Endocannabinoids, Related Compounds and Their Metabolic Routes
Abstract
:1. Introduction
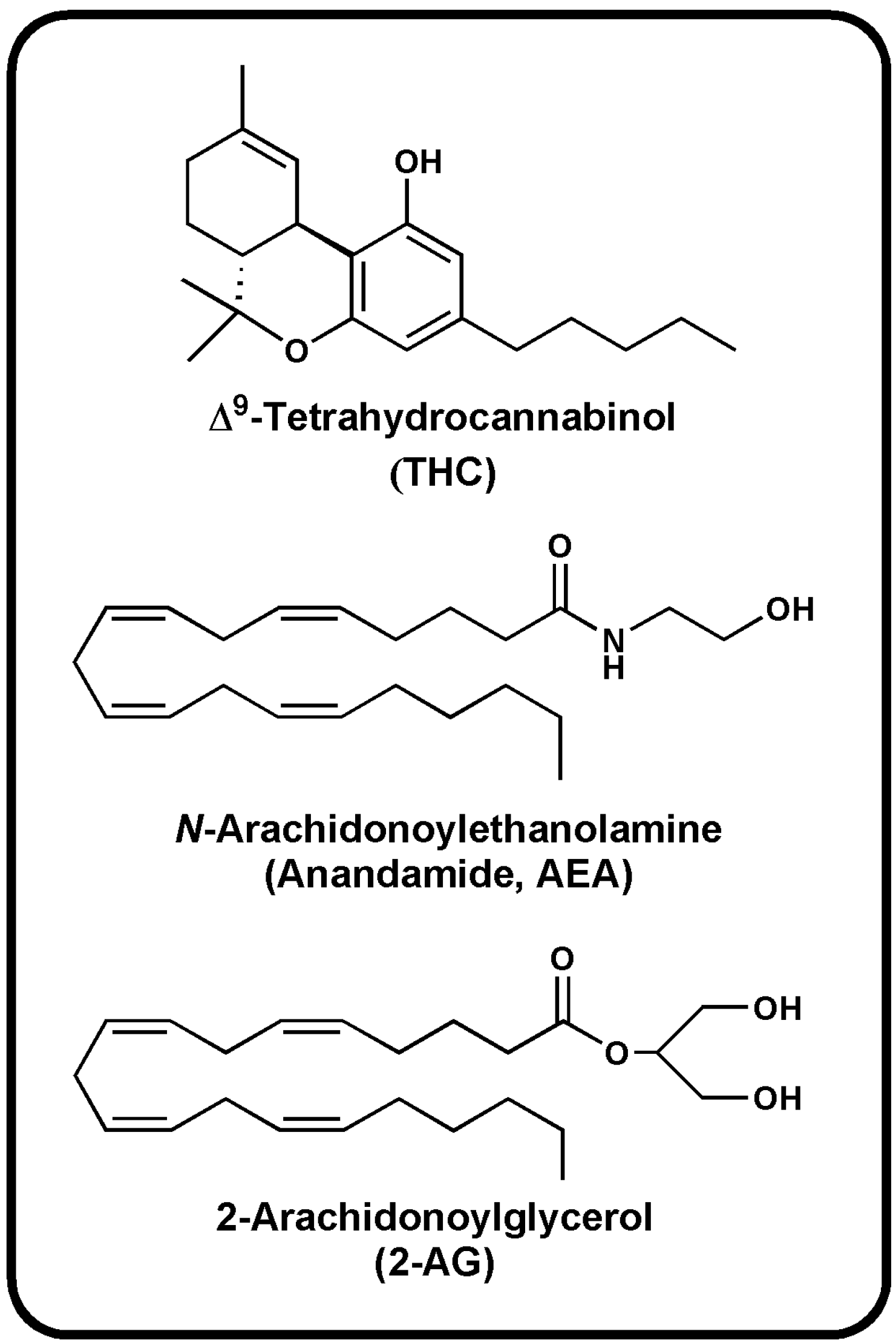
| Bioactive Lipids | Molecular Targets | Biosynthetic Enzymes | Catabolic Enzymes |
|---|---|---|---|
| n-6 eCBs derivatives | |||
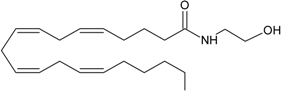 AEA | CB1 [1] CB2 [1] TRPV1 [8] PPARα [9] PPARγ [9] GPR55 [10] | NAT [11] iNAT [12,13,14] NAPE-PLD [15] ABHD4 [16,17,18] Lyso-PLD [16,17,18] GDE1[16,17,18] PTPN22 [16,17,18] | FAAH-1 [19] FAAH-2 [20] NAAH [21] LOXx [22] COX-2 [23,24] CytP450 [25] |
 2-AG | CB1 [1] CB2 [1] TRPV1 [26] | PLCβ [27,28] DAGLα [29] DAGLβ [29] | MAGL [30] FAAH-1[19] ABHD6 [31,32] ABHD12 [31,32] LOXx [22] COX-2 [23,24] |
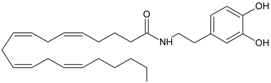 NADA | CB1 [1] TRPV1 [33] PPARγ [34] | Postulate condensation between the catecholamine with AA | Slow hydrolysis of the amide bond or the methylation of catecholamine |
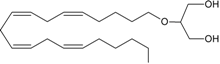 noladin ether | CB1 [35,36] CB2 [35] GPR55 [37] PPARα [9] | unknown | unknown |
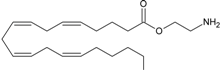 virodhamine | CB1 [38] CB2 [38] GPR55 [37] PPARα [9] | unknown | unknown |
| n-3 eCBs derivatives | |||
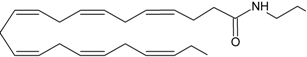 DHEA | CB1 [39] CB2 [39] PPARγ [40] | Postulate as other NAEs | Postulate as other NAEs |
 EPEA | CB1 [39] CB2 [39] PPARγ [41] | Postulate as other NAEs | Postulate as other NAEs |
| Monounsaturated and saturated fatty acids derivatives | |||
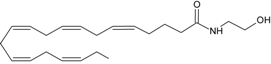
 PEA | PPARα [42,43,44,45,46,47,48,49,50,51] GPR55 [52] GPR119 [53] | NAT [11] iNAT [12,13,14] NAPE-PLD [15] Lyso-PLD [16,17,18] GDE1[16,17,18] PTPN22 [16,17,18] | FAAH-1 [54] FAAH-2 [21] NAAH [55] |
 OEA | PPARα [42,43,44,45,46,47,48,49,50,51] GPR119 [53] GPR55[52] | NAT [11] iNAT [12,13,14] NAPE-PLD [15] ABHD4 [16,17,18] Lyso-PLD [16,17,18] GDE1[16,17,18] PTPN22 [16,17,18] | FAAH-1 [54] FAAH-2 [21] NAAH [55] |
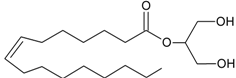 2-OG | GPR119 [53] | PLCβ [27,28] DAGLα [29] DAGLβ [29] | MAGL [30] FAAH-1 [54] |
2. Endocannabinoids System
2.1. Endogenous Ligands of Cannabinoid Receptors
2.1.1. Main Endocannabinoids
2.1.2. Additional n-6-Endocannabinoids
2.1.3. n-3-Endocannabinoids
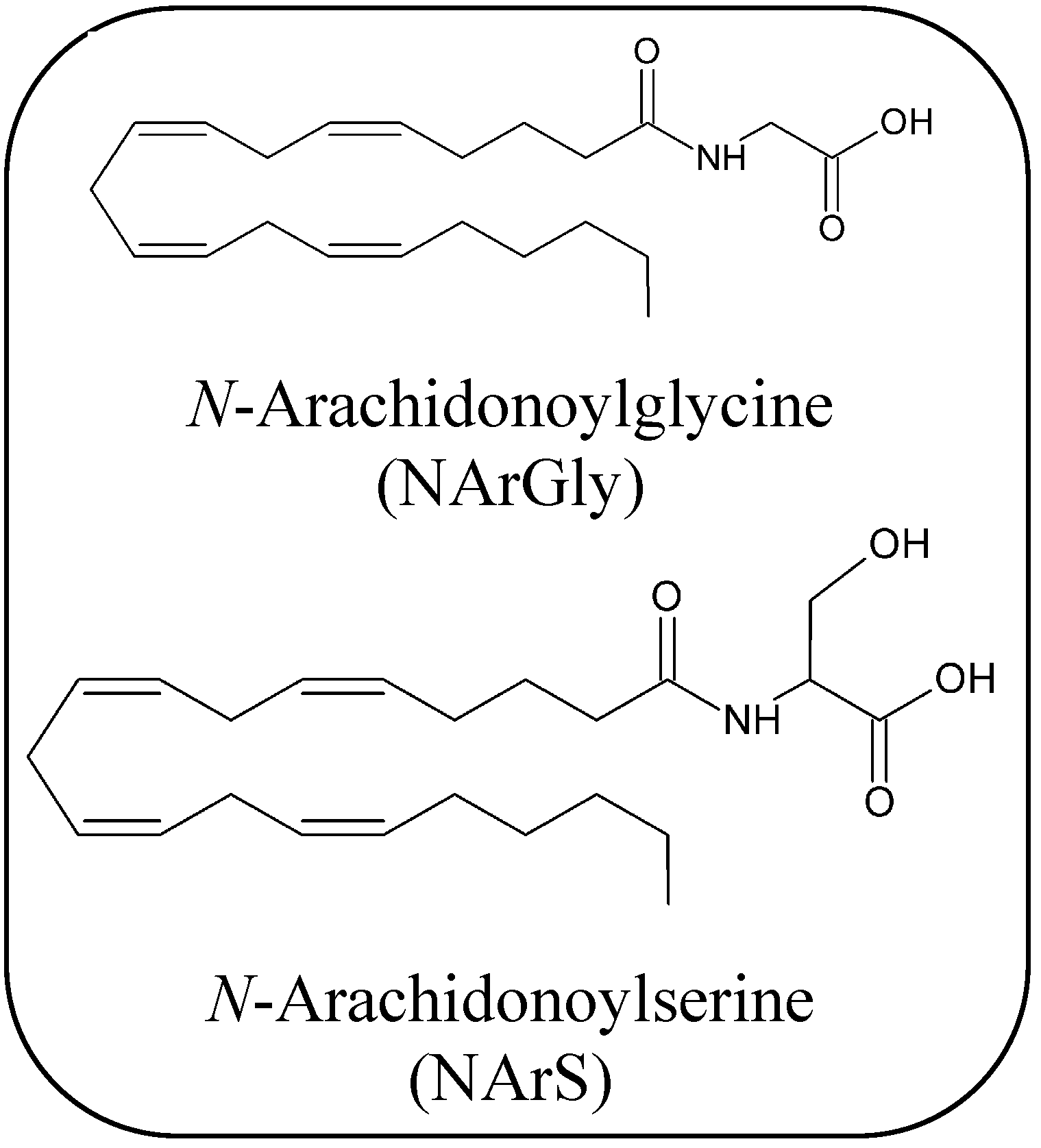
2.1.4. Endocannabinoid-Like Compounds
2.2. Metabolism of Endocannabinoids and Related Compounds
2.2.1. Biosynthesis of AEA and Congeners

2.2.2. Biosynthesis of 2-AG and Congeners
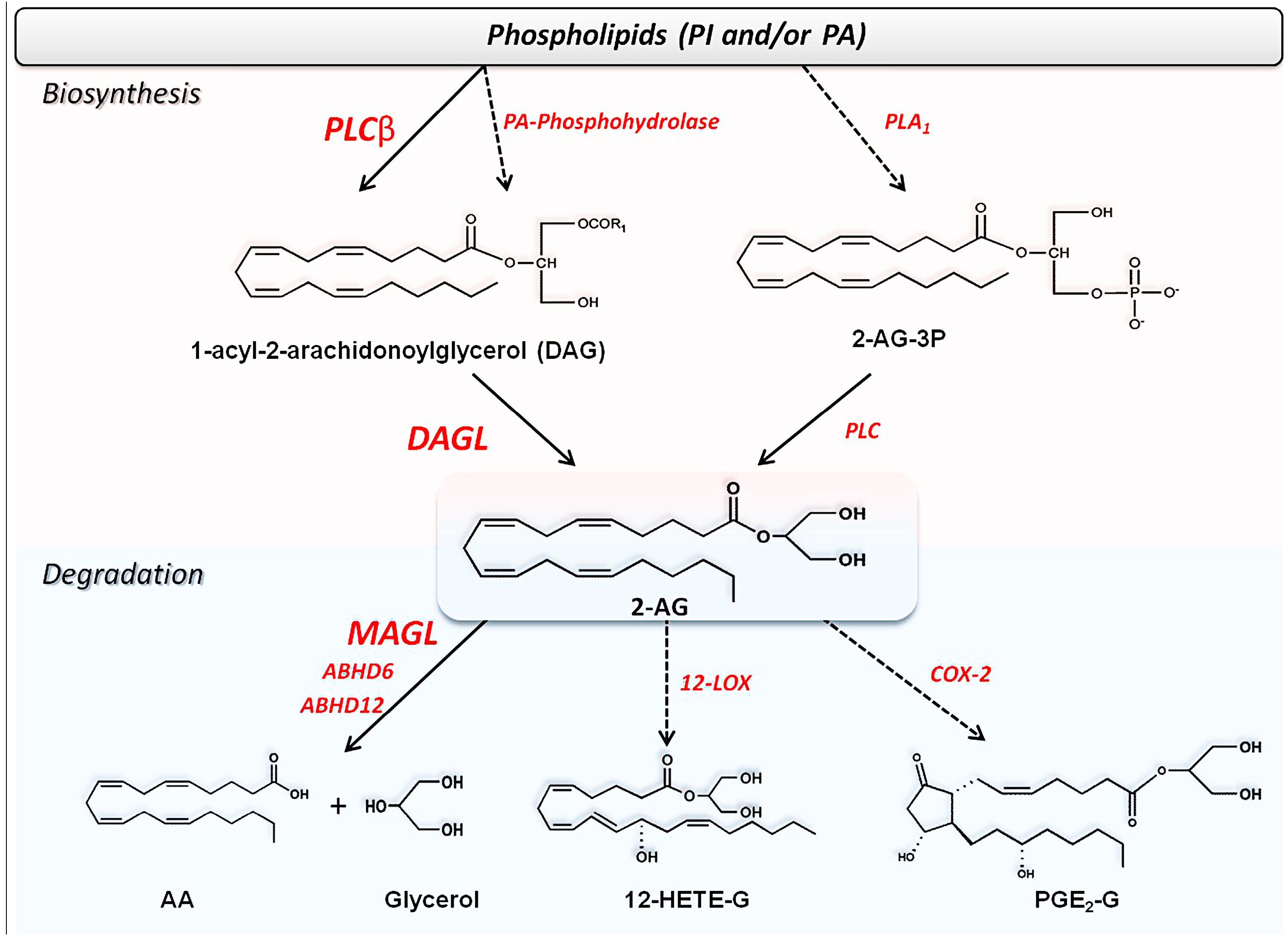
2.2.3. Degradation of Endocannabinoids and Congeners
Uptake of Endocannabinoids and Congeners
Hydrolysis of AEA and Congeners
Hydrolysis of 2-AG and Congeners
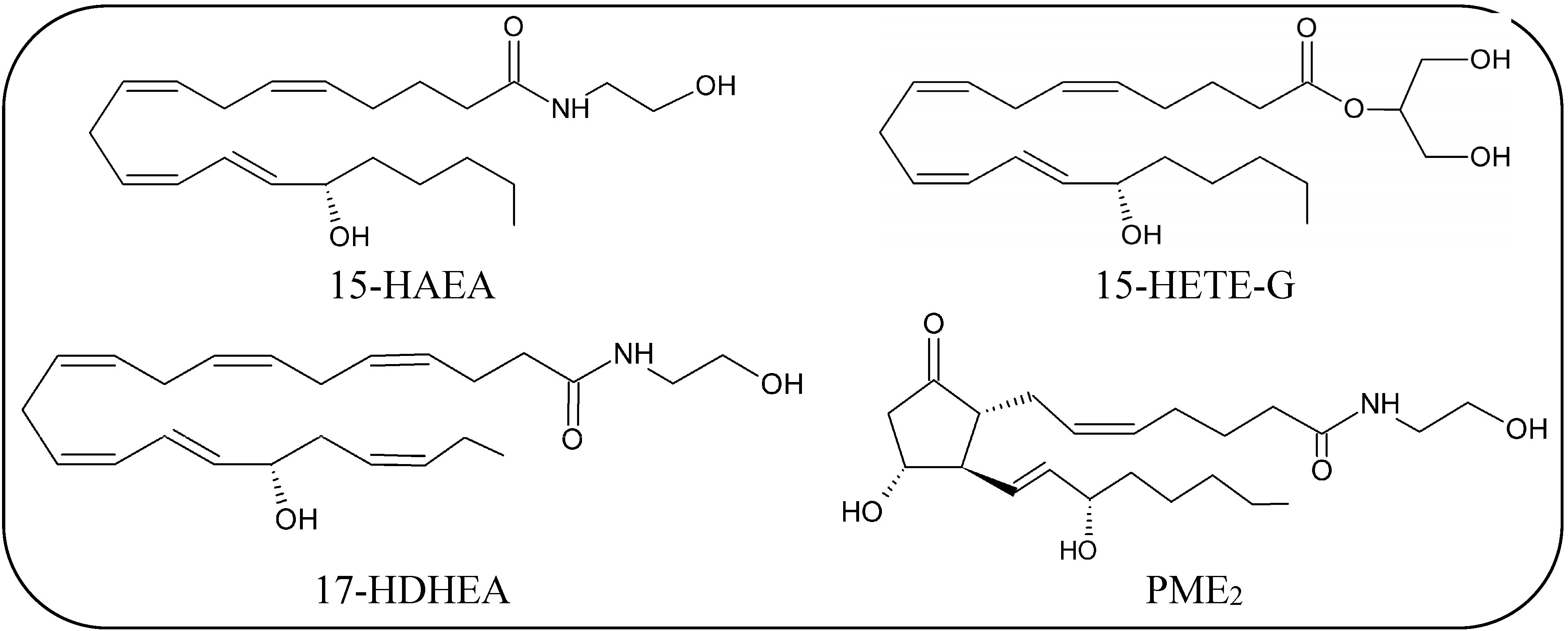
Oxidative Metabolism of eCBs and eCBs-Like Compounds
3. Conclusions
Acknowlwdgments
Author Contributions
Conflicts of Interest
References
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Dainese, E.; Oddi, S. Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem. Sci. 2010, 35, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 2014, 76, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A. Anandamide, an endogenous cannabinomimetic eicosanoid: ‘Killing two birds with one stone’. Prostaglandins Leukot. Essent. Fat. Acids 1995, 53, 1–11. [Google Scholar] [CrossRef]
- Devane, W.A.; Hannus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; de petrocellis, l. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, V.; Dainese, E.; Oddi, S.; Sabatucci, A.; Maccarrone, M. GPR55 and its interaction with membrane lipids: Comparison with other endocannabinoid-binding receptors. Curr. Med. Chem. 2013, 20, 64–78. [Google Scholar]
- Ueda, N.; Tsuboi, K.; Uyama, T. Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways. FEBS J. 2013, 280, 1874–1894. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Kiser, P.D.; Sears, A.E.; Lodowski, D.T.; Blaner, W.S.; Palczewski, K. Structural basis for the acyltransferase activity of lecithin:retinol acyltransferase-like proteins. Biol. Chem. 2012, 287, 23790–23807. [Google Scholar] [CrossRef]
- Jin, X.H.; Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J. Biol. Chem. 2007, 282, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Uyama, T.; Wang, J.; Okamoto, Y.; Tonai, T.; Ueda, N. cDNA cloning and characterization of human and mouse Ca(2+)-independent phosphatidylethanolamine N-acyltransferases. Biochim. Biophys. Acta 2009, 1791, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.M.; Cravatt, B.F. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol. Biosyst. 2010, 6, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Tsuboi, K.; Okamoto, Y.; Tonai, T.; Murakami, M.; Kudo, I.; Ueda, N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem. J. 2004, 380, 749–756. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.K.; Cravatt, B.F. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005, 74, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Takezaki, N.; Ueda, N. The N-acylethanolamine-hydrolyzing acid amidase (NAAA). Chem. Biodivers. 2007, 4, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, M.; van Kuik, J.A.; Bari, M.; van Zadelhoff, G.; Leeflang, B.R.; Veldink, G.A.; Finazzi-Agrò, A.; Vliegenthart, J.F.; Maccarrone, M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: Conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J. Med. Chem. 2002, 45, 3709–3720. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Walker, V.J.; Hollenberg, P.F. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: Physiological and pharmacological implications. Pharmacol. Rev. 2010, 62, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols activate TRPV1-a link between phospholipase C and TRPV1. PLoS One 2013, 8, e81618. [Google Scholar] [CrossRef] [PubMed]
- Hashimotodani, Y.; Ohno-Shosaku, T.; Tsubokawa, H.; Ogata, H.; Emoto, K.; Maejima, T.; Araishi, K.; Shin, H.S.; Kano, M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 2005, 45, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Maejima, T.; Oka, S.; Hashimotodani, Y.; Ohno-Shosaku, T.; Aiba, A.; Wu, D.; Waku, K.; Sugiura, T.; Kano, M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J. Neurosci. 2005, 25, 6826–6835. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Chanda, P.K.; Gao, Y.; Mark, L.; Btesh, J.; Strassle, B.W.; Lu, P.; Piesla, M.J.; Zhang, M.Y.; Bingham, B.; Uveges, A.; et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol. Pharmacol. 2010, 78, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Marrs, W.R.; Blankman, J.L.; Horne, E.A.; Thomazeau, A.; Lin, Y.H.; Coy, J.; Bodor, A.L.; Muccioli, G.G.; Hu, S.S.; Woodruff, G.; et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010, 13, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Huang, S.M.; de Petrocellis, L.; Bisogno, T.; Ewing, S.A.; Miller, J.D.; Zipkin, R.E.; Daddario, N.; Appendino, G.; Di Marzo, V.; et al. Noleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003, 278, 13633–13639. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Petrosino, S.; Di Marzo, V. From endocannabinoid profiling to “endocannabinoid therapeutics”. Curr. Opin. Chem. Biol. 2009, 13, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013, 52, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.; Wortelboer, H.M.; Witkamp, R.F.; Verhoeckx, K.C. Liquid chromatography-tandem mass spectrometry analysis of free and esterified fatty acid N-acyl ethanolamines in plasma and blood cells. Anal. Biochem. 2013, 434, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; de Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J. Cell. Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Wahle, K.W.; Cascio, M.G.; Smoum-Jaouni, R.; Mechoulam, R.; Pertwee, R.G.; Heys, S.D. Omega-3 N-acylethanolamines are endogenously synthesised from omega-3 fatty acidsin different human prostate and breast cancer cell lines. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 305–310. [Google Scholar] [CrossRef]
- Meijerink, J.; Plastina, P.; Vincken, J.P.; Poland, M.; Attya, M.; Balvers, M.; Gruppen, H.; Gabriele, B.; Witkamp, R.F. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: Evidence for a new link between fish oil and inflammation. Br. J. Nutr. 2011, 4, 1–10. [Google Scholar]
- Balvers, M.G.; Verhoeckx, K.C.; Plastina, P.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim. Biophys. Acta 2010, 1801, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.; Manfé, S.; Capuzzo, D.; Gava, S.; Viganò, F.; Coronella, M.L.; Gangemi, M. DHEA pre-treated patients, poor responders to a first IVF (ICSI) cycle: Clinical results. Clin. Exp. Obstet. Gynecol. 2014, 41, 5–9. [Google Scholar] [PubMed]
- Hansen, H.S. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp. Neurol. 2010, 224, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. “Entourage” effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Comelli, F.; Bettoni, I.; Colleoni, M.; Giagnoni, G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: Involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 2008, 139, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Almási, R.; Szoke, E.; Bölcskei, K.; Varga, A.; Riedl, Z.; Sándor, Z.; Szolcsányi, J.; Petho, G. Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci. 2008, 82, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: Pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediat. Inflamm. 2013, 2013, 328797. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, A.; Cerbara, I.; Maccarrone, M.; Topai, A. GPR55: Current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr. Med. Chem. 2010, 17, 1411–1429. [Google Scholar] [CrossRef] [PubMed]
- Overton, H.A.; Fyfe, M.C.; Reynet, C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 2008, 153, S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog. Lipid Res. 2010, 49, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Lucanic, M.; Held, J.M.; Vantipalli, M.C.; Klang, I.M.; Graham, J.B.; Gibson, B.W.; Lithgow, G.J.; Gill, M.S. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 2011, 473, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Wahle, K.W.; Smoum, R.; Mechoulam, R.; Ross, R.A.; Pertwee, R.G.; Heys, S.D. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 2010, 31, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Di Patrizio, N.V.; Piomelli, D. The thrifty lipids: Endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012, 35, 403–411. [Google Scholar]
- Maccarrone, M. Endocannabinoids: Friends and foes of reproduction. Prog. Lipid Res. 2009, 48, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Costa, B.; di Marzo, V. Endocannabinoids: A unique opportunity to develop multitarget analgesics. Pain 2013, 154, S87–S93. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease—Successes and failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar]
- Bisogno, T.; Maccarrone, M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin. Drug Discov. 2013, 8, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, S.; Kaye, W.H.; Cuomo, V.; Piomelli, D. Role of endocannabinoids and their analogues in obesity and eating disorders. Eat. Weight Disord. 2008, 13, e42–e46. [Google Scholar] [PubMed]
- Maccarrone, M.; Bernardi, G.; Agrò, A.F.; Centonze, D. Cannabinoid receptor signalling in neurodegenerative diseases: A potential role for membrane fluidity disturbance. Br. J. Pharmacol. 2011, 163, 1379–1390. [Google Scholar] [CrossRef]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Guy, G.; Di Marzo, V. Care and feeding of the endocannabinoid system: A systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One 2014, 9, e89566. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Petrosino, S. Endocannabinoids and the regulation of their levels in health and disease. Curr. Opin. Lipidol. 2007, 18, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Gutzki, F.M.; Batkai, S.; May, M.; Rakers, C.; Engeli, S.; Jordan, J.; Tsikas, D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: A comprehensive review from an analytical and biological perspective. Biochim. Biophys. Acta 2011, 1811, 706–723. [Google Scholar] [CrossRef]
- Alger, B.E.; Kim, J. Supply and demand for endocannabinoids. Trends Neurosci. 2011, 34, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; de Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef]
- Jung, K.M.; Clapper, J.R.; Fu, J.; D’Agostino, G.; Guijarro, A.; Thongkham, D.; Avanesian, A.; Astarita, G.; Di Patrizio, N.V.; Frontini, A.; et al. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012, 15, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 173–192. [Google Scholar] [CrossRef]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.W.; Christie, M.J. Retrograde signalling by endocannabinoids. Handb. Exp. Pharmacol. 2005, 168, 367–383. [Google Scholar]
- Buczynski, M.W.; Parsons, L.H.B. Quantification of brain endocannabinoid levels: Methods, interpretations and pitfalls. J. Pharmacol. 2010, 160, 423–442. [Google Scholar]
- Maccarrone, M.; Gasperi, V.; Catani, M.V.; Diep, T.A.; Dainese, E.; Hansen, H.S.; Avigliano, L. The endocannabinoid system and its relevance for nutrition. Annu. Rev. Nutr. 2010, 30, 423–440. [Google Scholar] [CrossRef]
- Kleberg, K.; Hassing, H.A.; Hansen, H.S. Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors 2014. [Google Scholar] [CrossRef]
- Engeli, S.; Lehmann, A.C.; Kaminski, J.; Haas, V.; Janke, J.; Zoerner, A.A.; Luft, F.C.; Tsikas, D.; Jordan, J. Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity 2014, 22, E70–E76. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; de Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef]
- Blankman, J.L.; Cravatt, B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013, 65, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; de Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.; Verhoeckx, K.C.; Witkamp, R.F. Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 14–15. [Google Scholar] [CrossRef]
- Fezza, F.; Bisogno, T.; Minassi, A.; Appendino, G.; Mechoulam, R.; Di Marzo, V. Noladin ether, a putative novel endocannabinoid: Inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002, 513, 294–298. [Google Scholar]
- Sun, Y.; Alexander, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoids and PPARalpha signalling. Biochem. Soc. Trans. 2006, 34, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Alexander, S.P.; Garle, M.J. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br. J. Pharmacol. 2007, 152, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Console-Bram, L.; Mundy, C.; Popoff, S.N.; Kapur, A.; Abood, M.E. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J. Neuroimmune Pharmacol. 2012, 7, 856–865. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Di Marzo, V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium 2009, 45, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Bradshaw, H.B.; Benton, V.M.; Chen, J.S.; Huang, S.M.; Minassi, A.; Bisogno, T.; Masuda, K.; Tan, B.; Roskoski, R., Jr.; et al. The biosynthesis of N-arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 291–301. [Google Scholar] [CrossRef]
- Lehtonen, M.; Storvik, M.; Malinen, H.; Hyytiä, P.; Lakso, M.; Auriola, S.; Wong, G.; Callaway, J.C. Determination of endocannabinoids in nematodes and human brain tissue by liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 677–694. [Google Scholar] [CrossRef]
- Bystrowska, B.; Smaga, I.; Tyszka-Czochara, M.; Filip, M. Troubleshooting in LC-MS/MS method for determining endocannabinoid and endocannabinoid-like molecules in rat brain structures applied to assessing the brain endocannabinoid/endovanilloid system significance. Toxicol. Mech. Methods 2014, 24, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.O.; Burr, M.M. Nutrition classics from The Journal of Biological Chemistry 82:345-67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr. Rev. 1973, 31, 248–249. [Google Scholar] [PubMed]
- Barrett, S.J. The role of omega-3 polyunsaturated fatty acids in cardiovascular health. Altern. Ther. Health Med. 2013, 1, 26–30. [Google Scholar]
- Maskrey, B.H.; Megson, I.L.; Rossi, A.G.; Whitfield, P.D. Emerging importance of omega-3 fatty acids in the innate immune response: Molecular mechanisms and lipidomic strategies for their analysis. Mol. Nutr. Food Res. 2013, 57, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Peskin, B.S. Why fish oil fails: A comprehensive 21st century lipids-based physiologic analysis. J. Lipids 2014, 2014, 495761. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lázaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar] [PubMed]
- Park, C.K.; Xu, Z.Z.; Liu, T.; Serhan, C.N.; Ji, R.R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: Distinct roles of resolvin D1, D2, and E1. J. Neurosci. 2011, 31, 18433–18438. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Schwarze, J. The role of pro-resolution lipid mediators in infectious disease. Immunology 2014, 141, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Russo, E.; Scicchitano, F.; van Rijn, C.M.; Cosco, D.; Avagliano, C.; Russo, R.; D’Agostino, G.; Petrosino, S.; Guida, F.; et al. Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-α receptor activation in a genetic model of absence epilepsy. Neuropharmacology 2013, 69, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Impellizzeri, D.; Mazzon, E.; Paterniti, I.; Cuzzocrea, S. Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson’s disease. PLoS One 2012, 7, e41880. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Bottegoni, G.; Sasso, O.; Bertorelli, R.; Rocchia, W.; Masetti, M.; Guijarro, A.; Lodola, A.; Armirotti, A.; Garau, G.; et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci. 2011, 15, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Lo Verme, J.; Fu, J.; Oveisi, F.; Blázquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef] [PubMed]
- Suardíaz, M.; Estivill-Torrús, G.; Goicoechea, C.; Bilbao, A.; Rodríguez de Fonseca, F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 2007, 133, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R.; McKillop, A.M. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol. Chem. 2014, 395, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Rosenkilde, M.M.; Holst, J.J.; Schwartz, T.W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 2012, 33, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Vassileva, G.; Corona, A.; Liu, L.; Baker, H.; Golovko, A.; Abbondanzo, S.J.; Hu, W.; Yang, S.; Ning, Y.; et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J. Endocrinol. 2009, 201, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Cartoni, A.; Parolaro, D.; Margonelli, A.; Massi, P.; Bari, M.; Battista, N.; Finazzi-Agrò, A. Cannabimimetic activity, binding, and degradation of stearoylethanolamide within the mouse central nervous system. Mol. Cell. Neurosci. 2002, 21, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, M.; Del Giudice, E.; Stecca, A.; Colavito, D.; Fabris, M.; D’Arrigo, A.; Bernardini, D.; Dam, M.; Leon, A. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: A neglected story. J. Neuroendocrinol. 2008, 1, 26–34. [Google Scholar] [CrossRef]
- Ghafouri, N.; Ghafouri, B.; Larsson, B.; Stensson, N.; Fowler, C.J.; Gerdle, B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 2013, 154, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wright, R.; Kirchhoff, A.M.; Chester, J.A.; Cooper, B.R.; Davisson, V.J.; Barker, E. Quantitative LC-MS/MS analysis of arachidonoyl amino acids in mouse brain with treatment of FAAH inhibitor. Anal. Biochem. 2013, 432, 74–81. [Google Scholar]
- Ottria, R.; Ravelli, A.; Gigli, F.; Ciuffreda, P. Simultaneous ultra-high performance liquid chromathograpy-electrospray ionization-quadrupole-time of flight mass spectrometry quantification of endogenous anandamide and related N-acylethanolamides in bio-matrices. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 958, 83–89. [Google Scholar] [CrossRef]
- Terrazzino, S.; Berto, F.; Dalle Carbonare, M.; Fabris, M.; Guiotto, A.; Bernardini, D.; Leon, A. Stearoylethanolamide exerts anorexic effects in mice via down-regulation of liver stearoyl-coenzyme A desaturase-1 mRNA expression. FASEB J. 2004, 18, 1580–1582. [Google Scholar] [PubMed]
- Huang, S.M.; Bisogno, T.; Petros, T.J.; Chang, S.Y.; Zavitsanos, P.A.; Zipkin, R.E.; Sivakumar, R.; Coop, A.; Maeda, D.Y.; De Petrocellis, L.; et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001, 276, 42639–42644. [Google Scholar] [CrossRef] [PubMed]
- Milman, G.; Maor, Y.; Abu-Lafi, S.; Horowitz, M.; Gallily, R.; Batkai, S.; Mo, F.M.; Offertaler, L.; Pacher, P.; Kunos, G.; et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. USA 2006, 103, 2428–2433. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.; Shohami, E.; Bab, I.; Mechoulam, R. N-Acyl amino acids and their impact on biological processes. Biofactors 2014. [Google Scholar] [CrossRef]
- Kohno, M.; Hasegawa, H.; Inoue, A.; Muraoka, M.; Miyazaki, T.; Oka, K.; Yasukawa, M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006, 347, 827–832. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Roskowski, D.; Xie, S.; Bradshaw, H.B. Δ(9)-THC and N-arachidonoyl glycine regulate BV-2 microglial morphology and cytokine release plasticity: Implications for signaling at GPR18. Front. Pharmacol. 2014, 4, 162. [Google Scholar] [CrossRef] [PubMed]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Yoon, J.M.; Moon, M.J.; Hwang, J.I.; Choe, H.; Lee, J.Y.; Kim, J.I.; Kim, S.; Rhim, H.; O’Dell, D.K.; et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J. Biol. Chem. 2008, 283, 21054–21064. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.G.; Minassi, A.; Ligresti, A.; Appendino, G.; Burstein, S.; Di Marzo, V. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 2004, 314, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Yeshurun, A.; Willner, D.; Trembovler, V.; Alexandrovich, A.; Mechoulam, R.; Shohami, E.; Leker, R.R. N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.; Hekker, T.A. Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: A case series. J. Pain Res. 2012, 5, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Ligresti, A.; Morera, E.; Nalli, M.; Ortar, G. The anandamide membrane transporter. Structure-activity relationships of anandamide and oleoylethanolamine analogs with phenyl rings in the polar head group region. Bioorg. Med. Chem. 2004, 12, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Min, R.; di Marzo, V.; Mansvelder, H.D. DAG lipase involvement in depolarization-induced suppression of inhibition: Does endocannabinoid biosynthesis always meet the demand? Neuroscientist 2010, 16, 608–613. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Okamoto, Y.; Ikematsu, N.; Inoue, M.; Shimizu, Y.; Uyama, T.; Wang, J.; Deutsch, D.G.; Burns, M.P.; Ulloa, N.M.; et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim. Biophys. Acta 2011, 1811, 565–577. [Google Scholar] [CrossRef]
- Fukami, K.; Inanobe, S.; Kanemaru, K.; Nakamura, Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res. 2010, 49, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.V.; Schmid, P.C.; Krebsbach, R.J.; Schmid, H.H. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J. 2001, 15, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Yanagimoto, S.; Ikeda, S.; Gokoh, M.; Kishimoto, S.; Waku, K.; Ishima, Y.; Sugiura, T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J. Biol. Chem. 2005, 280, 18488–18497. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Vasilyev, D.V.; Goncalves, M.B.; Howell, F.V.; Hobbs, C.; Reisenberg, M.; Shen, R.; Zhang, M.Y.; Strassle, B.W.; Lu, P.; et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 2010, 30, 2017–2024. [Google Scholar]
- Tanimura, A.; Yamazaki, M.; Hashimotodani, Y.; Uchigashima, M.; Kawata, S.; Abe, M.; Kita, Y.; Hashimoto, K.; Shimizu, T.; Watanabe, M.; et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 2010, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Rossi, S.; Bari, M.; de Chiara, V.; Fezza, F.; Musella, A.; Gasperi, V.; Prosperetti, C.; Bernardi, G.; Finazzi-Agrò, A.; et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 2008, 11, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Shonesy, B.C.; Wang, X.; Rose, K.L.; Ramikie, T.S.; Cavener, V.S.; Rentz, T.; Baucum, A.J.; Jalan-Sakrikar, N.; Mackie, K.; Winder, D.G.; et al. CaMKII regulates diacylglycerol lipase-α and striatal endocannabinoid signaling. Nat. Neurosci. 2013, 16, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Jeske, N.A.; Flores, C.M.; Hargreaves, K.M. Pharmacological interactions between calcium/calmodulin-dependent kinase II alpha and TRPV1 receptors in rat trigeminal sensory neurons. Neurosci. Lett. 2005, 389, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; Oddi, S.; Di Tommaso, M.; de Simone, C.; Rapino, C.; Pasquariello, N.; Dainese, E.; Finazzi-Agrò, A.; Maccarrone, M. Characterization of biotin-anandamide, a novel tool for the visualization of anandamide accumulation. J. Lipid Res. 2008, 49, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.; Jarrahian, A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem. Phys. Lipids 2000, 108, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013, 3280, 1895–1904. [Google Scholar] [CrossRef]
- Deutsch, D.G.; Glaser, S.T.; Howell, J.M.; Kunz, J.S.; Puffenbarger, R.A.; Hillard, C.J.; Abumrad, N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J. Biol. Chem. 2001, 276, 6967–6973. [Google Scholar]
- Kaczocha, M.; Hermann, A.; Glaser, S.T.; Bojesen, I.N.; Deutsch, D.G. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J. Biol. Chem. 2006, 281, 9066–9075. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Fezza, F.; Pasquariello, N.; de Simone, C.; Rapino, C.; Dainese, E.; Finazzi-Agrò, A.; Maccarrone, M. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell. Mol. Life Sci. 2008, 65, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, E.; Chahinian, H.; Sanchez, P.; Fantini, J. The insertion and transport of anandamide in synthetic lipid membranes are both cholesterol-dependent. PLoS One 2009, 4, e4989. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Fezza, F.; Pasquariello, N.; D’Agostino, A.; Catanzaro, G.; De Simone, C.; Rapino, C.; Finazzi-Agrò, A.; Maccarrone, M. Molecular identification of albumin and Hsp70 as cytosolic 12 anandamide-binding proteins. Chem. Biol. 2009, 16, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Vivieca, S.; Sun, J.; Glaser, S.T.; Deutsch, D.G. Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J. Biol. Chem. 2012, 287, 3415–3424. [Google Scholar]
- Chicca, A.; Marazzi, J.; Nicolussi, S.; Gertsch, J. Evidence for bidirectional endocannabinoid transport across cell membranes. J. Biol. Chem. 2012, 287, 34660–34682. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bari, M.; Battista, N.; Finazzi-Agrò, A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood 2002, 100, 4040–4048. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Jarrahian, A. Accumulation of anandamide: Evidence for cellular diversity. Neuropharmacology 2005, 48, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Beltramo, M.; Glasnapp, S.; Lin, S.Y.; Goutopoulos, A.; Xie, X.Q.; Makriyannis, A. Structural determinants for recognition and translocation by the anandamide transporter. Proc. Natl. Acad. Sci. USA 1999, 96, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Maccarrone, M.; de Petrocellis, L.; Jarrahian, A.; Finazzi-Agrò, A.; Hillard, C.; di Marzo, V. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur. J. Biochem. 2001, 268, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Kaczocha, M.; Deutsch, D.G. 2-Arachidonoylglycerol (2-AG) membrane transport: History and outlook. AAPS J. 2006, 8, 409–412. [Google Scholar] [CrossRef]
- Ehehalt, R.; Füllekrug, J.; Pohl, J.; Ring, A.; Herrmann, T.; Stremmel, W. Translocation of long chain fatty acids across the plasma membrane—Lipid rafts and fatty acid transport proteins. Mol. Cell. Biochem. 2006, 284, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Fezza, F.; de Simone, C.; Amadio, D.; Maccarrone, M. Fatty acid amide hydrolase: A gate-keeper of the endocannabinoid system. Subcell. Biochem. 2008, 49, 101–132. [Google Scholar] [PubMed]
- McKinney, M.K.; Cravatt, B.F. Evidence for distinct roles in catalysis for residues of the serine-serine-lysine catalytic triad of fatty acid amide hydrolase. J. Biol. Chem. 2003, 278, 37393–37399. [Google Scholar] [CrossRef]
- Kaczocha, M.; Glaser, S.T.; Chae, J.; Brown, D.A.; Deutsch, D.G. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J. Biol. Chem. 2010, 285, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Ponzano, S.; Bertozzi, F.; Mengatto, L.; Dionisi, M.; Armirotti, A.; Romeo, E.; Berteotti, A.; Fiorelli, C.; Tarozzo, G.; Reggiani, A.; et al. Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J. Med. Chem. 2013, 56, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.; Ottonello, G.; Petracca, R.; Bertozzi, S.M.; Ponzano, S.; Armirotti, A.; Berteotti, A.; Dionisi, M.; Cavalli, A.; Piomelli, D.; et al. Synthesis, structure-activity, and structure-stability relationships of 2-substituted-N-(4-oxo-3-oxetanyl) N-acylethanolamine acid amidase (NAAA) inhibitors. Chem. Med. Chem. 2014, 9, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, W.; Zhong, P.; Blankman, J.L.; Cravatt, B.F.; Liu, Q.S. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J. Neurosci. 2011, 31, 13420–13430. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Maccarrone, M. FAAH and anandamide: Is 2-AG really the odd one out? Trends Pharmacol. Sci. 2008, 29, 229–233. [Google Scholar]
- Long, J.Z.; Li, W.; Booker, L.; Burston, J.J.; Kinsey, S.G.; Schlosburg, J.E.; Pavón, F.J.; Serrano, A.M.; Selley, D.E.; Parsons, L.H.; et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Delgado, L.; Storch, J. Monoacylglycerol metabolism in human intestinal Caco-2 cells: Evidence for metabolic compartmentation and hydrolysis. J. Biol. Chem. 2002, 277, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Contreras, J.A.; Hellman, U.; Tornqvist, H.; Holm, C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. Biol. Chem. 1997, 272, 27218–27223. [Google Scholar] [CrossRef]
- Karlsson, M.; Reue, K.; Xia, Y.R.; Lusis, A.J.; Langin, D.; Tornqvist, H.; Holm, C. Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene 2001, 272, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.W.; Cravatt, B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.G.; Multhaupt, H.A.; Carrara, M.; de Lichtenberg, K.H.; Christensen, I.B.; Linnemann, D.; Santoni-Rugiu, E.; Calogero, R.A.; Lund, A.H. Prdm5 suppresses Apc(Min)-driven intestinal adenomas and regulates monoacylglycerol lipase expression. Oncogene 2014, 33, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Ruan, Z.H. The Role of Monoacylglycerol Lipase (MAGL) in the Cancer Progress. Cell Biochem. Biophys. 2014, 70, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2012, 204, 267–276. [Google Scholar] [CrossRef]
- Tchantchou, F.; Zhang, Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 2013, 30, 565–79. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Masquelier, J.; Cani, P.D.; Lambert, D.M.; Muccioli, G.G. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc. Natl. Acad. Sci. USA 2013, 110, 17558–17563. [Google Scholar] [CrossRef] [PubMed]
- Fiskerstrand, T.; H’mida-Ben Brahim, D.; Johansson, S.; M’zahem, A.; Haukanes, B.I.; Drouot, N.; Zimmermann, J.; Cole, A.J.; Vedeler, C.; Bredrup, C.; et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 2010, 87, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Long, J.Z.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 2013, 110, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.R.; Marnett, L.J. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 211–220. [Google Scholar] [CrossRef]
- McHugh, D.; McMaster, R.S.; Pertwee, R.G.; Roy, S.; Mahadevan, A.; Razdan, R.K.; Ross, R.A. Novel compounds that interact with both leukotriene B4 receptors and vanilloid TRPV1 receptors. J. Pharmacol. Exp. Ther. 2006, 316, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Przewlocka, B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3286–3299. [Google Scholar] [CrossRef] [PubMed]
- Amadio, D.; Fezza, F.; Catanzaro, G.; Incani, O.; van Zadelhoff, G.; Finazzi Agrò, A.; Maccarrone, M. Methylation and acetylation of 15-hydroxyanandamide modulate its interaction with the endocannabinoid system. Biochimie 2010, 92, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Dainese, E.; Sabatucci, A.; Angelucci, C.B.; Barsacchi, D.; Chiarini, M.; Maccarrone, M. Impact of embedded endocannabinoids and their oxygenation by lipoxygenase on membraneproperties. ACS Chem. Neurosci. 2012, 3, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fredman, G.; Krishnamoorthy, S.; Agrawal, N.; Irimia, D.; Piomelli, D.; Serhan, C.N. Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J. Biol. Chem. 2011, 286, 31532–31541. [Google Scholar] [CrossRef] [PubMed]
- Prusakiewicz, J.J.; Turman, M.V.; Vila, A.; Ball, H.L.; Al-Mestarihi, A.H.; Di Marzo, V.; Marnett, L.J. Oxidative metabolism of lipoamino acids and vanilloids by lipoxygenases and cyclooxygenases. Arch. Biochem. Biophys. 2007, 464, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Turman, M.V.; Kingsley, P.J.; Rouzer, C.A.; Cravatt, B.F.; Marnett, L.J. Oxidative metabolism of a fatty acid amide hydrolase-regulated lipid, arachidonoyltaurine. Biochemistry 2008, 47, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Muccioli, G.G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Carling, R.W.; Cornell, C.L.; Fliri, H.G.; Martos, J.L.; Pettit, S.N.; Liang, Y.; Wang, J.W. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 2008, 120, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Duggan, K.C.; Hermanson, D.J.; Musee, J.; Prusakiewicz, J.J.; Scheib, J.L.; Carter, B.D.; Banerjee, S.; Oates, J.A.; Marnett, L.J. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 2011, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Prusakiewicz, J.J.; Duggan, K.C.; Rouzer, C.A.; Marnett, L.J. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry 2009, 48, 7353–7355. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Piscitelli, F.; Giordano, C.; Boccella, S.; Lichtman, A.; Maione, S.; Di Marzo, V. Discovery of prostamide F2α and its role in inflammatory pain and dorsal horn nociceptive neuron hyperexcitability. PLoS One 2012, 7, e31111. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Wang, J.W.; Poloso, N.J. Recent progress in prostaglandin F2α ethanolamide (prostamide F2α) research and therapeutics. Pharmacol. Rev. 2013, 65, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Nast, J.A.; Tesmer, L.A.; Hollenberg, P.F. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol. Pharmacol. 2009, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Dostalek, M.; Guengerich, F.P. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS J. 2008, 275, 3706–3717. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, W.; Long, J.Z.; Sun, D.; Hillard, C.J.; Cravatt, B.F.; Liu, Q.S. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J. Pharmacol. Exp. Ther. 2009, 331, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoidsystem. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078-17106. https://doi.org/10.3390/molecules191117078
Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules. 2014; 19(11):17078-17106. https://doi.org/10.3390/molecules191117078
Chicago/Turabian StyleFezza, Filomena, Monica Bari, Rita Florio, Emanuela Talamonti, Monica Feole, and Mauro Maccarrone. 2014. "Endocannabinoids, Related Compounds and Their Metabolic Routes" Molecules 19, no. 11: 17078-17106. https://doi.org/10.3390/molecules191117078
APA StyleFezza, F., Bari, M., Florio, R., Talamonti, E., Feole, M., & Maccarrone, M. (2014). Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules, 19(11), 17078-17106. https://doi.org/10.3390/molecules191117078





