Ethanolic Extract of Taheebo Attenuates Increase in Body Weight and Fatty Liver in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of TBE on Body Weight and Fat Tissue Weight and Serum Biochemical Parameters
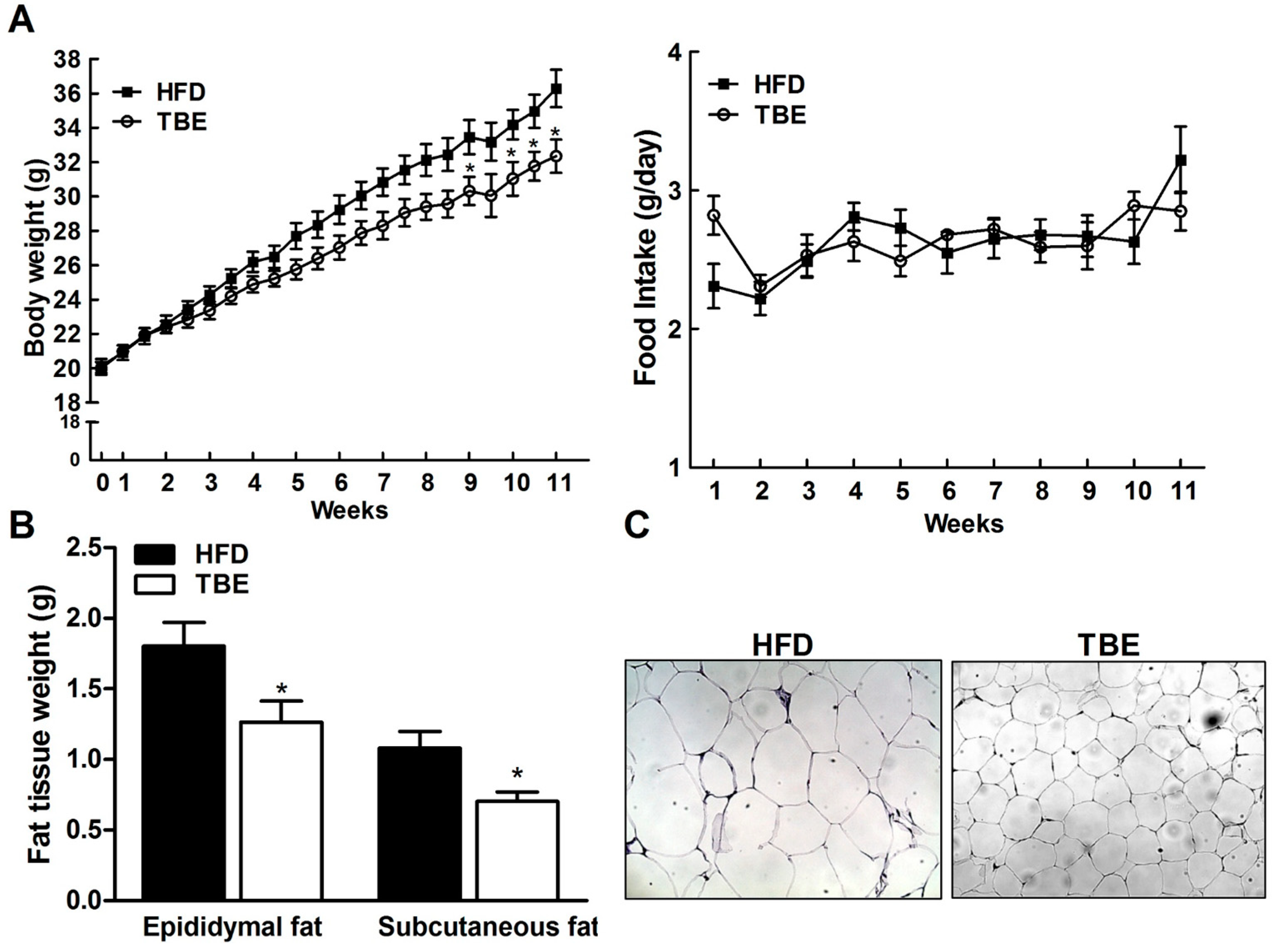
| Marker | HFD | TBE |
|---|---|---|
| Triglyceride (mg/mL) | 68.96 ± 5.14 | 55.11 ± 1.69 * |
| Total cholesterol (mg/mL) | 146.8 ± 7.92 | 134.4 ± 5.27 |
| HDL-cholesterol (mg/mL) | 63.12 ± 10.00 | 66.46 ± 6.69 |
| Insulin (ng/mL) | 1.73 ± 0.28 | 0.83 ± 0.15 * |
| Leptin (ng/mL) | 15880 ± 2803 | 6829 ± 1420 * |
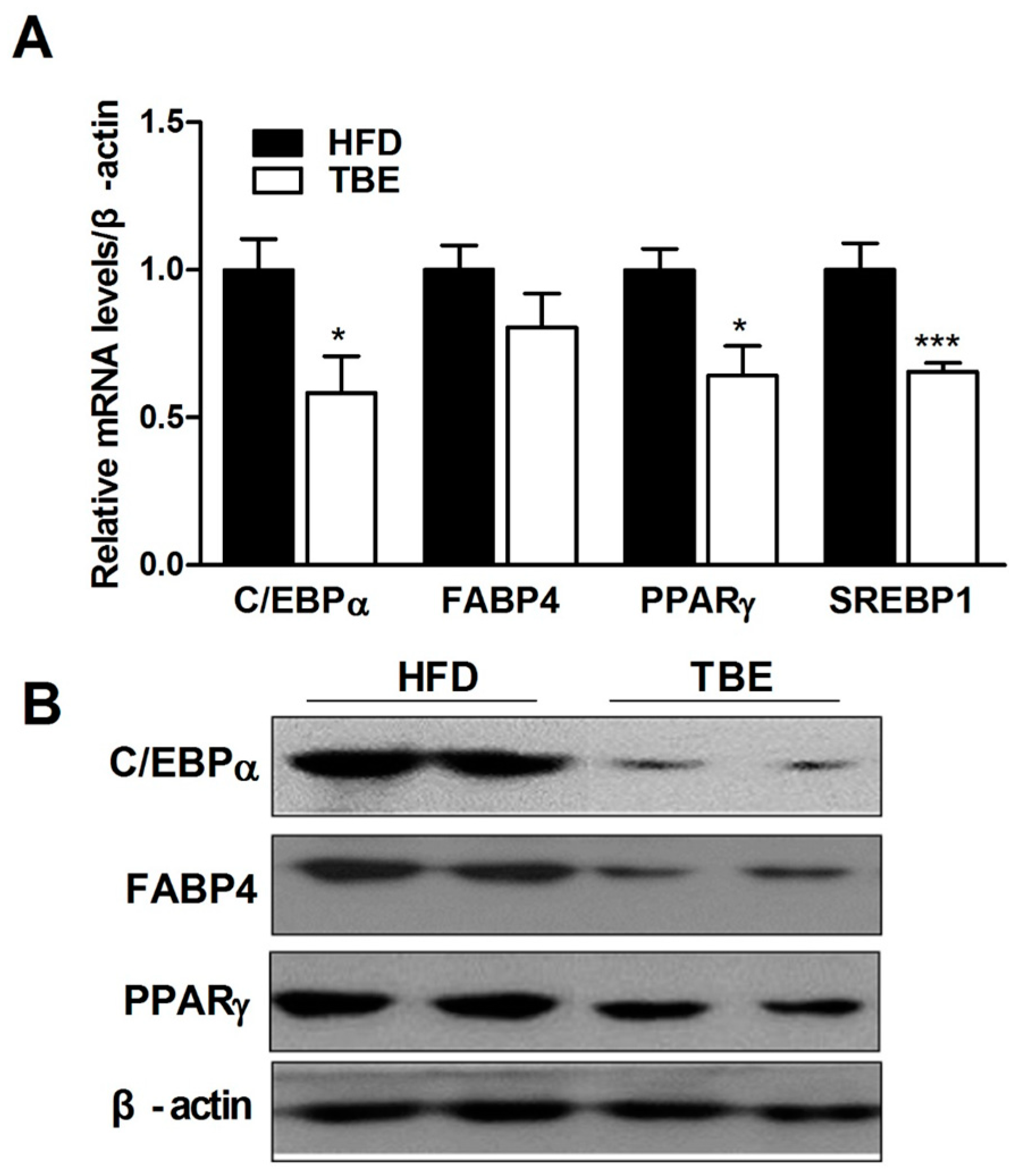
2.2. Effect of TBE on the Expression of Adipogenic Genes in the White Adipose Tissue (WAT)
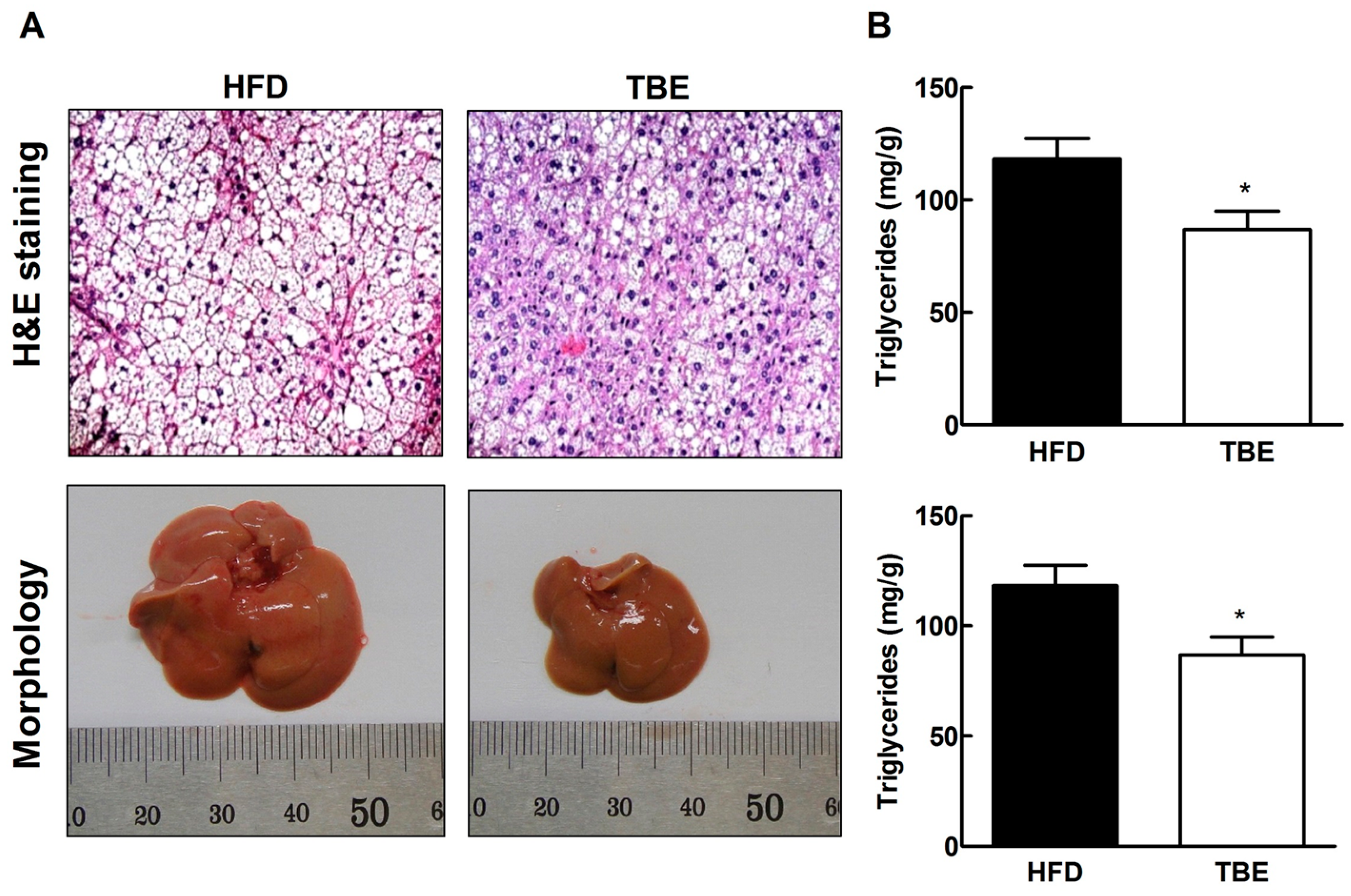
2.3. Effect of TBE on Lipid Content and Expression of Lipogenic Genes in the Live
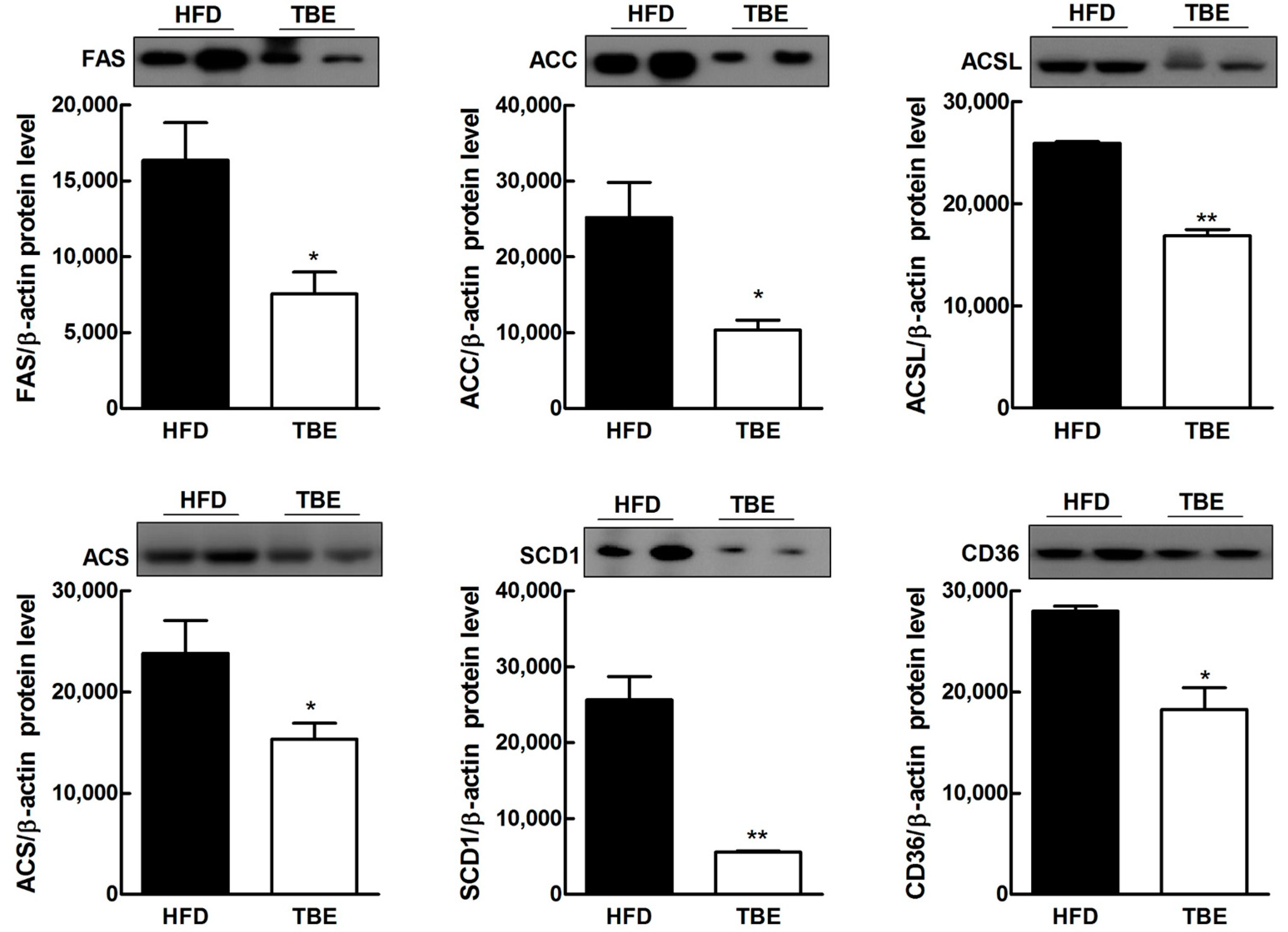
| Genes | Relative mRNA Levels | |
|---|---|---|
| HFD | TBE | |
| ACSL | 1.00 ± 0.123 | 0.8237 ± 0.084 |
| ACS | 1.00 ± 0.036 | 0.7346 ± 0.015 ** |
| GPAM | 1.00 ± 0.723 | 0.9089 ± 0.101 |
| FAS | 1.00 ± 0.063 | 0.7141 ± 0.044 * |
| SCD1 | 1.00 ± 0.057 | 0.6109 ± 0.0742 ** |
2.4. Discussion
3. Experimental Section
3.1. Preparation of TBE
3.2. Animal Models
3.3. Tissue Analysis
3.4. Histopathological Evaluation
3.5. qRT-PCR Analysis
| Primer | Direction | Sequence |
|---|---|---|
| FAS | Forward Reverse | 5'-GGAGGTGGTGATAGCCGGTAT-3' 5'-TGGGTAATCCATAGAGCCCAG-3' |
| SCD1 | Forward Reverse | 5'-TTCTTGCGATACACTCTGGTGC-3' 5'-CGGGATTGAATGTTCTTGTCGT-3' |
| ACSL | Forward Reverse | 5'-CTCACCATTATATTGCTGCCTGT-3' 5'-TCTCTTTGCCATAGCGTTTTTCT-3' |
| ACS | Forward Reverse | 5'-AAACACGCTCAGTAGCACCAC-3' 5'-AGCCAAGTAGGAAGCTCTCTC-3' |
| GPAM | Forward Reverse | 5'-ACAGTTGGCACAATAGACGTTT-3' 5'-CCTTCCATTTCAGTGTTGCAGA-3' |
| PPARγ | Forward Reverse | 5'-TCGCTGATGCACTGCCTATG-3' 5'-GAGAGGTCCACAGAGCTGATT-3' |
| C/EBPα | Forward Reverse | 5'-CAAGAACAGCAACGAGTACCG-3' 5'-GTCACTGGTCAACTCCAGCAC-3' |
| FABP4 | Forward Reverse | 5'-AAGGTGAAGAGCATCATAACCCT-3' 5'-TCACGCCTTTCATAACACATTCC-3' |
| β-actin | Forward Reverse | 5'-GCAGGAGTACGATGAGTCCG-3' 5'-ACGCAGCTCAGTAACAGTCC-3' |
3.6. Western Blotting
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253.
- Kang, Y.S. Obesity associated hypertension: New insights into mechanism. Electrolyte Blood Press. 2013, 11, 46–52. [Google Scholar] [PubMed]
- Ashraf, M.J.; Baweja, P. Obesity: The ‘huge’ problem in cardiovascular diseases. Mo. Med. 2013, 110, 499–504. [Google Scholar] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- McAninch, E.A.; Bianco, A.C. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. N. Y. Acad. Sci. 2014, 1311, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, L.Y.; Hong, Z.F.; Peng, J. Ethanol extract of Cirsium japonicum attenuates hepatic lipid accumulation via AMPK activation in human HepG2 cells. Exp. Ther. Med. 2014, 8, 79–84. [Google Scholar] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Um, M.Y.; Ahn, J.; Jung, C.H.; Ha, T.Y. Cooked rice inhibits hepatic fat accumulation by regulating lipid metabolism-related gene expression in mice fed a high-fat diet. J. Med. Food 2014, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Cui, A.; Xue, Y.; Cui, Y.; Dong, X.; Gao, Y.; Yang, H.; Fang, F.; Chang, Y. Hepatic differentiated embryo-chondrocyte expressed gene 1 (DEC1) inhibits sterol regulatory element-binding protein-1c (SREBP-1c) expression and alleviates fatty liver phenotype. J. Biol. Chem. 2014, 289, 23332–23342. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Choi, H.M.; Hahm, D.H.; Her, E.; Yang, H.I.; Yoo, M.C.; Kim, K.S. Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol. Med. Rep. 2012, 6, 791–796. [Google Scholar] [PubMed]
- Byeon, S.E.; Chung, J.Y.; Lee, Y.G.; Kim, B.H.; Kim, K.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of Taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol. 2008, 119, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.T.; Cheong, J.; Choi, Y.H. Beta-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anticancer Drugs 2003, 14, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.B.; Pinto, A.V.; Pinto, M.C.; Leal, I.C.; Silva, M.G.; Amaral, A.C.; Kuster, R.M.; Netto-dosSantos, K.R. In vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2003, 21, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Romero-Gomez, M.; Castellano-Megias, V.M.; Grande, L.; Irles, J.A.; Cruz, M.; Nogales, M.C.; Alcon, J.C.; Robles, A. Serum leptin levels correlate with hepatic steatosis in chronic hepatitis C. Am. J. Gastroenterol. 2003, 98, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Y.; Hu, B.; Huang, M.; Xie, W.; Zhai, Y. Activation of human stearoyl-coenzyme A desaturase 1 contributes to the lipogenic effect of PXR in HepG2 cells. PLoS One 2013, 8, e67959. [Google Scholar] [CrossRef] [PubMed]
- Frick, F.; Hume, R.; Robinson, I.C.; Eden, S.; Oscarsson, J. Hepatic and adipose tissue depot-specific changes in lipid metabolism in late-onset obese (LOB) rats. Lipids 2008, 43, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Amy, C.M.; Williams-Ahlf, B.; Naggert, J.; Smith, S. Molecular cloning of the mammalian fatty acid synthase gene and identification of the promoter region. Biochem. J. 1990, 271, 675–679. [Google Scholar] [PubMed]
- Gong, Z.; Huang, C.; Sheng, X.; Zhang, Y.; Li, Q.; Wang, M.W.; Peng, L.; Zang, Y.Q. The role of tanshinone IIA in the treatment of obesity through peroxisome proliferator-activated receptor gamma antagonism. Endocrinology 2009, 150, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Yokoyama, T.; Sekiguchi, K.; Iijima, D.; Sunaga, H.; Maniwa, M.; Ueno, M.; Iso, T.; Arai, M.; Kurabayashi, M. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS One 2012, 7, e33283. [Google Scholar] [CrossRef] [PubMed]
- Gomez Castellanos, J.R.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity. J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, D.W.; Jo, E.J.; Kim, Y.K.; Jo, Y.S.; Park, J.H.; Yoo, S.K.; Park, M.K.; Kwak, T.H.; Kho, Y.L.; et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 2009, 58, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the TBE is not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.H.; Um, M.Y.; Ahn, J.; Jung, C.H.; Park, M.K.; Ha, T.Y. Ethanolic Extract of Taheebo Attenuates Increase in Body Weight and Fatty Liver in Mice Fed a High-Fat Diet. Molecules 2014, 19, 16013-16023. https://doi.org/10.3390/molecules191016013
Choi WH, Um MY, Ahn J, Jung CH, Park MK, Ha TY. Ethanolic Extract of Taheebo Attenuates Increase in Body Weight and Fatty Liver in Mice Fed a High-Fat Diet. Molecules. 2014; 19(10):16013-16023. https://doi.org/10.3390/molecules191016013
Chicago/Turabian StyleChoi, Won Hee, Min Young Um, Jiyun Ahn, Chang Hwa Jung, Myung Kyu Park, and Tae Youl Ha. 2014. "Ethanolic Extract of Taheebo Attenuates Increase in Body Weight and Fatty Liver in Mice Fed a High-Fat Diet" Molecules 19, no. 10: 16013-16023. https://doi.org/10.3390/molecules191016013
APA StyleChoi, W. H., Um, M. Y., Ahn, J., Jung, C. H., Park, M. K., & Ha, T. Y. (2014). Ethanolic Extract of Taheebo Attenuates Increase in Body Weight and Fatty Liver in Mice Fed a High-Fat Diet. Molecules, 19(10), 16013-16023. https://doi.org/10.3390/molecules191016013




