | 16.0 | 27.5 | -- | 21.8 | 21.8 | 21.3 | 15.5 | 15.5 | 15.3 | -- | 19.6 | 20.8 | 21.3 | 21.3 | 21.4 |
| C30 | 23.3 | 17.9 | 25.0 | 25.2 | 15.3 | 28.4 | 24.4 | 24.3 | 24.4 | 33.2 | 33.2 | 33.0 | 28.2 | 20.7 | 25.1 | 24.8 | 26.1 | 24.9 |
| C31 | C1' | Glc | C31 | C31 | C31 | CO- | CO- | |||||||||||
| 16.2 | 102.7 | C1' | 51.9 | 170.7 | 15.5 | OCH3 | OCH3 | |||||||||||
| C32 | C2' | 95.8 | C32 | C32 | 51.4 | 52.0 | ||||||||||||
| 24.3 | 71.9 | 20.7 | 24.8 | |||||||||||||||
| NO. | 136b) | 137b) | 138b) | 139b) | 140 | 141 | 142c) | 143b) | 144b) | 145b) | 148a) | 149e) | 150b) | 151g) | 152b) | 153c) | 155b) | |
| C1 | 34.7 | 34.8 | 34.7 | 34.7 | 35.9 | 35.6 | 37.2 | 36.3 | 37.2 | 39.2 | 34.1 | 31.1 | 35.2 | 30.5 | 33.7 | 30.5 | 35.9 | |
| C2 | 24.2 | 24.1 | 24.2 | 24.2 | 33.6 | 27.6 | 35.2 | 34.0 | 34.5 | 18.2 | 28.5 | 23.6 | 24.1 | 24.6 | 27.4 | 23.0 | 33.8 | |
| C3 | 80.2 | 79.3 | 80.2 | 80.2 | 216.4 | 78.2 | 217.5 | 214.2 | 214.5 | 41.3 | 78.3 | 77.8 | 80.8 | 78.8 | 77.6 | 78.2 | 215.3 | |
| C4 | 37.8 | 37.9 | 37.7 | 37.8 | 46.0 | 41.6 | 49.0 | 47.8 | 47.4 | 38.3 | 39.4 | 37.0 | 37.8 | 38.1 | 40.5 | 36.5 | 47.7 | |
| C5 | 49.2 | 49.9 | 49.5 | 49.2 | 40.5 | 39.1 | 53.6 | 52.8 | 50.9 | 59.5 | 52.4 | 45.8 | 50.3 | 46.9 | 50.8 | 45.1 | 54.4 | |
| C6 | 26.7 | 37.0 | 26.7 | 26.7 | 23.1 | 22.2 | 40.9 | 40.0 | 33.4 | 22.3 | 19.1 | 18.3 | 26.3 | 19.4 | 36.2 | 17.7 | 40.3 | |
| C7 | 66.9 | 202.8 | 66.9 | 66.9 | 62.4 | 62.6 | 215.6 | 212.8 | 198.2 | 32.4 | 22.5 | 26.4 | 18.0 | 27.3 | 199.3 | 25.8 | 204.8 | |
| C8 | 157.0 | 154.7 | 157.0 | 157.0 | 63.6 | 62.8 | 55.0 | 54.0 | 150.0 | 42.8 | 51.2 | 134.7 | 132.9 | 135.6 | 146.8 | 133.9 | 46.2 | |
| C9 | 142.8 | 149.9 | 142.8 | 142.8 | 167.7 | 158.2 | 60.4 | 59.5 | 149.5 | 53.1 | 44.9 | 134.9 | 135.4 | 136.3 | 151.4 | 134.3 | 59.1 | |
| C10 | 38.9 | 40.1 | 38.9 | 38.9 | 40.5 | 37.9 | 38.0 | 36.5 | 39.2 | 37.5 | 35.7 | 37.2 | 37.0 | 38.4 | 39.1 | 36.6 | 37.5 | |
| C11 | 197.9 | 199.5 | 197.9 | 197.5 | 130.0 | 128.7 | 210.1 | 207.6 | 197.0 | 22.3 | 27.1 | 21.0 | 20.8 | 22.2 | 199.8 | 21.0 | 207.7 | |
| C12 | 50.4 | 52.3 | 50.4 | 50.4 | 203.6 | 78.1 | 53.7 | 52.6 | 192.6 | 41.5 | 49.0 | 29.6 | 31.1 | 30.8 | 49.9 | 28.6 | 50.6 | |
| C13 | 45.6 | 48.1 | 45.6 | 45.6 | 50.2 | 50.2 | 51.0 | 50.0 | 59.0 | 42.1 | 145.2 | 46.2 | 44.7 | 45.8 | 44.9 | 45.6 | 46.2 | |
| C14 | 59.6 | 53.0 | 59.6 | 59.6 | 64.8 | 64.4 | 51.3 | 49.7 | 61.0 | 59.5 | 47.9 | 48.8 | 51.0 | 51.0 | 57.2 | 49.3 | 54.7 | |
| C15 | 217.6 | 72.3 | 217.6 | 217.6 | 78.4 | 204.0 | 75.5 | 74.1 | 203.8 | 35.6 | 33.8 | 43.6 | 76.0 | 28.3 | 207.7 | 42.1 | 210.4 | |
| C16 | 41.1 | 36.5 | 41.1 | 41.1 | 127.2 | 127.2 | 39.0 | 38.4 | 38.9 | 30.5 | 31.3 | 76.6 | 36.5 | 28.7 | 35.0 | 76.6 | 40.2 | |
| C17 | 45.7 | 48.3 | 45.8 | 45.7 | 167.7 | 168.9 | 49.0 | 47.7 | 38.3 | 53.1 | 137.3 | 57.3 | 49.0 | 48.6 | 47.8 | 56.1 | 44.5 | |
| C18 | 17.6 | 17.5 | 17.6 | 17.6 | 30.9 | 31.0 | 17.1 | 16.6 | 12.4 | 14.6 | 179.2 | 17.8 | 16.1 | 17.0 | 17.8 | 17.0 | 15.8 | |
| C19 | 18.7 | 18.0 | 18.7 | 18.7 | 24.7 | 25.7 | 13.7 | 13.1 | 18.5 | 18.6 | 19.7 | 19.0 | 19.1 | 20.0 | 17.8 | 21.4 | 13.0 | |
| C20 | 32.2 | 32.6 | 32.2 | 32.2 | 72.2 | 72.2 | 33.5 | 32.0 | 32.2 | 36.0 | 35.4 | 48.7 | 36.2 | 49.0 | 73.9 | 47.9 | 32.1 | |
| C21 | 19.9 | 19.6 | 19.9 | 19.9 | 28.6 | 28.7 | 20.0 | 19.4 | 23.3 | 17.9 | 18.6 | 178.8 | 18.2 | 178.1 | 26.5 | 179.7 | 19.4 | |
| C22 | 49.5 | 49.5 | 49.5 | 49.5 | 53.9 | 53.9 | 50.5 | 49.6 | 48.6 | 35.3 | 35.1 | 31.6 | 34.6 | 34.3 | 42.1 | 32.0 | 49.1 | |
| C23 | 207.8 | 208.5 | 207.8 | 207.8 | 208.0 | 208.7 | 211.2 | 208.3 | 207.3 | 22.3 | 23.2 | 33.2 | 25.9 | 32.2(24R) | 23.4 | 24.9 | 207.7 | |
| C24 | 46.8 | 46.8 | 46.9 | 46.8 | 48.0 | 48.2 | 47.8 | 46.8 | 46.5 | 58.2 | 125.6 | 156.1 | 145.2 | 76.5(24R) | 143.6 | 33.6 | 47.1 | |
| C25 | 34.6 | 34.8 | 35.0 | 34.6 | 34.1 | 34.5 | 36.1 | 34.6 | 33.6 | 213.2 | 131.5 | 34.1 | 126.7 | 150.0(24R) | 127.6 | 155.1 | 34.5 | |
| C26 | 179.9 | 179.3 | 175.8 | 179.9 | 180.1 | 179.6 | 179.6 | 176.2 | 180.3 | 32.1 | 25.8 | 21.9 | 172.6 | 110.8(24R) | 173.0 | 106.4 | 176.2 | |
| C27 | 17.1 | 17.1 | 17.4 | 17.1 | 16.8 | 16.9 | 17.8 | 17.1 | 16.8 | 31.8 | 17.7 | 21.9 | 12.0 | 18.7(24R) | 12.1 | 18.6 | 16.7 | |
| C28 | 28.3 | 17.3 | 28.3 | 28.3 | 24.0 | 24.6 | 25.9 | 25.2 | -- | 20.3 | 26.7 | 27.8 | 18.2 | 28.7 | 27.9 | 27.3 | 25.4 | |
| C29 | 16.7 | 20.3 | 16.7 | 16.7 | 21.7 | 22.2 | 21.9 | 21.2 | -- | 35.0 | 16.4 | 22.0 | 27.9 | 22.9 | 15.5 | 21.5 | 20.7 | |
| C30 | 24.5 | 28.3 | 24.6 | 24.5 | 26.9 | 27.0 | 12.8 | 12.5 | 27.4 | 28.7 | 25.7 | 16.5 | 25.3 | 22.4 | 24.8 | 12.7 | ||
| CO | CO | OCH3 | C31 | C31 | CH3CO | COCH3 | ||||||||||||
| 171.1 | 170.9 | 51.9 | 20.3 | 107.0 | 171.1 | 171.3 | ||||||||||||
| CH3 | CH3 | C32 | CH3CO | CH3CO | COCH3 | |||||||||||||
| 21.5 | 21.4 | 19.3 | 21.1 | 171.1 | 21.8 | |||||||||||||
| NO. | 156b) | 157d) | 158d) | 160b) | 161d) | 162b) | 163b) | 164 | 165 | 166 | 167 | 168b) | 170b) | 171 | 172b) | 173b) | 174b) | 175b) |
| C1 | 36.6 | 29.8 | 29.7 | 36.6 | 35.8 | 30.9 | 30.7 | 36.6 | 35.8 | 35.4 | 35.3 | 36.4 | 30.6 | 36.9 | 30.8 | 29.8 | 30.5 | 36.6 |
| C2 | 34.8 | 25.7 | 25.6 | 34.9 | 34.8 | 23.2 | 23.2 | 34.8 | 28.2 | 28.1 | 24.1 | 34.7 | 23.1 | 35.0 | 23.3 | 25.5 | 23.0 | 34.8 |
| C3 | 216.6 | 73.8 | 73.8 | 216.9 | 216.6 | 78.1 | 78.1 | 217.0 | 79.0 | 80.8 | 80.6 | 216.4 | 78.1 | 215.2 | 78.2 | 76.0 | 78.0 | 216.9 |
| C4 | 47.4 | 37.2 | 37.0 | 47.5 | 47.3 | 36.6 | 36.6 | 47.4 | 38.7 | 38.0 | 37.4 | 47.4 | 36.5 | 47.5 | 36.8 | 37.1 | 36.4 | 47.5 |
| C5 | 50.5 | 42.8 | 42.8 | 50.7 | 50.4 | 44.0 | 44.1 | 50.7 | 50.4 | 50.3 | 48.8 | 50.6 | 44.0 | 51.0 | 44.1 | 43.1 | 44.0 | 50.7 |
| C6 | 23.6 | 22.6 | 22.6 | 23.7 | 23.6 | 22.9 | 22.8 | 23.6 | 23.1 | 24.3 | 22.7 | 23.6 | 22.8 | 23.9 | 22.7 | 27.5 | 27.5 | 23.7 |
| C7 | 121.2 | 120.6 | 120.6 | 119.9 | 121.0 | 121.2 | 121.3 | 119.8 | 120.3 | 120.0 | 120.9 | 121.7 | 121.6 | 121.6 | 121.6 | 120.3 | 120.2 | 120.3 |
| C8 | 141.0 | 142.0 | 141.9 | 142.9 | 141.0 | 140.3 | 140.8 | 142.8 | 142.7 | 142.6 | 140.0 | 139.9 | 140.5 | 142.1 | 140.2 | 142.2 | 142.2 | 142.5 |
| C9 | 144.8 | 146.0 | 145.9 | 144.5 | 144.7 | 145.9 | 146.2 | 144.5 | 145.9 | 145.6 | 145.5 | 145.0 | 146.2 | 145.4 | 146.2 | 145.9 | 145.8 | 144.6 |
| C10 | 37.3 | 37.1 | 37.1 | 37.2 | 37.2 | 37.4 | 37.4 | 37.2 | 37.4 | 37.2 | 37.2 | 37.4 | 37.3 | 37.6 | 37.5 | 37.3 | 37.1 | 37.2 |
| C11 | 116.9 | 115.1 | 115.1 | 117.3 | 117.0 | 115.7 | 115.7 | 117.2 | 116.3 | 116.5 | 116.0 | 116.8 | 115.3 | 117.3 | 115.5 | 115.5 | 115.5 | 116.9 |
| C12 | 38.5 | 35.3 | 35.3 | 37.8 | 38.5 | 38.0 | 38.5 | 37.8 | 37.9 | 37.8 | 37.9 | 36.6 | 38.4 | 38.9 | 38.1 | 37.6 | 37.6 | 37.8 |
| C13 | 44.4 | 43.9 | 43.9 | 43.8 | 44.3 | 44.2 | 44.5 | 43.7 | 43.8 | 43.8 | 43.9 | 44.3 | 44.3 | 44.6 | 44.1 | 43.6 | 43.6 | 43.7 |
| C14 | 52.0 | 48.5 | 48.4 | 50.3 | 51.9 | 51.5 | 52.1 | 50.3 | 49.2 | 49.3 | 51.2 | 42.9 | 52.1 | 52.6 | 51.6 | 50.3 | 50.3 | 50.3 |
| C15 | 74.6 | 43.4 | 43.3 | 31.5 | 74.6 | 77.5 | 74.7 | 27.9 | 28.0 | 27.9 | 77.2 | 47.0 | 74.5 | 73.7 | 77.4 | 31.2 | 31.3 | 31.4 |
| C16 | 40.0 | 75.1 | 75.0 | 27.9 | 40.1 | 37.0 | 40.1 | 31.4 | 31.5 | 31.5 | 36.8 | 219.2 | 39.6 | 40.5 | 36.7 | 22.9 | 22.8 | 27.6 |
| C17 | 48.9 | 56.2 | 56.2 | 50.8 | 48.8 | 48.9 | 48.9 | 50.9 | 50.9 | 50.8 | 48.7 | 60.5 | 45.5 | 49.4 | 45.6 | 47.3 | 47.3 | 47.4 |
| C18 | 16.0 | 16.9 | 16.9 | 15.7 | 15.9 | 16.0 | 16.0 | 15.7 | 15.7 | 15.7 | 15.8 | 16.8 | 15.8 | 16.5 | 15.9 | 15.4 | 15.4 | 15.5 |
| C19 | 22.2 | 22.7 | 22.7 | 22.1 | 22.1 | 22.7 | 22.5 | 22.4 | 22.8 | 22.9 | 22.7 | 22.1 | 22.7 | 22.2 | 23.0 | 22.7 | 22.4 | 21.1 |
| C20 | 35.9 | 46.9 | 46.9 | 36.2 | 35.8 | 36.0 | 36.0 | 36.0 | 36.1 | 36.1 | 35.8 | 31.5 | 39.3 | 36.3 | 39.8 | 39.3 | 39.3 | 39.4 |
| C21 | 18.3 | 177.2 | 177.2 | 18.3 | 18.3 | 18.5 | 18.3 | 18.4 | 18.4 | 18.4 | 18.0 | 18.6 | 12.8 | 18.7 | 12.8 | 12.6 | 12.6 | 12.7 |
| C22 | 34.7 | 31.9 | 31.7 | 35.8 | 36.6 | 34.7 | 34.8 | 35.9 | 36.0 | 35.8 | 34.5 | 35.7 | 74.7 | 30.0 | 74.6 | 74.6 | 74.6 | 74.7 |
| C23 | 25.8 | 26.0 | 25.4 | 26.9 | 25.4 | 26.0 | 25.9 | 24.5 | 24.6 | 24.8 | 25.8 | 24.8 | 31.7 | 24.7 | 32.1 | 31.7 | 31.8 | 31.9 |
| C24 | 145.1 | 124.3 | 123.1 | 147.1 | 126.6 | 144.9 | 145.2 | 126.9 | 127.0 | 128.6 | 144.9 | 131.2 | 139.1 | 127.6 | 139.2 | 139.5 | 139.6 | 139.5 |
| C25 | 126.8 | 131.2 | 135.7 | 125.7 | 134.6 | 126.8 | 126.8 | 134.3 | 134.3 | 137.4 | 126.7 | 137.3 | 129.4 | 140.8 | 129.4 | 129.0 | 129.0 | 129.1 |
| C26 | 172.2 | 25.7 | 66.5 | 172.1 | 69.0 | 172.0 | 172.1 | 69.0 | 69.1 | 66.8 | 173.0 | 67.7 | 172.2 | 65.5 | 172.0 | 172.2 | 172.3 | 171.9 |
| C27 | 12.0 | 17.7 | 13.6 | 20.6 | 13.5 | 12.1 | 12.1 | 13.6 | 13.7 | 59.8 | 11.8 | 60.5 | 12.3 | 58.6 | 12.4 | 12.2 | 12.2 | 12.3 |
| C28 | 17.0 | 28.7 | 28.7 | 25.4 | 16.9 | 27.8 | 27.8 | 25.3 | 25.6 | 25.6 | 18.2 | 22.4 | 27.8 | 25.7 | -- | -- | -- | 25.3 |
| C29 | 25.4 | 22.8 | 22.8 | 25.3 | 25.4 | 22.5 | 22.7 | 25.4 | 27.8 | 28.2 | 28.0 | 25.3 | 22.5 | 22.4 | -- | -- | -- | 22.5 |
| C30 | 22.5 | 26.2 | 26.1 | 22.5 | 22.1 | 18.2 | 17.2 | 22.0 | 15.8 | 17.0 | 16.8 | 25.7 | 17.3 | 18.0 | 27.9 | 28.1 | 27.7 | 25.5 |
| COCH3 | COCH3 | AcCH3 | COCH3 | C31 | C31 | C31 | 22-O-COCH3 | |||||||||||
| 171.2 | 170.8 | 21.1 | 170.8 | 22.6 | 22.5 | 22.5 | 170.7 | |||||||||||
| COCH3 | COCH3 | AcCH3 | COCH3 | C32 | C32 | C32 | 22-O-COCH3 | |||||||||||
| 21.4 | 21.3 | 21.2 | 21.3 | 18.6 | 25.7 | 25.6 | 21.1 | |||||||||||
| NO. | 176b) | 177b) | 178b) | 179b) | 180b) | 181b) | 182b) | 183b) | 184b) | 185b) | 186b) | 187a) | 188a) | 189b) | 190b) | 191b) | 192b) | 193b) |
| C1 | 35.4 | 29.9 | 30.5 | 30.6 | 30.5 | 35.6 | 35.3 | 36.6 | 36.6 | 29.9 | 30.6 | 36.8 | 36.8 | 35.6 | 36.1 | 35.8 | 35.4 | 30.5 |
| C2 | 24.2 | 25.5 | 23.0 | 23.1 | 23.1 | 27.6 | 24.1 | 37.5 | 34.8 | 25.5 | 23.1 | 35.0 | 29.2 | 27.8 | 34.9 | 36.5 | 36.5 | 23.1 |
| C3 | 80.7 | 76.7 | 78.0 | 78.1 | 78.0 | 78.8 | 80.7 | 216.6 | 216.4 | 76.1 | 78.1 | 215.3 | 78.4 | 78.8 | 216.8 | 216.5 | 216.5 | 78.0 |
| C4 | 37.6 | 37.3 | 36.4 | 36.5 | 36.4 | 38.5 | 37.4 | 47.4 | 47.4 | 37.0 | 36.5 | 47.5 | 39.8 | 38.6 | 47.5 | 47.6 | 47.6 | 36.4 |
| C5 | 48.9 | 42.9 | 43.8 | 44.0 | 43.9 | 48.7 | 48.9 | 50.4 | 50.4 | 42.9 | 45.0 | 50.9 | 50.1 | 49.0 | 50.8 | 60.7 | 60.8 | 44.2 |
| C6 | 22.8 | 23.0 | 22.7 | 22.8 | 22.7 | 22.9 | 22.6 | 23.6 | 23.7 | 23.0 | 22.8 | 23.8 | 23.9 | 22.9 | 23.7 | 23.8 | 23.8 | 22.7 |
| C7 | 121.3 | 121.5 | 121.3 | 121.4 | 121.2 | 121.3 | 121.0 | 121.0 | 121.3 | 121.5 | 121.6 | 121.6 | 122.5 | 120.3 | 120.0 | 121.8 | 121.8 | 121.5 |
| C8 | 140.0 | 140.0 | 140.0 | 140.6 | 140.6 | 140.0 | 140.6 | 140.4 | 140.2 | 140.0 | 140.5 | 142.0 | 142.4 | 142.4 | 142.9 | 140.1 | 140.2 | 140.4 |
| C9 | 145.8 | 146.0 | 145.8 | 146.1 | 146.1 | 145.8 | 145.7 | 145.0 | 144.7 | 146.0 | 146.2 | 145.3 | 147.4 | 145.9 | 144.6 | 145.1 | 145.1 | 146.1 |
| C10 | 37.3 | 37.3 | 37.3 | 37.3 | 37.2 | 37.3 | 37.2 | 37.3 | 37.3 | 37.3 | 37.3 | 37.5 | 38.3 | 37.3 | 37.3 | 37.6 | 37.6 | 37.2 |
| C11 | 115.9 | 115.5 | 115.4 | 115.5 | 115.4 | 115.8 | 116.0 | 116.9 | 116.7 | 115.3 | 115.3 | 117.2 | 116.6 | 116.0 | 117.3 | 117.0 | 117.0 | 115.2 |
| C12 | 38.0 | 37.9 | 37.8 | 38.3 | 38.3 | 37.9 | 38.3 | 38.0 | 38.0 | 37.9 | 38.4 | 38.8 | 39.2 | 37.7 | 37.9 | 36.8 | 36.8 | 38.4 |
| C13 | 43.9 | 44.1 | 44.1 | 44.5 | 44.3 | 44.0 | 44.2 | 44.1 | 43.9 | 43.9 | 44.2 | 44.5 | 45.0 | 43.7 | 43.8 | 44.5 | 44.5 | 43.9 |
| C14 | 51.3 | 51.7 | 51.4 | 52.2 | 52.0 | 51.2 | 51.8 | 51.3 | 51.4 | 51.4 | 52.0 | 52.6 | 53.0 | 50.2 | 50.4 | 43.1 | 43.1 | 52.0 |
| C15 | 77.0 | 77.0 | 77.2 | 74.6 | 74.5 | 77.3 | 74.5 | 77.2 | 76.7 | 76.9 | 74.5 | 73.6 | 74.2 | 31.4 | 27.9 | 34.9 | 34.9 | 74.4 |
| C16 | 36.6 | 37.2 | 37.1 | 40.1 | 40.2 | 36.9 | 39.8 | 37.0 | 36.6 | 36.7 | 39.8 | 40.9 | 41.0 | 27.7 | 31.5 | 25.0 | 25.3 | 39.6 |
| C17 | 45.4 | 48.7 | 48.6 | 48.8 | 49.2 | 48.7 | 48.7 | 48.9 | 45.5 | 45.4 | 45.0 | 50.1 | 49.8 | 50.7 | 50.9 | 31.6 | 31.7 | 45.4 |
| C18 | 15.7 | 16.0 | 15.9 | 16.0 | 15.7 | 15.9 | 15.8 | 16.0 | 15.8 | 15.8 | 15.8 | 16.3 | 16.8 | 15.7 | 15.8 | 17.0 | 17.1 | 15.7 |
| C19 | 22.8 | 22.6 | 22.5 | 22.7 | 22.5 | 22.7 | 22.7 | 22.4 | 22.1 | 22.7 | 22.6 | 22.1 | 23.6 | 22.6 | 22.5 | 22.3 | 22.3 | 22.6 |
| C20 | 39.6 | 32.8 | 32.8 | 33.0 | 33.4 | 35.8 | 35.8 | 35.9 | 39.6 | 39.6 | 39.3 | 34.4 | 36.8 | 36.0 | 36.1 | 50.8 | 50.8 | 39.2 |
| C21 | 12.6 | 19.4 | 19.3 | 19.6 | 19.4 | 18.1 | 18.1 | 18.2 | 12.7 | 12.7 | 12.8 | 20.3 | 19.0 | 18.2 | 18.4 | 18.7 | 18.8 | 12.7 |
| C22 | 74.4 | 51.5 | 51.5 | 51.9 | 67.0 | 34.5 | 34.7 | 34.6 | 74.4 | 74.4 | 74.6 | 44.7 | 37.3 | 34.6 | 36.7 | 219.3 | 219.0 | 74.5 |
| C23 | 31.9 | 201.6 | 201.4 | 201.8 | 43.6 | 25.8 | 25.7 | 25.9 | 31.9 | 31.9 | 31.7 | 67.1 | 25.1 | 25.9 | 24.4 | 47.1 | 47.1 | 31.7 |
| C24 | 139.0 | 133.8 | 133.9 | 134.1 | 144.8 | 144.9 | 145.0 | 144.5 | 139.0 | 139.1 | 139.2 | 145.5 | 127.9 | 155.4 | 131.7 | 131.4 | 126.8 | 139.2 |
| C25 | 129.2 | 139.5 | 139.4 | 139.3 | 128.3 | 126.7 | 126.8 | 126.8 | 129.2 | 129.2 | 129.4 | 128.7 | 141.3 | 139.1 | 136.8 | 137.4 | 134.8 | 129.2 |
| C26 | 171.3 | 171.2 | 171.8 | 171.0 | 172.0 | 172.9 | 172.9 | 172.1 | 171.3 | 171.6 | 172.0 | 170.3 | 65.8 | 195.3 | 67.7 | 60.2 | 69.3 | 172.1 |
| C27 | 12.3 | 14.1 | 13.9 | 14.1 | 12.6 | 11.9 | 11.9 | 12.0 | 12.3 | 12.3 | 12.3 | 13.5 | 58.8 | 9.0 | 60.2 | 67.9 | 13.8 | 12.2 |
| C28 | 18.4 | 18.5 | 18.3 | 17.2 | 17.1 | 18.3 | 17.0 | 18.2 | 25.4 | -- | -- | 25.6 | 29.3 | 25.4 | 25.5 | 25.5 | 25.5 | 17.2 |
| C29 | 28.1 | 28.2 | 27.7 | 27.8 | 27.7 | 28.1 | 28.0 | 25.4 | 22.4 | -- | -- | 22.3 | 17.1 | 28.0 | 22.1 | 22.6 | 22.6 | 27.6 |
| C30 | 16.9 | 22.6 | 22.3 | 22.5 | 22.3 | 15.7 | 16.8 | 22.1 | 18.3 | 28.2 | 27.7 | 18.0 | 18.6 | 15.5 | 25.4 | 25.8 | 25.8 | 22.4 |
| AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | 15-O- | C31 | C31 | C1' | |||||||
| 171.1 | 171.0 | 171.1 | 170.9 | 170.7 | 171.1 | 170.9 | 171.2 | COCH3 | 22.8 | 22.5 | 170.8 | |||||||
| AcCO | AcCH3 | AcCO | AcCH3 | AcCH3 | AcCO | AcCO | AcCO | 171.1 | C32 | C32 | C2' | |||||||
| 170.6 | 21.4 | 170.7 | 21.3 | 21.2 | 21.3 | 21.2 | 21.4 | 18.5 | 17.3 | 170.5 | ||||||||
| AcCH3 | AcCH3 | C3' | ||||||||||||||||
| 21.0 | 21.6 | 21.2 | ||||||||||||||||
| NO. | 194b) | 196d) | 197b) | 198b) | 201b) | 202b) | 203 | 204b) | 205b) | 207b) | 208b) | 209 | 210b) | 214b) | 215b) | 216b) | 217b) | 218b) |
| C1 | 35.6 | 36.1 | 35.7 | 35.4 | 35.7 | 36.6 | 36.6 | 36.5 | 29.9 | 30.0 | 35.5 | 30.5 | 36.7 | 29.8 | 35.6 | 30.6 | 35.4 | 29.9 |
| C2 | 27.7 | 34.4 | 28.0 | 22.8 | 27.9 | 34.8 | 28.7 | 34.9 | 25.6 | 25.6 | 27.0 | 23.0 | 34.8 | 25.4 | 27.4 | 23.1 | 24.2 | 25.6 |
| C3 | 78.8 | 215.3 | 78.9 | 80.8 | 78.9 | 216.7 | 78.1 | 216.9 | 76.1 | 76.3 | 78.3 | 78.0 | 217.0 | 75.8 | 78.6 | 78.0 | 80.7 | 76.1 |
| C4 | 38.6 | 46.9 | 38.7 | 37.6 | 38.7 | 47.4 | 39.4 | 47.5 | 37.3 | 37.3 | 38.3 | 36.4 | 47.5 | 37.2 | 38.5 | 36.5 | 37.6 | 37.4 |
| C5 | 48.7 | 50.5 | 49.1 | 49.3 | 49.1 | 50.7 | 49.8 | 50.3 | 42.9 | 43.1 | 48.5 | 43.8 | 50.3 | 42.9 | 49.0 | 43.9 | 49.0 | 43.0 |
| C6 | 22.9 | 23.2 | 23.0 | 24.3 | 23.0 | 23.6 | 23.5 | 23.7 | 23.0 | 23.0 | 22.6 | 22.7 | 23.6 | 22.8 | 22.8 | 22.8 | 22.9 | 22.9 |
| C7 | 121.6 | 120.1 | 120.4 | 120.0 | 120.2 | 119.9 | 121.0 | 119.9 | 121.3 | 121.4 | 121.0 | 121.0 | 119.9 | 121.2 | 121.2 | 121.2 | 121.2 | 121.7 |
| C8 | 139.9 | 142.1 | 142.5 | 142.7 | 142.6 | 142.8 | 143.0 | 142.8 | 140.2 | 140.9 | 140.5 | 140.1 | 142.8 | 140.6 | 140.7 | 140.1 | 140.2 | 140.5 |
| C9 | 146.0 | 144.2 | 146.0 | 146.7 | 145.9 | 144.5 | 146.6 | 144.5 | 146.0 | 146.3 | 145.9 | 145.4 | 144.4 | 146.1 | 146.0 | 145.9 | 145.7 | 146.3 |
| C10 | 37.4 | 37.0 | 37.4 | 37.8 | 37.3 | 37.2 | 37.8 | 37.8 | 37.3 | 37.3 | 37.1 | 37.2 | 37.8 | 37.2 | 37.3 | 37.3 | 37.3 | 37.4 |
| C11 | 115.5 | 116.7 | 116.1 | 116.5 | 116.2 | 117.2 | 116.6 | 117.2 | 115.6 | 115.7 | 115.5 | 115.5 | 117.2 | 115.4 | 115.8 | 115.5 | 116.1 | 115.3 |
| C12 | 37.9 | 35.3 | 37.8 | 37.2 | 37.8 | 37.8 | 38.1 | 37.2 | 38.0 | 38.5 | 38.2 | 37.9 | 37.2 | 38.3 | 38.4 | 37.9 | 38.0 | 38.5 |
| C13 | 43.9 | 43.8 | 43.8 | 43.7 | 43.7 | 43.7 | 44.1 | 43.8 | 44.1 | 44.4 | 44.0 | 44.0 | 43.7 | 44.2 | 44.1 | 44.1 | 44.1 | 44.2 |
| C14 | 51.3 | 48.3 | 50.3 | 50.3 | 50.3 | 50.3 | 50.7 | 50.7 | 51.4 | 52.2 | 51.6 | 51.3 | 50.7 | 51.9 | 51.9 | 51.4 | 51.4 | 52.1 |
| C15 | 76.9 | 43.3 | 31.5 | 26.0 | 31.5 | 27.6 | 28.1 | 27.9 | 77.4 | 74.8 | 73.9 | 77.3 | 27.9 | 74.2 | 74.3 | 77.3 | 77.1 | 74.6 |
| C16 | 36.6 | 75.0 | 27.8 | 31.5 | 27.8 | 28.8 | 31.9 | 28.7 | 37.0 | 40.0 | 39.2 | 36.9 | 28.8 | 39.8 | 38.7 | 37.2 | 37.2 | 39.8 |
| C17 | 45.4 | 56.2 | 50.9 | 50.8 | 50.9 | 51.0 | 51.5 | 50.9 | 48.9 | 48.8 | 48.7 | 48.8 | 51.0 | 49.2 | 45.0 | 49.4 | 49.4 | 45.4 |
| C18 | 15.7 | 16.9 | 15.7 | 16.9 | 15.7 | 15.7 | 16.6 | 15.7 | 16.0 | 15.9 | 15.5 | 15.9 | 15.3 | 15.7 | 15.7 | 15.9 | 15.9 | 15.8 |
| C19 | 22.7 | 21.8 | 22.7 | 22.8 | 22.7 | 22.4 | 23.1 | 22.5 | 22.7 | 22.7 | 22.4 | 22.5 | 22.4 | 22.6 | 22.7 | 22.6 | 22.9 | 22.7 |
| C20 | 39.5 | 46.9 | 36.2 | 36.5 | 36.5 | 36.5 | 37.1 | 36.6 | 36.0 | 35.9 | 35.6 | 35.9 | 36.6 | 33.3 | 40.7 | 33.6 | 33.6 | 39.3 |
| C21 | 12.6 | 177.2 | 18.3 | 18.6 | 18.6 | 18.6 | 19.0 | 18.6 | 18.2 | 18.3 | 17.9 | 18.1 | 18.6 | 19.3 | 12.4 | 19.3 | 19.3 | 12.8 |
| C22 | 74.4 | 31.8 | 34.7 | 32.6 | 33.5 | 31.4 | 34.4 | 31.5 | 34.7 | 34.8 | 34.5 | 34.5 | 31.5 | 66.5 | 72.1 | 67.2 | 67.2 | 74.6 |
| C23 | 31.8 | 26.0 | 26.1 | 27.8 | 28.7 | 33.5 | 28.9 | 33.5 | 25.9 | 25.7 | 25.4 | 25.8 | 33.5 | 43.4 | 34.8 | 43.3 | 43.4 | 31.7 |
| C24 | 138.9 | 124.2 | 155.4 | 76.6 | 79.6 | 79.2 | 77.2 | 79.6 | 145.1 | 145.2 | 143.3 | 145.0 | 79.1 | 143.6 | 139.9 | 144.7 | 144.7 | 139.2 |
| C25 | 129.2 | 131.1 | 139.1 | 73.3 | 73.2 | 73.9 | 74.8 | 73.3 | 126.6 | 127.0 | 127.0 | 126.7 | 74.1 | 128.6 | 129.0 | 128.1 | 128.1 | 129.1 |
| C26 | 171.5 | 25.6 | 195.4 | 68.5 | 23.6 | 67.6 | 69.3 | 25.5 | 171.9 | 172.8 | 170.5 | 172.9 | 67.6 | 170.3 | 170.4 | 171.2 | 171.2 | 171.0 |
| C27 | 12.2 | 17.6 | 9.2 | 20.2 | 26.5 | 22.0 | 20.1 | 25.3 | 12.0 | 12.0 | 11.7 | 11.8 | 22.0 | 12.7 | 11.4 | 12.8 | 12.8 | 12.3 |
| C28 | 18.3 | 25.4 | 28.1 | 28.1 | 28.1 | -- | 28.8 | 23.2 | 18.5 | 17.4 | 16.8 | 18.3 | 25.4 | 17.0 | 17.1 | 18.4 | 18.4 | 17.3 |
| C29 | 28.1 | 22.1 | 15.8 | 15.7 | 15.8 | -- | 16.0 | 26.6 | 28.2 | 28.2 | 27.7 | 27.7 | 25.4 | 28.0 | 28.0 | 27.8 | 28.1 | 28.1 |
| C30 | 15.7 | 25.8 | 25.6 | 25.5 | 25.6 | 25.3 | 25.9 | 22.1 | 22.8 | 22.8 | 15.4 | 22.3 | 20.9 | 22.7 | 15.7 | 22.4 | 16.9 | 22.8 |
| C1' | OCOCH3 | C31 | C31 | AcCH3 | CH3CO | CH3CO | CH3CO | |||||||||||
| 171.0 | 170.64 | 20.9 | 171.2 | 21.2 | 170.8 | 170.5 | 170.6 | |||||||||||
| C2' | 170.93 | C32 | C32 | AcCH3 | CH3CO | CH3CO | CH3CO | |||||||||||
| 170.5 | 171.11 | 25.4 | 21.4 | 21.3 | 170.6 | 171.0 | 21.03 | |||||||||||
| C3' | OCOCH3 | AcCO | CH3CO | CH3CO | ||||||||||||||
| 21.3 | 20.83 | 170.7 | 21.4 | 21.3 | ||||||||||||||
| C4' | 20.99 | AcCO | CH3CO | CH3CO | ||||||||||||||
| 20.9 | 21.29 | 171.0 | 21.3 | 21.4 | ||||||||||||||
| NO. | 219b) | 220b) | 221b) | 222b) | 223b) | 224b) | 225b) | 226b) | 227b) | 228b) | 229b) | 230b) | 231b) | 232b) | 233b) | 234b) | 238b) | 239b) |
| C1 | 35.6 | 36.6 | 35.7 | 35.0 | 35.8 | 34.7 | 35.8 | 37.4 | 35.7 | 36.0 | 34.8 | 37.4 | 36.7 | 37.3 | 35.6 | 34.2 | 35.3 | 34.4 |
| C2 | 27.3 | 34.8 | 27.8 | 27.9 | 34.5 | 27.5 | 34.1 | 34.0 | 34.3 | 34.5 | 27.6 | 34.0 | 27.4 | 34.6 | 34.2 | 23.9 | 34.3 | 27.2 |
| C3 | 78.6 | 216.8 | 78.9 | 78.5 | 216.8 | 78.3 | 216.6 | 214.8 | 216.5 | 215.9 | 78.3 | 215.2 | 77.5 | 215.3 | 218.1 | 79.9 | 214.7 | 78.0 |
| C4 | 38.5 | 47.5 | 38.7 | 38.8 | 47.0 | 38.6 | 46.7 | 46.9 | 46.8 | 46.8 | 38.6 | 46.9 | 40.5 | 43.9 | 46.7 | 37.5 | 47.2 | 38.5 |
| C5 | 48.9 | 50.7 | 49.1 | 49.3 | 49.1 | 49.1 | 48.8 | 51.0 | 49.0 | 48.9 | 49.1 | 50.9 | 51.4 | 51.0 | 48.7 | 49.2 | 50.4 | 49.1 |
| C6 | 22.8 | 23.7 | 23.0 | 26.8 | 27.8 | 26.5 | 27.6 | 33.8 | 27.7 | 29.1 | 26.6 | 33.7 | 33.3 | 33.9 | 27.6 | 26.6 | 37.1 | 26.6 |
| C7 | 121.6 | 120.0 | 120.3 | 67.0 | 66.5 | 66.9 | 66.2 | 198.5 | 66.3 | 65.7 | 66.9 | 198.7 | 198.8 | 199.5 | 66.2 | 66.1 | 198.1 | 66.1 |
| C8 | 140.4 | 142.8 | 142.6 | 157.2 | 158.1 | 156.8 | 157.4 | 149.7 | 157.8 | 159.8 | 156.8 | 149.8 | 151.6 | 149.7 | 157.9 | 157.4 | 139.5 | 155.9 |
| C9 | 146.2 | 144.5 | 146.0 | 142.9 | 141.4 | 142.6 | 141.1 | 146.2 | 141.3 | 140.9 | 142.7 | 146.2 | 146.0 | 146.9 | 141.1 | 141.6 | 162.7 | 142.9 |
| C10 | 37.3 | 37.2 | 37.4 | 38.8 | 38.4 | 38.8 | 38.2 | 39.3 | 38.3 | 38.5 | 38.8 | 39.3 | 39.2 | 39.4 | 38.2 | 37.5 | 39.4 | 38.5 |
| C11 | 115.4 | 117.2 | 116.2 | 198.2 | 197.9 | 197.8 | 196.9 | 194.1 | 197.5 | 198.0 | 198.0 | 194.1 | 194.1 | 199.4 | 197.8 | 199.5 | 23.8 | 192.3 |
| C12 | 38.3 | 37.8 | 37.8 | 50.6 | 50.4 | 49.1 | 49.9 | 79.1 | 49.1 | 49.8 | 50.3 | 79.1 | 79.4 | 49.0 | 50.2 | 78.2 | 30.1 | 79.8 |
| C13 | 44.0 | 43.8 | 43.8 | 45.5 | 45.1 | 46.0 | 45.0 | 47.6 | 45.3 | 45.5 | 45.3 | 47.6 | 48.0 | 47.0 | 44.9 | 51.9 | 44.9 | 50.4 |
| C14 | 51.8 | 50.3 | 50.3 | 59.6 | 59.5 | 58.8 | 59.2 | 58.6 | 58.8 | 58.3 | 59.4 | 58.6 | 58.5 | 57.2 | 59.3 | 60.2 | 47.8 | 60.6 |
| C15 | 74.0 | 31.5 | 31.5 | 218.1 | 218.2 | 217.7 | 215.8 | 205.8 | 217.7 | 215.5 | 217.5 | 205.9 | 206.0 | 207.3 | 216.8 | 217.1 | 28.5 | 216.7 |
| C16 | 39.1 | 27.9 | 27.9 | 41.2 | 41.3 | 38.4 | 35.6 | 37.4 | 38.7 | 39.5 | 41.0 | 37.6 | 37.6 | 39.9 | 41.1 | 38.0 | 31.8 | 37.4 |
| C17 | 45.3 | 50.9 | 50.9 | 46.4 | 46.5 | 46.1 | 49.5 | 45.2 | 46.3 | 46.7 | 46.1 | 45.2 | 45.5 | 45.2 | 46.2 | 46.5 | 48.9 | 46.0 |
| C18 | 15.6 | 15.7 | 15.7 | 17.6 | 17.9 | 18.4 | 19.0 | 12.0 | 18.8 | 19.3 | 17.4 | 12.0 | 12.1 | 16.1 | 17.6 | 12.0 | 15.9 | 13.1 |
| C19 | 22.7 | 22.5 | 22.7 | 18.6 | 18.3 | 18.5 | 18.1 | 18.6 | 18.2 | 18.6 | 18.4 | 18.7 | 18.0 | 18.6 | 18.2 | 18.8 | 17.9 | 18.6 |
| C20 | 39.1 | 36.1 | 36.0 | 35.4 | 35.4 | 143.9 | 85.9 | 33.0 | 143.9 | 145.8 | 35.1 | 33.1 | 33.0 | 35.4 | 35.2 | 31.7 | 35.9 | 31.8 |
| C21 | 12.5 | 18.4 | 18.4 | 18.3 | 18.2 | 112.2 | 25.9 | 20.1 | 112.3 | 111.6 | 18.0 | 20.0 | 20.2 | 18.3 | 18.0 | 20.5 | 18.3 | 20.4 |
| C22 | 74.9 | 32.7 | 32.7 | 30.8 | 30.9 | 31.3 | 34.2 | 30.1 | 31.3 | 31.8 | 30.4 | 29.9 | 30.2 | 30.8 | 30.6 | 29.4 | 30.8 | 29.5 |
| C23 | 31.5 | 25.3 | 25.3 | 31.3 | 31.3 | 32.3 | 27.4 | 31.6 | 31.9 | 32.6 | 30.7 | 31.6 | 31.8 | 31.0 | 31.1 | 31.5 | 30.9 | 30.0 |
| C24 | 137.2 | 60.6 | 60.6 | 173.8 | 173.8 | 177.3 | 175 | 173.6 | 175.1 | 173.2 | 178.2 | 178.7 | 173.7 | 173.8 | 173.5 | 177.8 | 178.1 | 178.2 |
| C25 | 129.9 | 60.7 | 60.7 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C26 | 169.9 | 65.4 | 65.4 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C27 | 12.3 | 14.2 | 14.2 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C28 | 17.1 | 25.4 | 28.1 | 28.4 | 27.2 | 24.2 | 25.0 | -- | 27.0 | 27.0 | 24.4 | -- | -- | -- | -- | 27.9 | 25.3 | 28.0 |
| C29 | 27.9 | 22.1 | 15.8 | 15.6 | 20.9 | 28.1 | 26.9 | -- | 20.8 | 20.9 | 28.1 | -- | -- | -- | -- | 16.4 | 21.4 | 15.3 |
| C30 | 15.6 | 25.4 | 25.6 | 24.6 | 24.9 | 15.4 | 20.7 | 27.6 | 24.6 | 25.2 | 15.4 | 27.6 | 27.9 | 27.7 | 27.0 | 23.1 | 24.9 | 24.0 |
| CH3CO | Bu1' | Bu1' | C31 | COOCH3 | C31 | C31 | C31 | C31 | C31 | COCH3 | ||||||||
| 171.1 | 64.6 | 64.6 | 20.8 | 51.5 | 20.4 | 15.6 | 20.9 | 20.7 | 161.0 | 170.5 | ||||||||
| CH3CO | Bu2' | Bu2' | C32 | C32 | C32 | C32 | C32 | COCH3 | ||||||||||
| 20.87 | 30.9 | 30.9 | 20.4 | 20.8 | 21.4 | 20.3 | 24.6 | 20.7 | ||||||||||
| NO. | 241b) | 242b) | 243b) | 244b) | 248b) | 249b) | 252b) | 253b) | 254b) | 255b) | 256b) | 261b) | 262a) | 263a) | 264a) | 266a) | 267b) | 268a) |
| C1 | 35.6 | 33.6 | 34.0 | 33.3 | 35.0 | 35.4 | 34.0 | 34.6 | 33.2 | 34.8 | 34.4 | 36.6 | 36.8 | 35.4 | 36.7 | 35.3 | 35.4 | 73.8 |
| C2 | 34.3 | 27.4 | 33.8 | 27.4 | 27.9 | 34.5 | 33.6 | 33.8 | 27.3 | 27.6 | 27.4 | 34.8 | 35.0 | 28.3 | 27.9 | 28.9 | 27.7 | 39.8 |
| C3 | 216.9 | 77.6 | 214.8 | 77.5 | 78.5 | 215.9 | 214.9 | 215.1 | 77.4 | 78.2 | 78.1 | 216.5 | 215.3 | 72.0 | 70.7 | 79.3 | 78.8 | 75.5 |
| C4 | 46.8 | 39.1 | 46.9 | 40.5 | 38.8 | 47.2 | 46.9 | 47.0 | 40.5 | 38.6 | 38.6 | 47.4 | 47.4 | 43.0 | 43.6 | 43.1 | 38.8 | 40.2 |
| C5 | 48.8 | 50.8 | 51.0 | 51.4 | 49.3 | 49.6 | 50.9 | 50.9 | 51.4 | 49.1 | 49.1 | 50.7 | 50.9 | 42.5 | 44.5 | 50.2 | 50.2 | 49.1 |
| C6 | 29.0 | 36.3 | 37.4 | 36.7 | 26.9 | 28.0 | 37.4 | 37.2 | 36.6 | 26.6 | 26.7 | 23.7 | 23.8 | 27.8 | 34.1 | 28.5 | 18.2 | 17.6 |
| C7 | 68.8 | 199.4 | 198.5 | 198.8 | 67.0 | 66.0 | 198.4 | 199.1 | 198.0 | 66.0 | 66.1 | 119.9 | 121.6 | 66.7 | 200.4 | 67.0 | 26.4 | 26.0 |
| C8 | 159.2 | 151.6 | 149.7 | 151.6 | 157.2 | 158.6 | 145.6 | 146.2 | 151.4 | 156.5 | 155.9 | 142.6 | 142.0 | 158.8 | 152.2 | 158.0 | 134.1 | 134.1 |
| C9 | 140.3 | 147.0 | 146.2 | 156.0 | 142.9 | 140.6 | 149.6 | 149.6 | 145.4 | 142.6 | 142.9 | 144.4 | 145.3 | 142.9 | 147.4 | 142.6 | 134.4 | 137.0 |
| C10 | 38.0 | 40.4 | 39.3 | 39.2 | 38.9 | 38.1 | 39.3 | 34.6 | 39.1 | 38.9 | 38.6 | 37.2 | 37.5 | 39.2 | 40.8 | 39.1 | 36.9 | 44.1 |
| C11 | 199.6 | 199.9 | 194.1 | 194.1 | 198.1 | 199.9 | 193.3 | 198.0 | 193.0 | 197.0 | 191.0 | 117.0 | 117.2 | 198.3 | 199.8 | 198.4 | 20.9 | 25.1 |
| C12 | 51.8 | 49.6 | 79.1 | 79.4 | 50.6 | 78.5 | 78.6 | 48.8 | 78.9 | 50.2 | 79.1 | 37.8 | 38.7 | 51.1 | 49.9 | 51.1 | 30.7 | 32.0 |
| C13 | 46.6 | 44.3 | 47.6 | 48.0 | 45.5 | 51.9 | 47.9 | 44.3 | 48.2 | 45.4 | 49.8 | 43.7 | 44.5 | 45.6 | 44.5 | 45.6 | 44.4 | 44.3 |
| C14 | 53.9 | 57.0 | 58.6 | 58.5 | 59.6 | 60.5 | 58.9 | 57.1 | 58.9 | 59.3 | 61.0 | 50.2 | 52.6 | 59.2 | 57.6 | 59.0 | 49.8 | 50.4 |
| C15 | 72.6 | 208.1 | 205.8 | 206.0 | 218.1 | 217.5 | 203.8 | 204.9 | 203.9 | 215.7 | 214.5 | 27.9 | 73.6 | 216.8 | 208.0 | 216.7 | 30.7 | 31.6 |
| C16 | 36.6 | 40.2 | 37.4 | 37.6 | 41.2 | 37.9 | 35.4 | 34.3 | 35.7 | 35.7 | 37.1 | 31.5 | 40.4 | 41.4 | 40.5 | 41.5 | 27.7 | 28.7 |
| C17 | 48.5 | 45.6 | 45.2 | 45.5 | 46.4 | 46.8 | 48.8 | 48.1 | 49.2 | 49.5 | 50.2 | 50.8 | 49.3 | 46.2 | 45.5 | 46.3 | 45.7 | 46.6 |
| C18 | 17.3 | 16.2 | 12.0 | 12.1 | 17.6 | 12.3 | 13.0 | 17.4 | 13.2 | 18.8 | 14.2 | 15.7 | 16.4 | 17.8 | 16.4 | 17.9 | 15.5 | 16.2 |
| C19 | 19.4 | 17.8 | 18.6 | 18.0 | 18.6 | 18.5 | 18.7 | 18.6 | 17.9 | 18.3 | 18.5 | 22.0 | 22.1 | 19.2 | 18.6 | 19.2 | 19.1 | 15.5 |
| C20 | 35.7 | 35.3 | 33.0 | 33.0 | 35.4 | 31.8 | 86.4 | 86.0 | 86.6 | 85.9 | 86.7 | 36.2 | 36.1 | 35.4 | 35.5 | 35.3 | 40.4 | 40.7 |
| C21 | 18.1 | 18.2 | 20.1 | 20.2 | 18.2 | 20.7 | 26.1 | 26.3 | 26.1 | 25.9 | 25.2 | 18.3 | 18.4 | 18.1 | 18.2 | 18.0 | 13.3 | 13.8 |
| C22 | 30.0 | 30.5 | 30.1 | 30.2 | 30.9 | 30.0 | 34.5 | 34.2 | 34.5 | 34.2 | 34.6 | 29.7 | 32.0 | 31.0 | 31.0 | 31.0 | 80.2 | 80.5 |
| C23 | 31.0 | 30.8 | 31.6 | 31.8 | 31.1 | 32.4 | 28.0 | 27.3 | 28.1 | 27.5 | 28.3 | 30.1 | 31.9 | 31.0 | 31.1 | 30.9 | 27.7 | 28.7 |
| C24 | 174.3 | 178.0 | 173.6 | 173.7 | 174.1 | 173.9 | 175.6 | 175.8 | 175.6 | 175.9 | 175.5 | 178.2 | 176.4 | 174.0 | 174.0 | 174.1 | 139.7 | 140.5 |
| C25 | -- | 27.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 66.6 | 65.1 | -- | 128.1 | 127.8 |
| C26 | -- | 15.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 13.2 | 13.1 | -- | 166.6 | 166.2 |
| C27 | -- | 21.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 24.8 | 21.6 | -- | 17.1 | 18.7 |
| C28 | 27.4 | -- | -- | -- | 28.4 | 26.5 | 27.6 | 27.6 | 27.9 | 28.1 | 28.1 | 25.4 | 25.6 | -- | -- | 23.7 | 27.9 | 28.2 |
| C29 | 20.7 | -- | -- | -- | 15.6 | 21.4 | 20.4 | 20.3 | 15.5 | 15.4 | 15.4 | 22.4 | 22.3 | -- | -- | 64.2 | 15.4 | 15.4 |
| C30 | 19.4 | -- | 27.6 | 27.9 | 24.6 | 23.5 | 21.1 | 21.3 | 21.6 | 24.7 | 24.5 | 25.3 | 17.9 | -- | -- | 24.9 | 24.3 | 24.9 |
| CO- | C31 | C31 | C1' | C1' | COCH3 | COCH3 | COCH3 | CO- | CO- | CO- | ||||||||
| OCH3 | 20.4 | 15.6 | 51.9 | 64.6 | 170.1 | 170.1 | 170.3 | OCH3 | OCH3 | OCH3 | ||||||||
| 51.6 | C32 | C32 | C2' | COCH3 | COCH3 | COCH3 | 51.4 | 51.4 | 51.4 | |||||||||
| 20.8 | 21.4 | 30.9 | 21.0 | 21.0 | 21.2 | |||||||||||||
| NO. | 269b) | 270b) | 271b) | 272b) | 273b) | 274b) | 275b) | 276b) | 277a) | 278b) | 279b) | 280b) | 281b) | 282b) | 286 | 287 | 288b) | 289b) |
| C1 | 143.7 | 143.6 | 144.2 | 147.9 | 35.2 | 35.0 | 36.6 | 36.6 | 35.5 | 35.2 | 36.0 | 35.7 | 35.7 | 35.4 | 36.2 | 36.1 | 36.7 | 29.7 |
| C2 | 118.0 | 118.1 | 118.5 | 116.7 | 34.3 | 28.3 | 34.8 | 34.8 | 29.3 | 34.3 | 33.6 | 27.7 | 35.4 | 27.5 | 33.7 | 33.6 | 34.9 | 22.8 |
| C3 | 167.1 | 167.0 | 166.8 | 163.9 | 213.2 | 77.0 | 217.0 | 217.0 | 78.1 | 217.2 | 216.2 | 78.0 | 215.7 | 77.9 | 216.5 | 216.0 | 216.9 | 77.5 |
| C4 | 80.5 | 80.5 | 80.6 | 77.5 | 47.0 | 38.6 | 47.5 | 47.5 | 40.1 | 44.8 | 45.9 | 41.5 | 46.0 | 41.8 | 46.0 | 45.9 | 47.5 | 36.4 |
| C5 | 49.0 | 49.0 | 49.2 | 92.6 | 49.8 | 49.6 | 50.8 | 50.8 | 50.5 | 49.4 | 40.8 | 39.3 | 40.7 | 39.4 | 39.4 | 40.7 | 50.7 | 47.7 |
| C6 | 38.7 | 38.4 | 37.3 | 44.1 | 27.9 | 27.9 | 23.6 | 23.6 | 27.2 | 27.2 | 23.2 | 21.8 | 22.9 | 21.8 | 23.2 | 22.7 | 23.7 | 20.4 |
| C7 | 27.6 | 27.2 | 27.2 | 26.8 | 65.8 | 66.6 | 119.4 | 119.4 | 71.7 | 70.1 | 60.5 | 58.7 | 59.1 | 59.0 | 62.9 | 62.6 | 119.4 | 25.7 |
| C8 | 147.6 | 147.0 | 149.3 | 149.5 | 157.8 | 156.8 | 142.8 | 142.7 | 158.3 | 158.3 | 66.0 | 71.4 | 62.1 | 62.9 | 64.3 | 57.6 | 142.7 | 137.6 |
| C9 | 130.5 | 129.9 | 131.8 | 127.6 | 140.9 | 143.0 | 144.4 | 144.4 | 140.7 | 138.7 | 163.4 | 165.6 | 167.6 | 168.9 | 164.8 | 164.8 | 144.3 | 128.3 |
| C10 | 139.1 | 139.3 | 140.1 | 132.9 | 37.8 | 39.2 | 37.2 | 37.1 | 39.3 | 37.5 | 40.2 | 37.9 | 40.8 | 38.0 | 40.3 | 40.7 | 37.2 | 39.4 |
| C11 | 26.9 | 26.8 | 67.7 | 28.0 | 199.4 | 200.5 | 117.3 | 117.2 | 200.0 | 199.7 | 130.8 | 128.7 | 129.8 | 128.5 | 130.1 | 129.7 | 117.2 | 22.5 |
| C12 | 31.4 | 31.1 | 42.5 | 31.1 | 78.1 | 79.0 | 37.7 | 37.7 | 53.3 | 52.1 | 201.2 | 200.9 | 199.8 | 200.2 | 202.8 | 200.5 | 37.7 | 31.0 |
| C13 | 43.7 | 44.3 | 43.2 | 44.3 | 49.9 | 50.5 | 43.7 | 43.7 | 40.1 | 46.9 | 50.3 | 54.9 | 54.5 | 54.3 | 53.3 | 55.0 | 43.8 | 44.3 |
| C14 | 56.4 | 54.9 | 55.6 | 55.1 | 63.3 | 63.0 | 50.3 | 50.2 | 53.7 | 52.9 | 59.3 | 62.0 | 62.8 | 61.5 | 60.2 | 58.8 | 50.3 | 50.4 |
| C15 | 76.2 | 78.0 | 77.6 | 78.4 | 215.5 | 215.0 | 27.8 | 27.8 | 71.9 | 70.8 | 71.7 | 209.6 | 202.8 | 203.1 | 76.6 | 209.5 | 27.8 | 31.1 |
| C16 | 40.6 | 38.6 | 38.5 | 38.4 | 60.4 | 60.6 | 31.5 | 31.4 | 33.3 | 32.5 | 33.6 | 38.5 | 125.2 | 125.2 | 39.5 | 38.3 | 31.5 | 27.6 |
| C17 | 45.6 | 45.3 | 45.1 | 45.7 | 54.2 | 54.0 | 50.8 | 50.7 | 49.6 | 48.9 | 45.8 | 43.0 | 181.6 | 181.9 | 48.7 | 42.9 | 50.9 | 45.7 |
| C18 | 17.3 | 16.7 | 18.9 | 16.8 | 12.8 | 13.3 | 15.7 | 15.7 | 18.1 | 17.6 | 18.1 | 18.1 | 30.3 | 29.8 | 19.6 | 18.1 | 15.8 | 15.7 |
| C19 | 143.0 | 142.8 | 141.0 | 139.4 | 18.3 | 19.2 | 22.0 | 22.0 | 20.7 | 19.6 | 21.6 | 18.3 | 24.9 | 25.9 | 21.3 | 21.6 | 22.1 | 104.0 |
| C20 | 40.0 | 39.9 | 39.9 | 39.8 | 33.0 | 33.3 | 36.4 | 36.5 | 33.6 | 32.7 | 157.3 | 154.9 | 73.0 | 72.9 | 158.6 | 158.6 | 36.5 | 40.4 |
| C21 | 13.4 | 13.3 | 13.2 | 13.3 | 19.2 | 19.6 | 18.5 | 18.5 | 20.3 | 19.8 | 20.6 | 20.4 | 29.5 | 29.4 | 20.9 | 20.4 | 18.7 | 13.3 |
| C22 | 80.0 | 79.8 | 79.6 | 79.7 | 52.5 | 52.3 | 33.1 | 33.1 | 50.6 | 49.9 | 126.0 | 126.7 | 52.9 | 52.9 | 126.3 | 126.8 | 33.5 | 80.1 |
| C23 | 27.8 | 27.8 | 27.7 | 27.7 | 87.0 | 87.5 | 25.8 | 25.7 | 209.3 | 208.5 | 198.8 | 198.7 | 206.2 | 206.3 | 199.2 | 198.5 | 29.8 | 27.9 |
| C24 | 139.6 | 139.4 | 139.4 | 139.3 | 40.6 | 40.4 | 78.8 | 76.8 | 47.8 | 46.9 | 47.6 | 47.7 | 47.8 | 47.8 | 47.6 | 47.6 | 77.2 | 139.0 |
| C25 | 128.3 | 128.4 | 128.4 | 128.4 | 34.3 | 34.8 | 73.6 | 73.5 | 36.3 | 34.8 | 34.8 | 34.9 | 34.5 | 33.1 | 33.7 | 34.9 | 73.9 | 128.0 |
| C26 | 166.4 | 166.3 | 166.2 | 166.2 | 178.6 | 179.0 | 66.5 | 68.7 | 178.6 | 176.4 | 179.6 | 179.7 | 179.9 | 179.9 | 180.1 | 180.2 | 69.6 | 166.5 |
| C27 | 17.1 | 17.1 | 17.1 | 17.1 | 15.6 | 15.6 | 18.1 | 18.3 | 18.3 | 17.3 | 17.0 | 17.0 | 16.9 | 16.9 | 17.0 | 17.0 | 20.3 | 17.2 |
| C28 | 29.0 | 28.8 | 28.1 | 24.9 | -- | -- | 25.3 | 25.3 | 29.3 | 27.8 | 14.6 | 17.1 | 24.8 | 17.1 | 21.7 | 24.8 | 25.4 | 23.8 |
| C29 | 26.3 | 26.4 | 26.7 | 24.8 | -- | -- | 22.5 | 22.4 | 17.4 | 20.8 | 24.6 | 25.8 | 21.6 | 25.7 | 24.8 | 21.7 | 22.5 | 25.7 |
| C30 | 26.6 | 26.4 | 25.4 | 24.4 | 25.8 | 28.5 | 25.4 | 25.4 | 20.2 | 19.8 | 28.7 | 28.7 | 28.7 | 28.7 | 28.6 | 28.6 | 25.5 | 23.2 |
| C1' | C1' | C1' | C31 | C31 | C1' | C1' | C1' | COCH3 | C1' | OCH3 | ||||||||
| 170.4 | 170.3 | 170.1 | 21.3 | 16.0 | 195.5 | 31.8 | 102.7 | 52.1 | 195.3 | 55.2 | ||||||||
| C2' | C2' | C2' | C32 | C32 | C2' | C2' | C2' | C(CH3)2 | C2' | |||||||||
| 21.4 | 21.4 | 21.3 | 25.2 | 24.6 | 127.8 | 140.6 | 24.1 | 102.3 | 126.6 | |||||||||
| NO. | 290b) | 291b) | 292b) | 293a) | 294b) | 295b) | 296b) | 297e) | 298b) | 299a) | 300a) | 301a) | 302b) | 303b) | 304b) | 305b) | 306b) | 307b) |
| C1 | 27.5 | 143.8 | 34.1 | 35.5 | 35.7 | 31.2 | 28.7 | 31.3 | 32.6 | 57.0 | 57.0 | 57.0 | 30.4 | 31.0 | 28.8 | 28.8 | 29.7 | 35.2 |
| C2 | 27.1 | 118.0 | 27.4 | 28.6 | 34.2 | 27.7 | 28.4 | 23.6 | 28.0 | 36.5 | 36.5 | 36.5 | 23.3 | 34.6 | 27.7 | 25.5 | 28.3 | 34.0 |
| C3 | 177.3 | 167.0 | 76.5 | 77.6 | 216.4 | 78.7 | 179.0 | 78.3 | 78.9 | 216.5 | 216.5 | 216.4 | 78.1 | 216.6 | 175.6 | 174.5 | 175.5 | 214.9 |
| C4 | 74.5 | 77.8 | 39.2 | 49.7 | 46.8 | 38.9 | 75.3 | 37.0 | 39.0 | 47.0 | 47.0 | 47.1 | 36.7 | 47.1 | 75.2 | 75.0 | 74.8 | 46.6 |
| C5 | 55.1 | 48.9 | 50.3 | 49.7 | 48.9 | 50.2 | 47.9 | 46.0 | 50.1 | 55.4 | 55.4 | 55.5 | 45.3 | 39.7 | 47.9 | 48.0 | 47.8 | 49.3 |
| C6 | 33.8 | 35.8 | 36.9 | 28.1 | 27.6 | 17.6 | 23.9 | 18.4 | 17.8 | 29.4 | 29.4 | 29.4 | 18.0 | 28.8 | 24.3 | 21.5 | 24.8 | 36.9 |
| C7 | 27.1 | 27.1 | 203.8 | 66.7 | 66.2 | 25.7 | 26.7 | 26.3 | 26.2 | 68.3 | 68.4 | 68.3 | 25.9 | 66.2 | 24.3 | 117.9 | 26.0 | 204.5 |
| C8 | 139.2 | 146.0 | 146.2 | 158.7 | 157.5 | 137.1 | 143.3 | 134.4 | 137.9 | 136.4 | 136.3 | 136.3 | 133.7 | 132.9 | 143.1 | 134.1 | 139.6 | 150.9 |
| C9 | 121.7 | 147.0 | 155.9 | 142.7 | 141.2 | 131.7 | 126.1 | 135.1 | 130.4 | 154.7 | 154.7 | 154.8 | 134.7 | 76.3 | 126.1 | 141.1 | 126.1 | 153.2 |
| C10 | 91.5 | 142.8 | 40.4 | 39.3 | 38.3 | 39.6 | 45.4 | 37.3 | 42.2 | 48.6 | 48.6 | 48.6 | 36.9 | 41.4 | 45.4 | 42.2 | 45.7 | 39.4 |
| C11 | 33.0 | 26.3 | 198.5 | 198.0 | 196.8 | 23.0 | 20.8 | 21.2 | 22.0 | 83.5 | 83.6 | 83.5 | 20.8 | 26.2 | 22.6 | 120.4 | 22.5 | 200.9 |
| C12 | 30.7 | 31.1 | 79.3 | 50.9 | 50.1 | 31.0 | 31.0 | 30.8 | 31.0 | 37.4 | 37.4 | 37.4 | 30.8 | 34.2 | 31.2 | 39.4 | 31.2 | 49.6 |
| C13 | 44.5 | 44.3 | 54.1 | 45.8 | 45.1 | 44.4 | 44.3 | 45.0 | 44.4 | 45.6 | 45.6 | 45.7 | 44.3 | 45.2 | 51.4 | 43.7 | 44.1 | 48.2 |
| C14 | 50.5 | 54.9 | 50.6 | 59.0 | 59.3 | 50.2 | 51.5 | 49.9 | 50.4 | 62.3 | 62.4 | 62.4 | 49.5 | 155.0 | 51.8 | 49.9 | 51.4 | 51.8 |
| C15 | 30.1 | 77.8 | 80.1 | 215.0 | 215.8 | 30.7 | 31.3 | 27.5 | 30.7 | 215.0 | 215.3 | 214.8 | 27.0 | 77.5 | 30.5 | 31.5 | 30.8 | 72.9 |
| C16 | 27.1 | 38.5 | 128.1 | 36.5 | 35.8 | 27.6 | 27.4 | 29.4 | 27.5 | 41.0 | 41.1 | 41.0 | 28.9 | 47.9 | 28.5 | 26.0 | 26.6 | 31.7 |
| C17 | 45.5 | 44.8 | 157.7 | 49.7 | 49.0 | 45.8 | 45.9 | 47.7 | 47.1 | 46.8 | 46.9 | 46.6 | 47.0 | 49.7 | 46.9 | 47.5 | 47.8 | 39.2 |
| C18 | 15.5 | 16.7 | 26.0 | 18.6 | 18.1 | 15.6 | 15.9 | 16.4 | 16.6 | 17.7 | 17.7 | 17.7 | 16.3 | 17.2 | 15.7 | 16.0 | 15.8 | 16.6 |
| C19 | 41.5 | 142.8 | 20.8 | 19.4 | 19.1 | 67.8 | 67.3 | 19.1 | 65.8 | 18.8 | 18.8 | 18.8 | 19.0 | 17.0 | 67.2 | 61.6 | 67.2 | 17.6 |
| C20 | 40.3 | 35.8 | 71.2 | 86.5 | 85.5 | 40.4 | 40.3 | 49.1 | 41.6 | 35.7 | 35.9 | 32.6 | 47.8 | 37.8 | 40.1 | 40.0 | 40.2 | 124.4 |
| C21 | 13.3 | 12.8 | 32.8 | 25.8 | 26.0 | 13.3 | 13.4 | 178.5 | 12.0 | 18.6 | 18.7 | 20.3 | 175.2 | 13.3 | 12.9 | 12.6 | 13.3 | 138.4 |
| C22 | 80.1 | 84.0 | 48.6 | 27.8 | 27.5 | 80.2 | 80.3 | 31.9 | 72.7 | 31.4 | 31.9 | 49.8 | 32.9 | 74.6 | 75.6 | 75.5 | 80.1 | 107.7 |
| C23 | 27.9 | 63.6 | 106.8 | 34.2 | 34.2 | 27.9 | 27.8 | 32.8 | 34.0 | 31.1 | 31.8 | 208.9 | 123.5 | 31.6 | 33.1 | 33.1 | 27.3 | 153.6 |
| C24 | 139.7 | 143.8 | 44.6 | 176.7 | 175.9 | 139.6 | 139.7 | 156.0 | 125.4 | 174.1 | 176.1 | 46.9 | 132.3 | 138.5 | 141.0 | 140.9 | 142.9 | 34.3 |
| C25 | 128.0 | 127.7 | 34.1 | -- | -- | 128.2 | 128.2 | 34.3 | 137.9 | -- | -- | 35.7 | 155.4 | 129.8 | 128.2 | 128.1 | 128.0 | 38.5 |
| C26 | 166.5 | 164.0 | 178.7 | -- | -- | 166.6 | 166.7 | 22.0 | 61.4 | -- | -- | 178.4 | 106.7 | 172.0 | 172.4 | 171.7 | 166.3 | 180.0 |
| C27 | 17.1 | 16.8 | 14.5 | -- | -- | 17.1 | 17.1 | 21.9 | 22.4 | -- | -- | 17.7 | 17.7 | 12.5 | 20.6 | 20.5 | 17.0 | 18.9 |
| C28 | 32.0 | 28.5 | 29.6 | 25.4 | 25.0 | 28.1 | 33.7 | 28.0 | 28.4 | 27.1 | 27.1 | 27.1 | 27.6 | 25.8 | 33.7 | 33.5 | 28.7 | 27.3 |
| C29 | 25.2 | 26.6 | 15.1 | 16.5 | 27.0 | 15.5 | 26.1 | 21.9 | 15.5 | 18.9 | 18.9 | 18.9 | 21.8 | 22.0 | 26.1 | 25.8 | 23.8 | 20.4 |
| C30 | 24.5 | 26.6 | 29.7 | 28.8 | 20.7 | 24.2 | 24.9 | 24.5 | 24.7 | 20.6 | 20.7 | 20.6 | 24.4 | 31.4 | 24.3 | 24.3 | 22.5 | 20.5 |
| CO | C1' | C1' | C1' | C1' | OCH3 | C1' | C1' | C1' | C1' | |||||||||
| 170.4 | 171.1 | 170.7 | 171.2 | 170.5 | 51.4 | 94.5 | 170.6 | 170.5 | 170.4 | |||||||||
| NO. | 308a) | 309b) | 310a) | 311b) | 312a) | 315a) | 316b) | NO. | 308a) | 309b) | 310a) | 311b) | 312a) | 315a) | 316b) | |||
| C1 | 32.7 | 31.8 | 38.0 | 36.6 | 37.1 | 35.5 | 32.4 | C18 | 17.7 | 17.7 | 18.0 | 17.8 | 17.7 | 17.7 | 19.6 | |||
| C2 | 30.8 | 30.4 | 29.6 | 29.9 | 30.1 | 28.8 | 32.2 | C19 | 22.4 | 22.4 | 25.5 | 24.9 | 23.0 | 20.0 | 16.3 | |||
| C3 | 176.5 | 173.9 | 176.4 | 174.7 | 175.6 | 77.6 | 214.1 | C20 | 36.2 | 36.2 | 36.2 | 36.0 | 139.9 | 40.1 | 205.2 | |||
| C4 | 146.5 | 146.4 | 87.3 | 86.1 | 145.0 | 39.4 | 47.0 | C21 | 18.5 | 18.5 | 18.1 | 18.1 | 19.0 | 17.5 | 31.2 | |||
| C5 | 45.0 | 45.0 | 48.0 | 48.4 | 44.8 | 49.9 | 43.5 | C22 | 31.9 | 27.7 | 31.9 | 31.3 | 126.3 | 66.9 | 26.9 | |||
| C6 | 35.5 | 35.4 | 32.1 | 32.5 | 27.0 | 29.0 | 36.7 | C23 | 31.9 | 32.4 | 31.6 | 31.0 | 75.2 | -- | 20.3 | |||
| C7 | 67.9 | 67.8 | 72.9 | 73.1 | 61.3 | 69.5 | 198.3 | C24 | 176.5 | 176.5 | 176.4 | 174.5 | 36.8 | -- | 17.5 | |||
| C8 | 165.3 | 165.7 | 161.4 | 161.2 | 66.5 | 160.5 | 66.8 | C25 | -- | -- | -- | -- | 34.3 | -- | -- | |||
| C9 | 137.4 | 137.1 | 135.4 | 135.1 | 163.0 | 141.9 | 68.1 | C26 | -- | -- | -- | -- | 179.0 | -- | -- | |||
| C10 | 41.3 | 41.2 | 41.6 | 41.1 | 43.8 | 39.1 | 37.4 | C27 | -- | -- | -- | -- | 15.5 | -- | -- | |||
| C11 | 200.4 | 200.4 | 199.8 | 200.2 | 130.0 | 200.3 | 200.6 | C28 | 115.6 | 115.7 | 71.3 | 71.3 | 115.2 | 28.8 | -- | |||
| C12 | 52.1 | 52.0 | 50.9 | 50.8 | 201.1 | 52.8 | 46.0 | C29 | 23.4 | 23.2 | 25.0 | 25.3 | 23.0 | 16.7 | ||||
| C13 | 47.3 | 41.2 | 45.3 | 45.2 | 59.1 | 47.7 | 45.5 | C30 | 27.6 | 27.7 | 24.5 | 23.8 | 15.1 | 20.2 | ||||
| C14 | 53.4 | 53.3 | 50.9 | 50.6 | 51.8 | 54.5 | 54.8 | CH2O | 3-OCH3 | OAc | ||||||||
| C15 | 32.4 | 31.9 | 30.1 | 29.3 | 71.8 | 72.6 | 205.9 | 60.4 | 51.9 | 20.7 | ||||||||
| C16 | 27.8 | 32.4 | 27.2 | 29.5 | 31.9 | 36.6 | 36.4 | CH3CH2O | 24-OCH3 | 169.9 | ||||||||
| C17 | 50.1 | 50.1 | 50.2 | 50.0 | 44.3 | 45.4 | 52.4 | 14.4 | 51.8 |
4. The Bioactivities of Ganoderma Triterpenes
4.1. Anti-Tumor Activity
4.2. Anti-HIV and Anti-HIV-1 Protease Activity
4.3. Neurotrophic Activity
4.4. Hepatoprotection
4.5. Antiobesity Activity
4.6. Hypoglycemic Activity
4.7. Other Bioactivities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, J.T.; Wang, C.Z.; Wicks, S.; Yin, J.J.; Kong, J.; Li, J.; Li, Y.C.; Yuan, C.S. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp. Oncol. 2006, 28, 25–29. [Google Scholar] [PubMed]
- Liu, G.Q.; Zhang, K.C. Mechanisms of the anticancer action of Ganoderma lucidum (Leyss. ex Fr.) Karst: A new understanding. J. Integr. Plant Biol. 2005, 47, 129–135. [Google Scholar] [CrossRef]
- Yuen, J.W.M.; Gohel, M.D.I. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer 2005, 53, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.D.; Wasser, S.P.; Mahajna, J.A.; Denchev, C.M.; Nevo, E. Potential role of medicinal mushrooms in breast cancer treatment: Current knowledge and future perspectives. Int. J. Med. Mushrooms 2005, 7, 141–155. [Google Scholar] [CrossRef]
- Lin, Z.B.; Zhang, H.N. Antitumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 2004, 25, 1387–1395. [Google Scholar] [PubMed]
- Gao, Y.H.; Zhou, S.F. Chemopreventive and tumoricidal properties of lingzhi mushroom Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromy cetideae). Part II. mechanism considerations (review). Int. J. Med. Mushrooms 2004, 6, 219–230. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, H.M. Cardiovascular effects of mycelium extract of Ganoderma lucidum: Inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem. Pharm. Bull. 1990, 38, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Chinese Pharmacopoeia, 2010 ed.; Chemical Industry Press: Beijing, China, 2010; Part 1; pp. 174–175. [Google Scholar]
- Kubota, T.; Asaka, Y.; Miura, I.; Mori, H. Structure of Ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) Karst. Helv. Chim. Acta 1982, 65, 611–619. [Google Scholar] [CrossRef]
- Luo, J.; Lin, Z.B. Advances of pharmacological effects of triterpenes from Ganoderma lucidum. Acta Pharmacol. Sin. 2002, 37, 574–578. [Google Scholar]
- Xie, J.T.; Wang, C.Z.; Wicks, S.; Yin, J.J.; Kong, J.; Li, J.; Li, Y.C.; Yuan, C.S. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Boh, B.; Berovic, M.; Zhang, J.; Lin, Z.B. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2012, 29, 780–818. [Google Scholar]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Seo, J.; Kim, J.; Kim, H.; Youn, U.; Lee, J.; Jung, H.; Na, M.; Hattori, M.; Min, B.; et al. Lanostane triterpenes from the fruiting bodies of Ganoderma lucidum and their inhibitory effects on adipocyte differentiation in 3T3-L1 cells. J. Nat. Prod. 2010, 73, 172–176. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Kanomi, S.; Murai, Y.; Kadota, S.; Tsubono, K.; Ogita, Z. Constituents of the fungus Ganoderma lucidum (FR.) Karst. III. Structure of Ganolucidic acids A and B, new lanostane-type triterpenoids. Chem. Pharm. Bull. 1986, 34, 4030–4036. [Google Scholar] [CrossRef]
- Kikuchi, T.; Matsuda, S.; Murai, Y.; Ogita, Z. Ganoderic acid G and I and Ganolucidic acid A and B, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2628–2631. [Google Scholar] [CrossRef]
- Kohda, H.; Tokumoto, W.; Sakamoto, K.; Fujii, M.; Hirai, Y.; Yamasaki, K.; Komoda, Y.; Nakamura, H.; Ishihara, S. The biologically active constituents of Ganoderma lucidum (FR.) Karst. histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 1985, 33, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Matsuda, S.; Kadota, S.; Murai, Y.; Tsubono, K.; Ogita, Z. Constituents of the fungus Ganoderma lucidum (FR.) Karst. I. structure of Ganoderic acids of C2, E, I, and K, Lucidenic acid F and related compounds. Chem. Pharm. Bull. 1986, 34, 3695–3712. [Google Scholar] [CrossRef]
- Morigiwa, A.; Kitabatake, K.; Fujimoto, Y.; Ikekawa, N. Angiotensin converting enzyme-inhibitory triterpenes from Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 3025–3028. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lin, Z.B. Srncture identification of triterpenes from fruiting bodies of Ganoderma lucidum by NMR spectra and X-ray diffraction analysis. Chin. Tradit. Herb. Drugs 2002, 33, 197–200. [Google Scholar]
- Gao, J.J.; Min, B.S.; Ahn, E.M.; Nakamura, N.; Lee, H.K.; Hattori, M. New triterpene aldehydes, lucialdehydes A-C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem. Pharm. Bull. 2002, 50, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.R.; Yue, Q.X.; Wu, Z.Y.; Song, X.Y.; Tao, S.J.; Wu, X.H.; Xu, P.P.; Liu, X.; Guan, S.H.; Guo, D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry 2010, 71, 1579–1585. [Google Scholar] [PubMed]
- Kinge, T.R.; Mih, A.M. Secondary metabolites of oil palm isolates of Ganoderma zonatum Murill. from Cameroon and their cytotoxicity against five human tumour cell lines. Afr. J Biotechnol. 2011, 10, 8440–8447. [Google Scholar]
- Guan, S.H.; Yang, M.; Liu, X.; Xia, J.M.; Wang, X.M.; Jin, H.; Guo, D.A. Two new lanostanoid triterpenes from fruit body of Ganoderma lucidum—The major componet of Sunrecome. Nat. Prod. Commun. 2006, 1, 177–181. [Google Scholar]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibirory activity from the fruiting body of Ganoderma lucidum. Fitoterapia 2010, 86, 1033–1036. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kanomi, S.; Murai, Y.; Kadota, S.; Tsubono, K.; Ogita, Z.I. Constituents of the fungus Ganoderma lucidum (FR.) Karst. II.: Structure of Ganoderic acids F, G, and H, Lucidenic acids D2 and E2, and related compounds. Chem. Pharm. Bull. 1986, 34, 4018–4029. [Google Scholar] [CrossRef]
- Komoda, Y.; Nakamura, H.; Ishihara, S.; Uchida, M.; Kohda, H.; Yamasaki, K. Structure of new terpenoid constituents of Ganoderma lucidum (FR.) Karst (polyporaceae). Chem. Pharm. Bull. 1985, 33, 4829–4835. [Google Scholar] [CrossRef]
- Guan, S.H.; Xia, J.M.; Yang, M.; Wang, X.M.; Liu, X.; Guo, D.A. Cytotoxic lanostanoid triterpenes from Ganoderma lucidum. J. Asian Nat. Prod. Res. 2008, 10, 695–700. [Google Scholar] [CrossRef]
- Sato, N.; Zhang, Q.; Ma, C.M.; Hattori, M. Anti-human immunodeficiency virus-1 protease activity of new lanos-tane-type triterpenoids from Ganoderma sinense. Chem. Pharm. Bull. 2009, 57, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.K. Highly oxygenated lanostane triterpenoids from the fungus Ganoderma applanatum. Chem. Pharm. Bull. 2008, 56, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Li, Y.M.; Sun, H.H. New ganoderic acids, bioactive triterpenoid metabolites from the mushroom Ganoder-ma lucidum. Nat. Prod. Res. 2006, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, Y.Y.; Lin, Z.B. A new lanostane-type triterpene from the fruiting bodies of Ganoderma lucidum. J. Asian Nat. Prod. Res. 2002, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Gao, J.J.; Nakamura, N.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against Meth-A and LLC tumor cells. Chem. Pharm. Bull. 2000, 48, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Bae, KW.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Oda, K.; Sato, H.; Sakamura, S. Novel triterpeniods from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1988, 52, 367–372. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Oda, K.; Sakamura, S. Novel triterpeniods and a steroid from the fungus Ganoderma luci-dum. Agric. Biol. Chem. 1988, 52, 211–216. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Shirasu, S.; Sakamura, S. Novel triterpeniods from the mycelial mat at the previous stage of fruiting of Ganoderma lucidum. Agric. Biol. Chem. 1987, 51, 619–622. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Lindequist, U.; Renate, M.; Gordes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Sato, H.; Sakamura, S. Novel mycelial components, Ganoderic acid Mg, Mh, Mi, Mj, and Mk, from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1987, 51, 1149–1153. [Google Scholar] [CrossRef]
- González, A.G.; León, F.; Rivera, A.; Muñoz, C.M.; Bermejo, J. Lanostanoid triterpenes from Ganoderma lucidum. J. Nat. Prod. 1999, 62, 1770–1701. [Google Scholar]
- Cai, H.; Wang, F.S.; Yang, J.S.; Zhang, Y.M.; Hu, K.; Zhao, Y.J.; Bai, Y.; Zhang, Z.X. Studies on the triterpenoid constituents from the fruiting body Ganoderma lucidum (FR) Karst. Chin. J. Vet. Sci. 1997, 17, 511–513. [Google Scholar]
- Isaka, M.; Chinthanom, P.; Kongthong, S.; Srichomthong, K.; Choeklin, R. Lanostane triterpenes from cultures of the basidiomycete Ganoderma orbiforme BCC 22324. Phytochemistry 2013, 87, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Wang, C.F.; Li, Y.; Luo, H.R.; Qiu, M.H. Isolation and bioactivity evaluation of terpenoids from the medicinal fungus Ganoderma sinense. Planta Med. 2012, 78, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.J.; Zhou, Y.; Ruan, Y.; Ma, J.C.; Ren, W.; Wen, C.N. Lanostane-type triterpenes from the sporoderm-broken spor-es of Ganoderma lucidum. J. Antibiot. 2012, 65, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Liu, R.M.; Zhong, J.J. A new ganoderic acid from Ganoderma lucidum mycelia and its stability. Fitoterapia 2013, 84, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Asaka, I.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acid derivatives and ergosta-4,7,22-triene-3,6-dione from Ganoderma lucidum. Phytochemistry 1987, 26, 2797–2803. [Google Scholar] [CrossRef]
- Shim, S.H.; Ryu, J.; Kim, J.S.; Kang, S.S.; Xu, Y.; Jung, S.H.; Lee, Y.S.; Lee, S.; Shin, K.H. New lanostane-type triterpenoids from Ganoderma applanatum. J. Nat. Prod. 2004, 67, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Goto, S.; Sato, H.; Sakamura, S. Bitter triterpenoids from the fungus Ganoderma applanatum. Phytochemistry 1989, 28, 193–197. [Google Scholar] [CrossRef]
- Hao, R.X.; Zhang, J.S.; Tang, Q.J.; Hu, L.H.; Zheng, Z.P.; Pan, Y.J. Isolation, purification and identification of two new triterpenoid constituents from the fruiting bodies of Ganoderma lucidum. Mycosystema 2006, 25, 599–602. [Google Scholar]
- Kikuchi, T.; Matsuda, S.; Kadota, S.; Murai, Y.; Ogita, Z. Ganoderic acid D, E, F, and H and Lucidenic acid D, E, and F, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2624–2627. [Google Scholar] [CrossRef]
- Hirotani, M.; Furuya, T. Ganoderic acid derivatives, highly oxygenated lanostane-type triterpenoids, from Ganoderma lucidum. Phytochemistry 1986, 25, 1189–1193. [Google Scholar] [CrossRef]
- Wang, C.F.; Liu, J.Q.; Yan, Y.X.; Chen, J.C.; Lu, Y.; Guo, Y.H.; Qiu, M.H. Three new triterpenoids containing four-membered ring from the fruiting body of Ganoderma sinense. Org. Lett. 2010, 12, 1656–1659. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Ye, Q.; Hua, Y.J.; Zhang, D.C.; Cooper, R.; Chang, M.N.; Chang, J.Y.; Sun, H.H. New lanostanoids from the mushroom Ganoderma lucidum. J. Nat. Prod. 2002, 65, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Sun, S.; Xia, B.; Zhang, H.Q. A new triterpene from the fruiting bodies of Ganoderma lucidum. Acta Pharm. Sin. 2009, 44, 768–770. [Google Scholar]
- Liu, C.; Chen, R.Y. A new triterpene from fungal fruiting bodies of Ganoderma sinense. Chin. Tradit. Herb. Drugs 2010, 41, 8–11. [Google Scholar]
- Su, H.J.; Fann, Y.F.; Chung, M.I.; Won, S.J.; Lin, C.N. New lanostanoids of Ganoderma tsugae. J. Nat. Prod. 2000, 63, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Fann, Y.F.; Chung, M.I. Steroids of formosan Ganoderma Tsugae. Phytochemistry 1997, 46, 1143–1146. [Google Scholar] [CrossRef]
- Niu, X.M.; Li, S.H.; Xiao, W.L.; Sun, H.D.; Che, C.T. Two new lanostanoids from Ganoderma resinaceum. J. Asian Nat. Prod. Res. 2007, 9, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.H.; Yang, M.; Wang, X.M.; Xia, J.M.; Zhang, Z.M.; Liu, X.; Guo, D.A. Structure elucidation and complete NMR spectral assignments of three new lanostanoid triterpenes with unprecedented Δ16,17 double bond from Ganoderma lucidum. Magn. Reson. Chem. 2007, 45, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Sato, H.; Sakamura, S. Triterpenoids from the fungus Ganoderma lucidum. Phytochemistry 1987, 26, 1777–1784. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mi, Z.Y.; Tang, Y.; Wang, G.; Li, D.S.; Tang, Y.J. Lanostanoids isolated from Ganoderma lucidum mycelium cultured by submerged fermentation. Helv. Chim. Acta 2009, 92, 1586–1593. [Google Scholar] [CrossRef]
- Chairul, S.M.; Hayashi, Y. Lanostanoid triterpenes from Ganoderma applanatum. Phytochemistry 1994, 35, 1305–1308. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yu, D.Q. Studies on the triterpenoid constituents of the spores from Ganoderma lucidum karst. J. Chin. Pharm. Sci. 1993, 2, 91–96. [Google Scholar]
- Gan, K.H.; Kuo, S.H.; Lin, C.N. Steroidal constituents of Ganoderma applanatum and Ganderma neo-japonicum. J. Nat. Prod. 1998, 61, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- De Silva, E.D.; van der Sar, S.A.; Santha, R.L.; Wijesundera, R.L.; Cole, A.L.; Blunt, J.W.; Munro, M.H. Lanostane triterpenoids from the Sri Lankan Basidiomycete Ganoderma applanatum. J. Nat. Prod. 2006, 69, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, X.M.; Dong, X.C.; Qiu, M.H. A new 18(13→12β)-abeo-lanostadiene triterpenoid from Ganoderma fornicatum. Helv. Chim. Acta 2006, 89, 1038–1041. [Google Scholar] [CrossRef]
- Lin, L.J.; Shiao, M.S. Seven new triterpenes from Ganoderma lucidum. J. Nat. Prod. 1988, 51, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Ino, C.; Furuya, T. Comparative study on the strain-specific triterpenoid components of Ganoderma lucidum. Phytochemistry 1993, 33, 379–382. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ip, F.C.F.; Zhang, D.M.; Chen, L.X.; Zhang, W.; Li, Y.L.; Ip, N.Y.; Ye, W.C. Triterpenoids with neurotrophic activity from Ganoderma lucidum. Nat. Prod. Res. 2011, 25, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Yin, J.H.; Guo, F.J.; Zhang, D.C.; Sun, H.H. Ganoderic acid Sz, a new lanostanoid from the mushroom Ganoderma lucidum. Nat. Prod. Res. 2005, 19, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.; Leon, F.; Rivera, A.; Padron, J.I.; Ganzalez-Plata, J.; Zuluaga, J.C.; Quintana, J.; Estevez, F.; Bermejo, J. New lanostanoids from the Fungus Ganoderma concinna. J. Nat. Prod. 2002, 65, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Fujita, A.; Saga, M.; Fukumura, H.; Hayashi, T.; Shimizu, M.; Morita, N. Three new lanostanoids from Ganoderma lucidum. J. Nat. Prod. 1986, 49, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nishitoba, T.; Shirasu, S.; Oda, K.; Sakamura, S. Ganoderiol A and B, new triteroenoids from the fungus Ganoderma lucidum (Reishi). Afr. Biol. Chem. 1986, 50, 2887–2890. [Google Scholar]
- Shiao, M.S.; Lin, L.J. Two new triterpenes of the fungus Ganoderma lucidum. J. Nat. Prod. 1987, 50, 886–890. [Google Scholar] [CrossRef]
- Keller, A.C.; Keller, J.; Maillard, M.P.; Hostettmann, K. A lanostane-type steroid from the fungus Ganoderma carnosum. Phytochemistry 1997, 46, 963–965. [Google Scholar] [CrossRef]
- Hirotani, M.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acids T, S and R, new triterpenoids from the cultured mycelia of Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 2282–2285. [Google Scholar] [CrossRef]
- Shiao, M.S.; Lin, L.J.; Yeh, S.F. Triterpenes from Ganoderma lucidum. Phytochemistry 1988, 27, 2911–2914. [Google Scholar] [CrossRef]
- Lin, L.J.; Shiao, M.S.; Yea, S.F. Triterpenes from Ganoderma lucidum. Phytochemistry 1988, 27, 2269–2271. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Zhang, W.D.; Ho-Chong-Line, N.; Fourasté, I. New lanostanoids from Ganoderma lucidum that induce NAD(P)H: Quinine oxidoreductase in cultured hepalclc 7 murine hepatoma cells. Planta Med. 2000, 66, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.S.; Lin, L.J.; Yea, S.F. Triterpenes in Ganoderma lucidum. Phytochemistry 1988, 27, 873–875. [Google Scholar] [CrossRef]
- Yang, S.X.; Yu, Z.C.; Lu, Q.Q.; Shi, W.Q.; Laatsch, H.; Gao, J.M. Toxic lanostane triterpenes from the basidiomycete Ganoderma amboinense. Phytochem. Lett. 2012, 5, 576–580. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, X.M.; Qiu, M.H. Two novel lanostane triterpenoids from Ganoderma Sinense. Molecules 2007, 12, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Arisawa, M.; Saga, M.; Hayashi, T.; Fukumura, H.; Morita, N. Two new lanostanoids from Ganoderma lucidum. J. Nat. Prod. 1986, 49, 1122–1125. [Google Scholar] [CrossRef]
- Li, C.H.; Chen, P.Y.; Chang, U.M.; Kan, L.S.; Fang, W.H.; Tsai, K.S.; Lin, S.B. Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci. 2005, 77, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kurashiki, K.; Shimizu, K.; Kondo, R. Structure–activity relationship for inhibition of 5α-reductase by triterpenoids isolated from Ganoderma lucidum. Bioorg. Med. Chem. 2006, 14, 8654–8660. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tagata, M.; Ukiya, M.; Tokuda, H.; Suzuki, T.; Kimura, Y. Oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. J. Nat. Prod. 2005, 68, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum Triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Chau, C.F.; Chen, K.D.; Chen, D.H.; Yen, G.C. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 2007, 51, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Deng, Y.P.; Wei, X.X.; Xu, J.H. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Nat. Prod. Res. 2013, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Sato, H.; Kasai, T.; Kawagishi, H.; Sakamura, S. New bitter C27 and C30 terpenoids from the fungus Ganodema lucidum(Reishi). Agric. Biol. Chem. 1984, 48, 2905–2907. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Oshikubo, M.; Kimura, Y.; Asano, T.; Nomura, A.; Nishino, H. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on epstein-barr virus activation. J. Nat. Prod. 2003, 66, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, H.; Youn, U.; Kim, J.; Min, B.; Jung, H.; Na, M.; Hattori, M.; Bae, K. Effect of lanostane triterpenes from the fruiting bodies of Ganoderma lucidum on adipocyte differentiation in 3T3-L1 cells. Planta Med. 2010, 76, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- El Dine, R.S.; el Halawany, A.M.; Nakamura, N.; Ma, C.M.; Hattori, M. New lanostane triterpene lactones from the vietnamese mushroom Ganoderma colossum (FR.) C. F. BAKER. Chem. Pharm. Bull. 2008, 56, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Kleinwächter, P.; Anh, N.; Kiet, T.T.; Schlegel, B.; Dahse, H.M.; Härtl, A.; Gräfe, U. Colossolactones, new triterpenoid Metabolites from a Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2001, 64, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Yu, D.Q. Application of 2D NMR techniques in the structure determination of Ganosporelactone A and B. Acta Pharm. Sin. 1991, 26, 430–436. [Google Scholar]
- Sato, N.; Ma, C.M.; Komatsu, K.; Hattori, M. Triterpene-farnesyl hydroquinone conjugates from Ganoderma sinense. J. Nat. Prod. 2009, 72, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, T.; Hayashi, Y.; Nishizawa, M.; Tokuda, H.; Chairul, S.M.; Hayashi, Y. Applanoxidic acids A, B, C, and D, biologically active tetracyclic triterpenes from Ganoderma applanatum. Phytochemistry 1991, 30, 4105–4109. [Google Scholar] [CrossRef]
- El Dine, R.S.; el Halawany, A.M.; Ma, C.M.; Hattori, M. Anti-HIV-1 protease activity of lanostane triterpenes from the vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2008, 71, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Leon, F.; Valenica, M.; Rivera, A.; Nieto, I.; Quintana, J.; Estevez, F.; Bermejo, J. Novel cytostatic lanostanoid triterpenes from Ganoderma australe. Helv. Chim. Acta 2003, 86, 3088–3095. [Google Scholar] [CrossRef]
- Wang, F.S.; Cai, H.; Yang, J.S.; Zhang, Y.M.; Zhao, Y.J. Triterpenoids from the fruiting body of Ganoderma lucidum. J. Chin. Pharm. Sci. 1997, 6, 192–197. [Google Scholar]
- Niu, X.M.; Qiu, M.H.; Lu, Z.G.; Lu, Y.; Cao, P.; Zheng, Q. Two novel 3,4-seco-trinorlanostane triterpenoids isolated from Ganoderma fornicatum. Tetrahedron Lett. 2004, 45, 2989–2993. [Google Scholar] [CrossRef]
- Peng, X.R.; Liu, J.Q.; Xia, J.J.; Yang, Y.H.; Qiu, M.H. Two new triterpenoids from Ganoderma cochlear. Chin. Tradit. Herb. Drugs 2012, 43, 1045–1049. [Google Scholar]
- Su, C.H.; Lai, M.N.; Chan, M.H. Hepato-protective triterpenoids from Ganoderma tsugae Murrill. In Mushroom Biology and Mushroom Products; Chang, S., Buswell, J.A., Chiu, S., Eds.; The Chinese University Press: Hong Kong, China, 1993; pp. 275–283. [Google Scholar]
- Fatmawati, S.; Shimizu, K.; Kondo, R.; Ganoderol, B. A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478-17535. https://doi.org/10.3390/molecules191117478
Xia Q, Zhang H, Sun X, Zhao H, Wu L, Zhu D, Yang G, Shao Y, Zhang X, Mao X, et al. A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp. Molecules. 2014; 19(11):17478-17535. https://doi.org/10.3390/molecules191117478
Chicago/Turabian StyleXia, Qing, Huazheng Zhang, Xuefei Sun, Haijuan Zhao, Lingfang Wu, Dan Zhu, Guanghui Yang, Yanyan Shao, Xiaoxue Zhang, Xin Mao, and et al. 2014. "A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp." Molecules 19, no. 11: 17478-17535. https://doi.org/10.3390/molecules191117478
APA StyleXia, Q., Zhang, H., Sun, X., Zhao, H., Wu, L., Zhu, D., Yang, G., Shao, Y., Zhang, X., Mao, X., Zhang, L., & She, G. (2014). A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp. Molecules, 19(11), 17478-17535. https://doi.org/10.3390/molecules191117478