Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
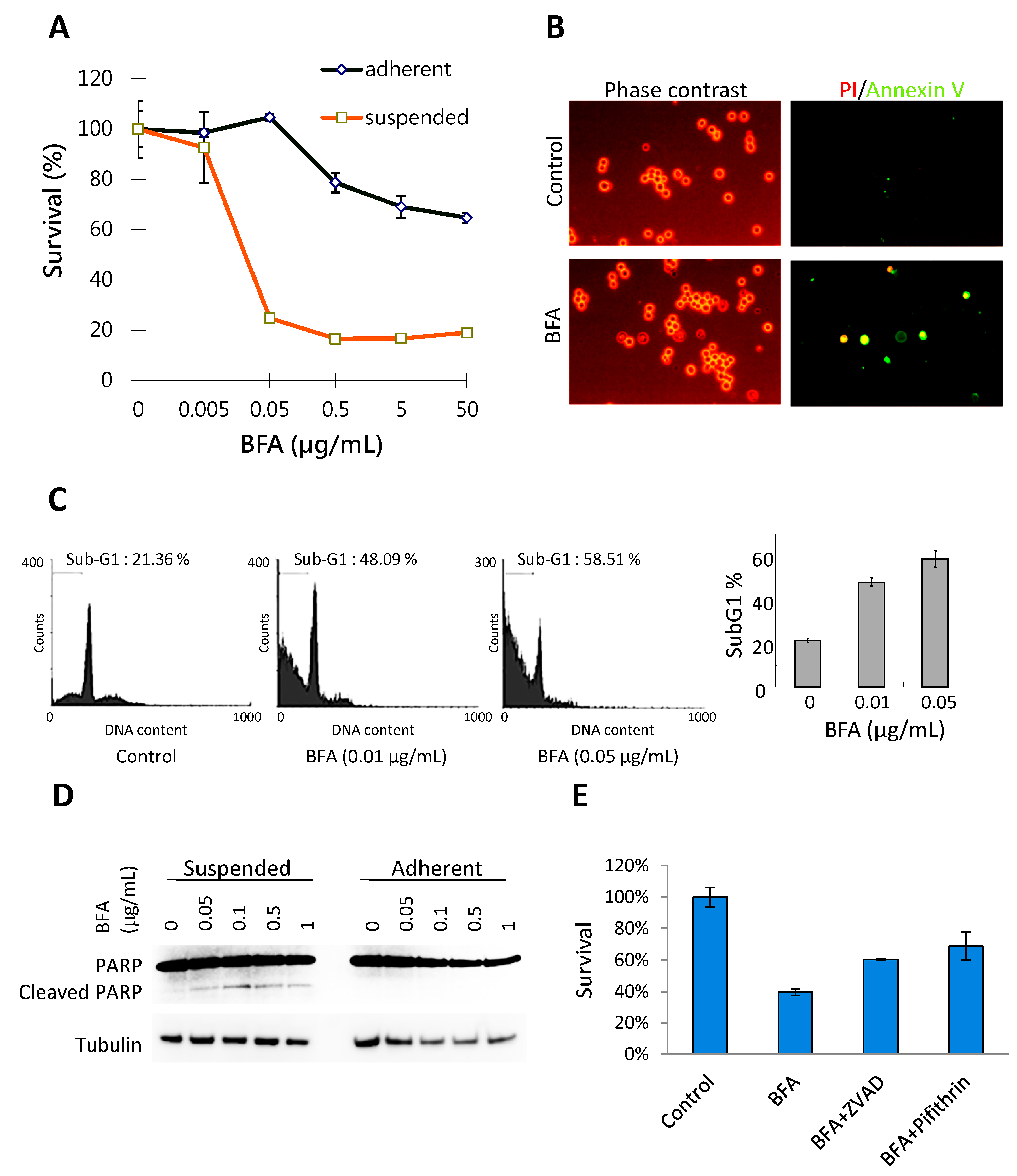
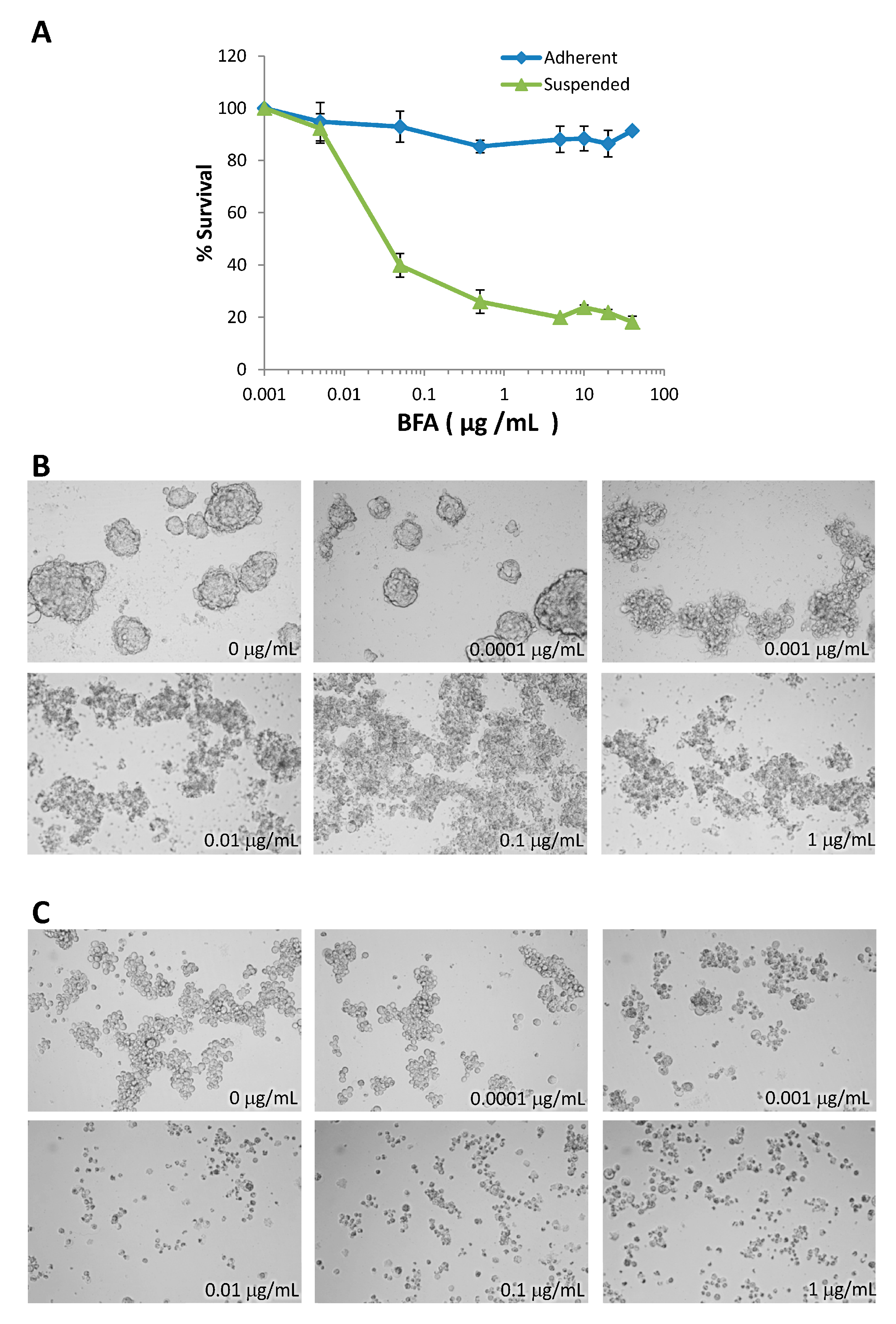
2.1. BFA Induces Anchorage-Independent Cell Death in MDA-MB-231 Breast Cancer Cells
2.2. BFA Inhibits the Formation of MDA-MB-231 Colonies in 3D and 2D Cultures

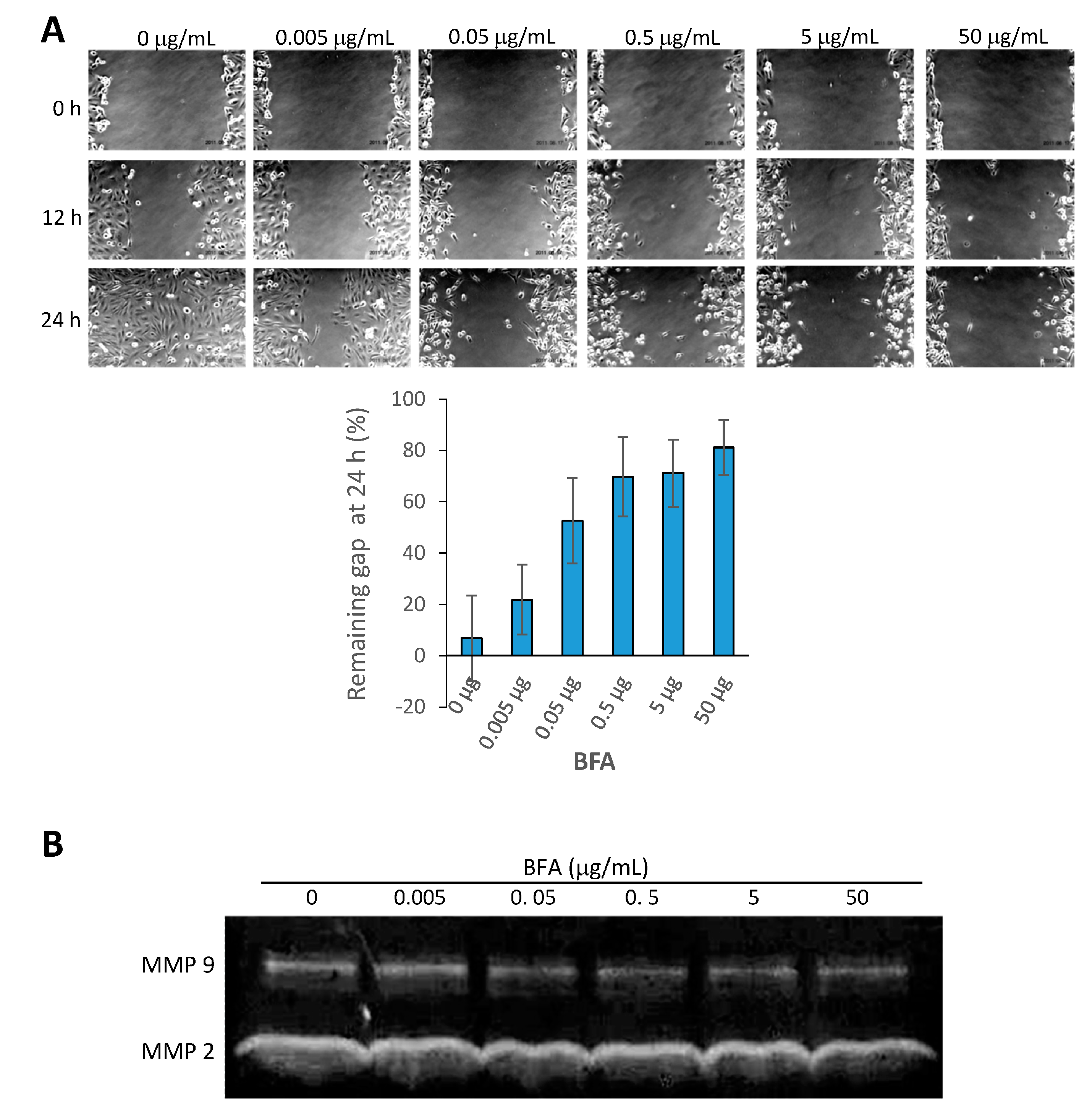
2.3. BFA Inhibits the Migration and MMP 9 (Matrix Metallopeptidase 9) Activity of MDA-MB-231
2.4. Modulation of Protein Expression by BFA

2.5. Inhibitory Effects of BFA on MDA-MB-468, T47D, and MDA-MB-453 Human Breast Cancer Cells
2.6. Discussion
3. Experimental Section
3.1. Adhesion and Suspension Cell Cultures
3.2. Trypan Blue Cellular Viability Assessment
3.3. WST-1 Cell Survival Assay
3.4. Flow Cytometic Analysis of Sub-G1 Population and Live Cell Annexin V/PI Staining
3.5. 3D Soft Agar Tumorsphere Formation Assay
3.6. 2D Clonogenic Assay
3.7. Wound-Healing Motility Assay
3.8. Gelatin Zymography
3.9. Western Blotting
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar]
- Korkaya, H.; Paulson, A.; Charafe-Jauffret, E.; Ginestier, C.; Brown, M.; Dutcher, J.; Clouthier, S.G.; Wicha, M.S. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009, 7, e1000121. [Google Scholar]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006, 66, 1883–1890. [Google Scholar]
- Soltysova, A.; Altanerova, V.; Altaner, C. Cancer stem cells. Neoplasma 2005, 52, 435–440. [Google Scholar]
- Ailles, L.E.; Weissman, I.L. Cancer stem cells in solid tumors. Curr. Opin. Biotechnol. 2007, 18, 460–466. [Google Scholar]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar]
- Yamashita, T.; Wang, X.W. Cancer stem cells in the development of liver cancer. J. Clin. Investig. 2013, 123, 1911–1918. [Google Scholar]
- Peng, C.Y.; Fong, P.C.; Yu, C.C.; Tsai, W.C.; Tzeng, Y.M.; Chang, W.W. Methyl Antcinate A suppresses the population of cancer stem-like cells in MCF7 human breast cancer cell line. Molecules 2013, 18, 2539–2548. [Google Scholar]
- Fillmore, C.M.; Kuperwasser, C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008, 10, R25. [Google Scholar]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in Vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–59. [Google Scholar]
- Tseng, C.N.; Huang, C.F.; Cho, C.L.; Chang, H.W.; Huang, C.W.; Chiu, C.C.; Chang, Y.F. Brefeldin a effectively inhibits cancer stem cell-like properties and MMP-9 activity in human colorectal cancer Colo 205 cells. Molecules 2013, 18, 10242–10253. [Google Scholar]
- De la Mare, J.A.; Sterrenberg, J.N.; Sukhthankar, M.G.; Chiwakata, M.T.; Beukes, D.R.; Blatch, G.L.; Edkins, A.L. Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int. 2013, 13, 39. [Google Scholar]
- Tamura, G.; Ando, K.; Suzuki, S.; Takatsuki, A.; Arima, K. Antiviral activity of brefeldin A and verrucarin A. J. Antibiot. 1968, 21, 160–161. [Google Scholar]
- Chardin, P.; McCormick, F. Brefeldin A: The advantage of being uncompetitive. Cell 1999, 97, 153–155. [Google Scholar]
- Natsume, Y.; Ito, S.; Satsu, H.; Shimizu, M. Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology 2009, 258, 164–175. [Google Scholar]
- Lee, M.S.; Chao, J.; Yen, J.C.; Lin, L.W.; Tsai, F.S.; Hsieh, M.T.; Peng, W.H.; Cheng, H.Y. Schizandrin protects primary rat cortical cell cultures from glutamate-induced apoptosis by inhibiting activation of the MAPK family and the mitochondria dependent pathway. Molecules 2012, 18, 354–372. [Google Scholar]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar]
- Farrar, W.L. Cancer Stem Cells; Cambridge University Press: New York, NY, USA, 2010; p. 171. [Google Scholar]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland. Biol. Neoplasia 2010, 15, 201–212. [Google Scholar]
- Lin, C.Y.; Tsai, P.H.; Kandaswami, C.C.; Lee, P.P.; Huang, C.J.; Hwang, J.J.; Lee, M.T. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011, 102, 815–827. [Google Scholar]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar]
- Chetty, C.; Vanamala, S.K.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell. Signal. 2012, 24, 549–559. [Google Scholar]
- Chang, H.W.; Wang, H.C.; Chen, C.Y.; Hung, T.W.; Hou, M.F.; Yuan, S.S.; Huang, C.J.; Tseng, C.N. 5-azacytidine induces anoikis, inhibits mammosphere formation and reduces metalloproteinase 9 activity in MCF-7 human breast cancer cells. Molecules 2014, 19, 3149–3159. [Google Scholar]
- Kopp, F.; Hermawan, A.; Oak, P.S.; Herrmann, A.; Wagner, E.; Roidl, A. Salinomycin treatment reduces metastatic tumor burden by hampering cancer cell migration. Mol. Cancer 2014, 13, 16. [Google Scholar]
- Woods, N.T.; Yamaguchi, H.; Lee, F.Y.; Bhalla, K.N.; Wang, H.G. Anoikis, initiated by Mcl-1 degradation and Bim induction, is deregulated during oncogenesis. Cancer Res. 2007, 67, 10744–10752. [Google Scholar]
- Sanchez-Tillo, E.; de Barrios, O.; Siles, L.; Cuatrecasas, M.; Castells, A.; Postigo, A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc. Natl. Acad. Sci. USA 2011, 108, 19204–19209. [Google Scholar]
- Soini, Y.; Tuhkanen, H.; Sironen, R.; Virtanen, I.; Kataja, V.; Auvinen, P.; Mannermaa, A.; Kosma, V.M. Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer 2011, 11, 73. [Google Scholar]
- Takeyama, Y.; Sato, M.; Horio, M.; Hase, T.; Yoshida, K.; Yokoyama, T.; Nakashima, H.; Hashimoto, N.; Sekido, Y.; Gazdar, A.F.; et al. Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells. Cancer Lett. 2010, 296, 216–224. [Google Scholar]
- Aigner, K.; Dampier, B.; Descovich, L.; Mikula, M.; Sultan, A.; Schreiber, M.; Mikulits, W.; Brabletz, T.; Strand, D.; Obrist, P.; et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007, 26, 6979–6988. [Google Scholar]
- Peinado, H.; Portillo, F.; Cano, A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004, 48, 365–375. [Google Scholar]
- Babina, I.S.; McSherry, E.A.; Donatello, S.; Hill, A.D.; Hopkins, A.M. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 2014, 16, R19. [Google Scholar]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar]
- Yakkundi, A.; McCallum, L.; O’Kane, A.; Dyer, H.; Worthington, J.; McKeen, H.D.; McClements, L.; Elliott, C.; McCarthy, H.O.; Hirst, D.G.; et al. The anti-migratory effects of FKBPL and its peptide derivative, AD-01: Regulation of CD44 and the cytoskeletal pathway. PLoS One 2013, 8, e55075. [Google Scholar]
- Nickerson, N.K.; Mohammad, K.S.; Gilmore, J.L.; Crismore, E.; Bruzzaniti, A.; Guise, T.A.; Foley, J. Decreased autocrine EGFR signaling in metastatic breast cancer cells inhibits tumor growth in bone and mammary fat pad. PLoS One 2012, 7, e30255. [Google Scholar]
- Jechlinger, M.; Sommer, A.; Moriggl, R.; Seither, P.; Kraut, N.; Capodiecci, P.; Donovan, M.; Cordon-Cardo, C.; Beug, H.; Grunert, S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Investig. 2006, 116, 1561–1570. [Google Scholar]
- Harrison, H.; Simoes, B.M.; Rogerson, L.; Howell, S.J.; Landberg, G.; Clarke, R.B. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res. 2013, 15, R21. [Google Scholar]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar]
- Sample Availability: Brefeldin A (B-8500) was purchased from LC Laboratories (Woburn, MA, USA).
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-N.; Hong, Y.-R.; Chang, H.-W.; Yu, T.-J.; Hung, T.-W.; Hou, M.-F.; Yuan, S.-S.F.; Cho, C.-L.; Liu, C.-T.; Chiu, C.-C.; et al. Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells. Molecules 2014, 19, 17464-17477. https://doi.org/10.3390/molecules191117464
Tseng C-N, Hong Y-R, Chang H-W, Yu T-J, Hung T-W, Hou M-F, Yuan S-SF, Cho C-L, Liu C-T, Chiu C-C, et al. Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells. Molecules. 2014; 19(11):17464-17477. https://doi.org/10.3390/molecules191117464
Chicago/Turabian StyleTseng, Chao-Neng, Yi-Ren Hong, Hsueh-Wei Chang, Tsai-Jung Yu, Ting-Wei Hung, Ming-Feng Hou, Shyng-Shiou F. Yuan, Chung-Lung Cho, Chien-Tsung Liu, Chien-Chih Chiu, and et al. 2014. "Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells" Molecules 19, no. 11: 17464-17477. https://doi.org/10.3390/molecules191117464
APA StyleTseng, C.-N., Hong, Y.-R., Chang, H.-W., Yu, T.-J., Hung, T.-W., Hou, M.-F., Yuan, S.-S. F., Cho, C.-L., Liu, C.-T., Chiu, C.-C., & Huang, C.-J. (2014). Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells. Molecules, 19(11), 17464-17477. https://doi.org/10.3390/molecules191117464





