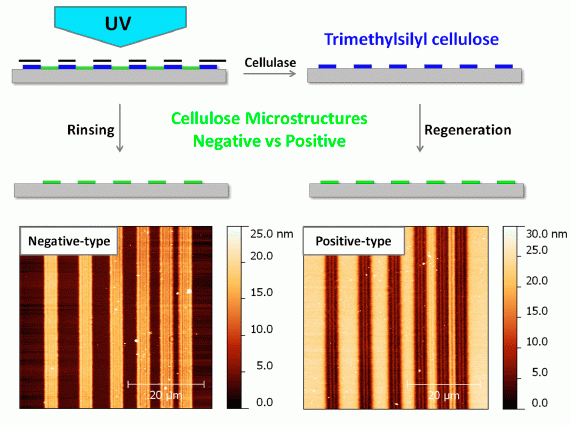
Photoregeneration of Trimethylsilyl Cellulose as a Tool for Microstructuring Ultrathin Cellulose Supports
Abstract
:1. Introduction
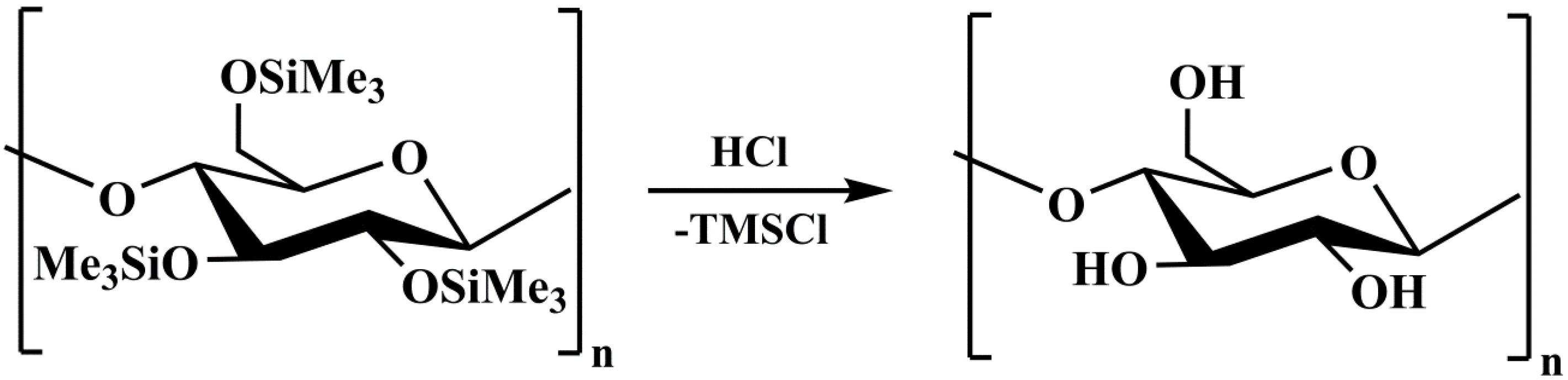
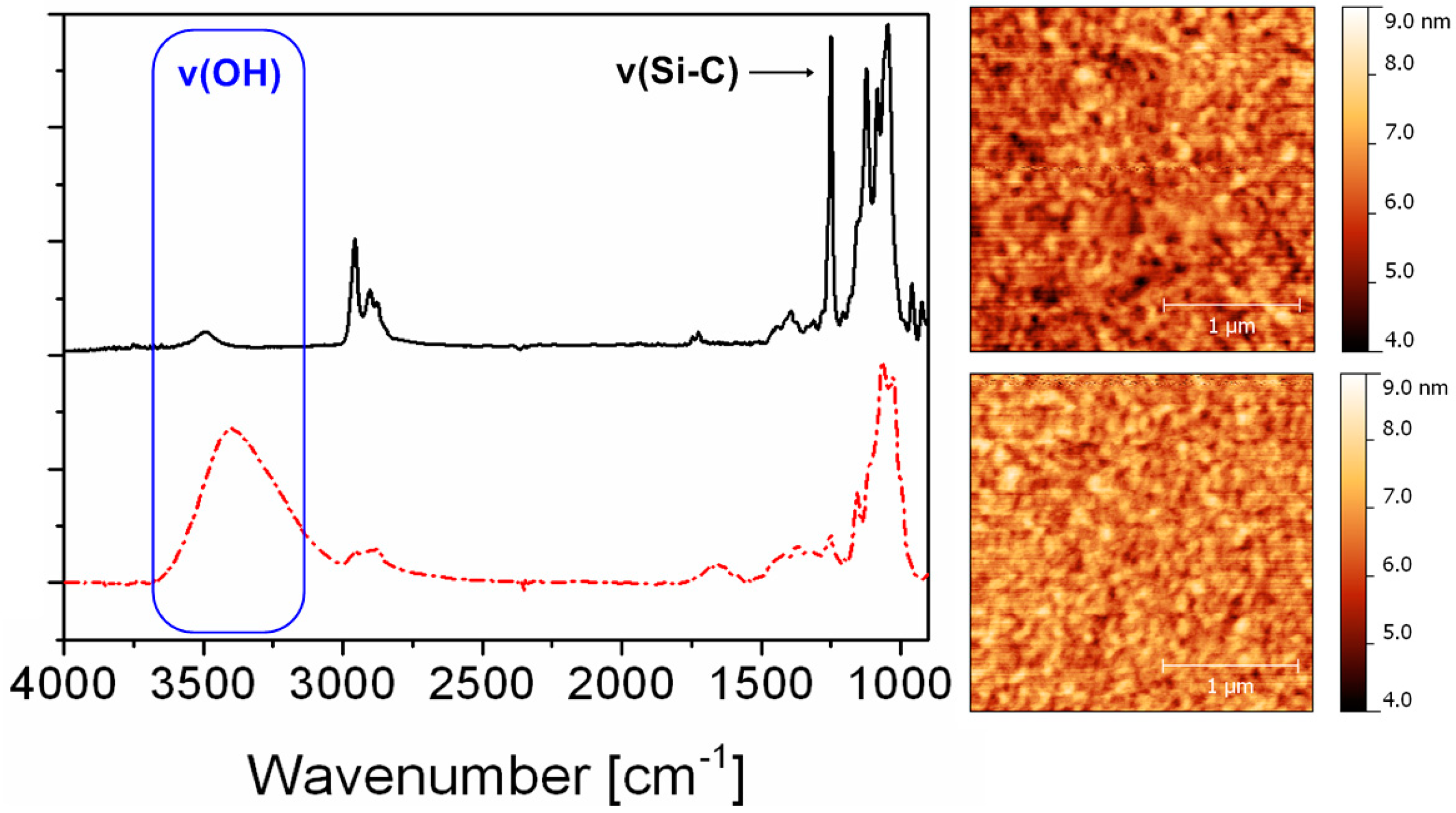
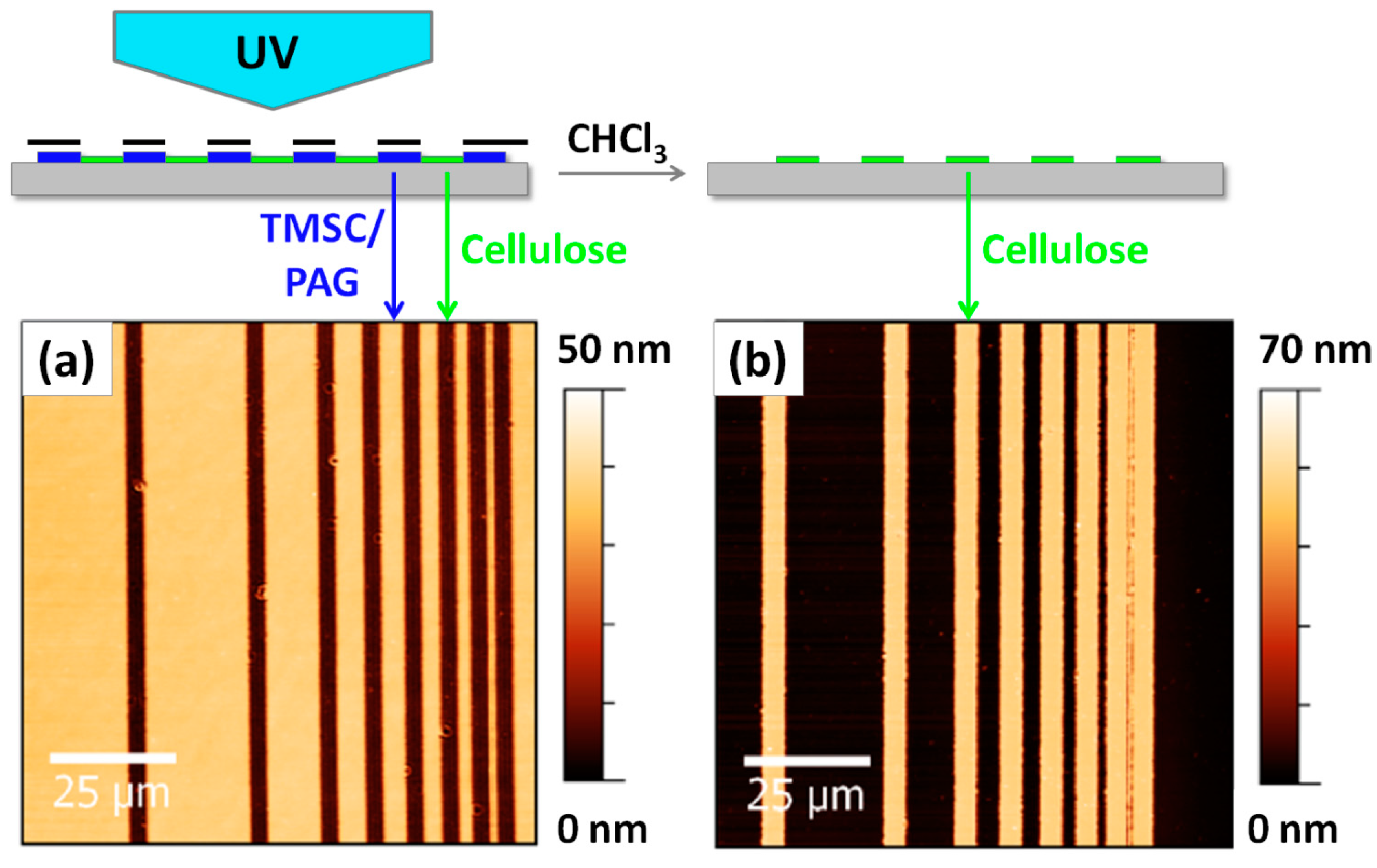
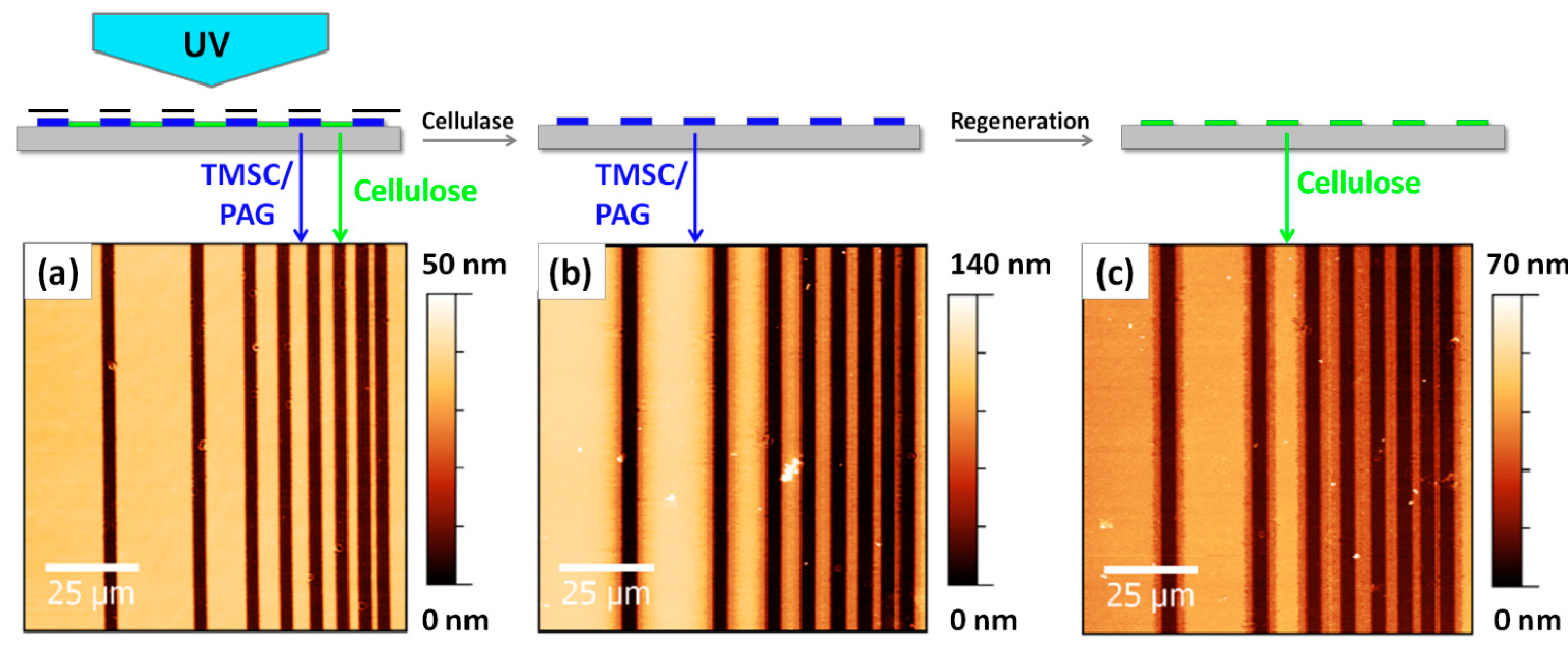
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Sample Preparation
3.3. Photoregeneration
3.4. FTIR Spectroscopy
3.5. Atomic Force Microscopy
3.6. Photopatterning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry: Fundamentals and Analytical Methods; Wiley-VCH Weinheim: Weinheim, Germany, 2004; Volume 1. [Google Scholar]
- Wyss, G.D. The ripening of viscose. Ind. Eng. Chem. 1925, 17, 1043–1045. [Google Scholar] [CrossRef]
- Greber, G.; Paschinger, O. Silylderivate der cellulose. Lenz. Ber. 1983, 55, 20–25. [Google Scholar]
- Cooper, G.K.; Sandberg, K.R.; Hinck, J.F. Trimethylsilyl cellulose as precursor for regenerated cellulose fiber. J. Appl. Polym. Sci. 1981, 26, 3827–3836. [Google Scholar] [CrossRef]
- Schaub, M.; Wenz, G.; Wegner, G.; Stein, A.; Klemm, D. Ultrathin films of cellulose on silicon wafers. Adv. Mat. 1993, 5, 919–922. [Google Scholar] [CrossRef]
- Kontturi, E.; Thune, P.C.; Niemantsverdriet, J.W. Novel method for preparing cellulose model surfaces by spin coating. Polymer 2003, 44, 3621–3625. [Google Scholar] [CrossRef]
- Kontturi, E.; Suchy, M.; Penttilä, P.; Jean, B.; Pirkkalainen, K.; Torkkeli, M.; Serimaa, R. Amorphous characteristics of an ultrathin cellulose film. Biomacromolecules 2011, 12, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliska, A.; Vesel, A.; Salzmann, I.; Resel, R.; Ribitsch, V.; Stana-Kleinschek, K. Exploring the rearrangement of amorphous cellulose model thin films upon heat treatment. Soft Matter 2012, 8, 9807–9815. [Google Scholar] [CrossRef]
- Mohan, T.; Zarth, C.; Doliska, A.; Kargl, R.; Grießer, T.; Spirk, S.; Heinze, T.; Stana-Kleinschek, K. Interactions of a cationic cellulose derivative with an ultrathin cellulose support. Carbohydr. Polym. 2013, 92, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Köhnke, T.; Östlund, Å.; Brelid, H. Adsorption of arabinoxylan on cellulosic surfaces: Influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 2011, 12, 2633–2641. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Bracic, M.; Kulterer, M.; Doliska, A.; Stana-Kleinschek, K.; Ribitsch, V. Adsorption of carboxymethyl cellulose on polymer surfaces: Evidence of a specific interaction with cellulose. Langmuir 2012, 28, 11440–11447. [Google Scholar] [CrossRef] [PubMed]
- Filpponen, I.; Kontturi, E.; Nummelin, S.; Rosilo, H.; Kolehmainen, E.; Ikkala, O.; Laine, J. Generic method for modular surface modification of cellulosic materials in aqueous medium by sequential “Click” reaction and adsorption. Biomacromolecules 2012, 13, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Laine, J.; Rojas, O.J. Modification of cellulose films by adsorption of CMC and chitosan for controlled attachment of biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Johansson, L.-S.; Filpponen, I.; Rojas, O.J.; Laine, J. Generic method for attaching biomolecules via avidin-biotin complexes immobilized on films of regenerated and nanofibrillar cellulose. Biomacromolecules 2012, 13, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Teerinen, T.; Johansson, L.-S.; Holappa, S.; Laine, J. CMC-Modified cellulose biointerface for antibody conjugation. Biomacromolecules 2012, 13, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Löscher, F.; Ruckstuhl, T.; Jaworek, T.; Wegner, G.; Seeger, S. Immobilization of biomolecules on Langmuir−Blodgett films of regenerative cellulose derivatives. Langmuir 1998, 14, 2786–2789. [Google Scholar] [CrossRef]
- Mohan, T.; Ristic, T.; Kargl, R.; Doliska, A.; Köstler, S.; Ribitsch, V.; Marn, J.; Spirk, S.; Stana-Kleinschek, K. Cationically rendered biopolymer surfaces for high protein affinity support matrices. Chem. Commun. 2013, 49, 11530–11532. [Google Scholar] [CrossRef]
- Rohm, S.; Hirn, U.; Ganser, C.; Teichert, C.; Schennach, R. Thin cellulose films as a model system for paper fibre bonds. Cellulose 2014, 21, 237–249. [Google Scholar] [CrossRef]
- Petritz, A.; Wolfberger, A.; Fian, A.; Irimia-Vladu, M.; Haase, A.; Gold, H.; Rothländer, T.; Griesser, T.; Stadlober, B. Cellulose as biodegradable high-k dielectric layer in organic complementary inverters. Appl. Phys. Lett. 2013, 103, 153303. [Google Scholar] [CrossRef]
- Spirk, S.; Ehmann, H.; Kargl, R.; Hurkes, N.; Reischl, M.; Novak, J.; Resel, R.; Wu, M.; Pietschnig, R.; Ribitsch, V. Surface modifications using a water-stable silanetriol in neutral aqueous media. ACS Appl. Mater. Interfaces 2010, 2, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Bračič, M.; Mohan, T.; Kargl, R.; Griesser, T.; Hribernik, S.; Köstler, S.; Stana-Kleinschek, K.; Fras-Zemljič, L. Preparation of PDMS ultrathin films and patterned surface modification with cellulose. RSC Adv. 2014, 4, 11955–11961. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Köstler, S.; Spirk, S.; Doliska, A.; Stana-Kleinschek, K.; Ribitsch, V. Functional patterning of biopolymer thin films using enzymes and lithographic methods. Adv. Funct. Mat. 2013, 23, 308–315. [Google Scholar] [CrossRef]
- Kontturi, E.; Lankinen, A. Following the kinetics of a chemical reaction in ultrathin supported polymer films by reliable mass density determination with X-ray reflectivity. J. Am. Chem. Soc. 2010, 132, 3678–3679. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Ehmann, H.M.A.; Köstler, S.; Ribitsch, V.; Stana-Kleinschek, K. Watching cellulose grow—Kinetic investigations on cellulose thin film formation at the gas–solid interface using a quartz crystal microbalance with dissipation (QCM-D). Colloids Surf. A 2012, 400, 67–72. [Google Scholar] [CrossRef]
- Woods, D.A.; Petkov, J.; Bain, C.D. Surfactant adsorption by total internal reflection Raman spectroscopy. Part III: Adsorption onto cellulose. Colloids Surf. A 2011, 391, 10–18. [Google Scholar] [CrossRef]
- Tanaka, M.; Wong, A.P.; Rehfeldt, F.; Tutus, M.; Kaufmann, S. Selective deposition of native cell membranes on biocompatible micropatterns. J. Am. Chem. Soc. 2004, 126, 3257–3260. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfberger, A.; Kargl, R.; Griesser, T.; Spirk, S. Photoregeneration of Trimethylsilyl Cellulose as a Tool for Microstructuring Ultrathin Cellulose Supports. Molecules 2014, 19, 16266-16273. https://doi.org/10.3390/molecules191016266
Wolfberger A, Kargl R, Griesser T, Spirk S. Photoregeneration of Trimethylsilyl Cellulose as a Tool for Microstructuring Ultrathin Cellulose Supports. Molecules. 2014; 19(10):16266-16273. https://doi.org/10.3390/molecules191016266
Chicago/Turabian StyleWolfberger, Archim, Rupert Kargl, Thomas Griesser, and Stefan Spirk. 2014. "Photoregeneration of Trimethylsilyl Cellulose as a Tool for Microstructuring Ultrathin Cellulose Supports" Molecules 19, no. 10: 16266-16273. https://doi.org/10.3390/molecules191016266
APA StyleWolfberger, A., Kargl, R., Griesser, T., & Spirk, S. (2014). Photoregeneration of Trimethylsilyl Cellulose as a Tool for Microstructuring Ultrathin Cellulose Supports. Molecules, 19(10), 16266-16273. https://doi.org/10.3390/molecules191016266