Enhancement of Cunninghamella elegans UCP/WFCC 0542 Biomass and Chitosan with Amino Acid Supply
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Sucrose, Corn Steep Liquor and Asparagine on the Production of Biomass and Chitosan
| Assay | pH Initial | pH Final | Biomass (g/L) | Chitosan (g/L) |
|---|---|---|---|---|
| 1 | 4.66 | 5.30 | 10.83 | 0.60 |
| 2 | 4.01 | 5.10 | 11.12 | 1.51 |
| 3 | 4.02 | 5.46 | 14.62 | 1.07 |
| 4 | 4.59 | 5.09 | 10.63 | 1.93 |
| 5 | 4.01 | 5.48 | 14.64 | 2.03 |
| 6 | 4.01 | 5.40 | 14.72 | 1.52 |
| 7 | 4.03 | 5.37 | 13.47 | 1.04 |
| 8 | 4.02 | 5.15 | 14.02 | 1.55 |
| 9 | 4.03 | 5.44 | 16.95 | 2.10 |
| 10 | 4.02 | 5.21 | 16.03 | 2.08 |
| 11 | 4.01 | 5.50 | 15.77 | 2.12 |
| 12 | 4.01 | 5.29 | 15.52 | 2.14 |
2.2. Assessment of Independent Variables on the Production of Biomass and Chitosan
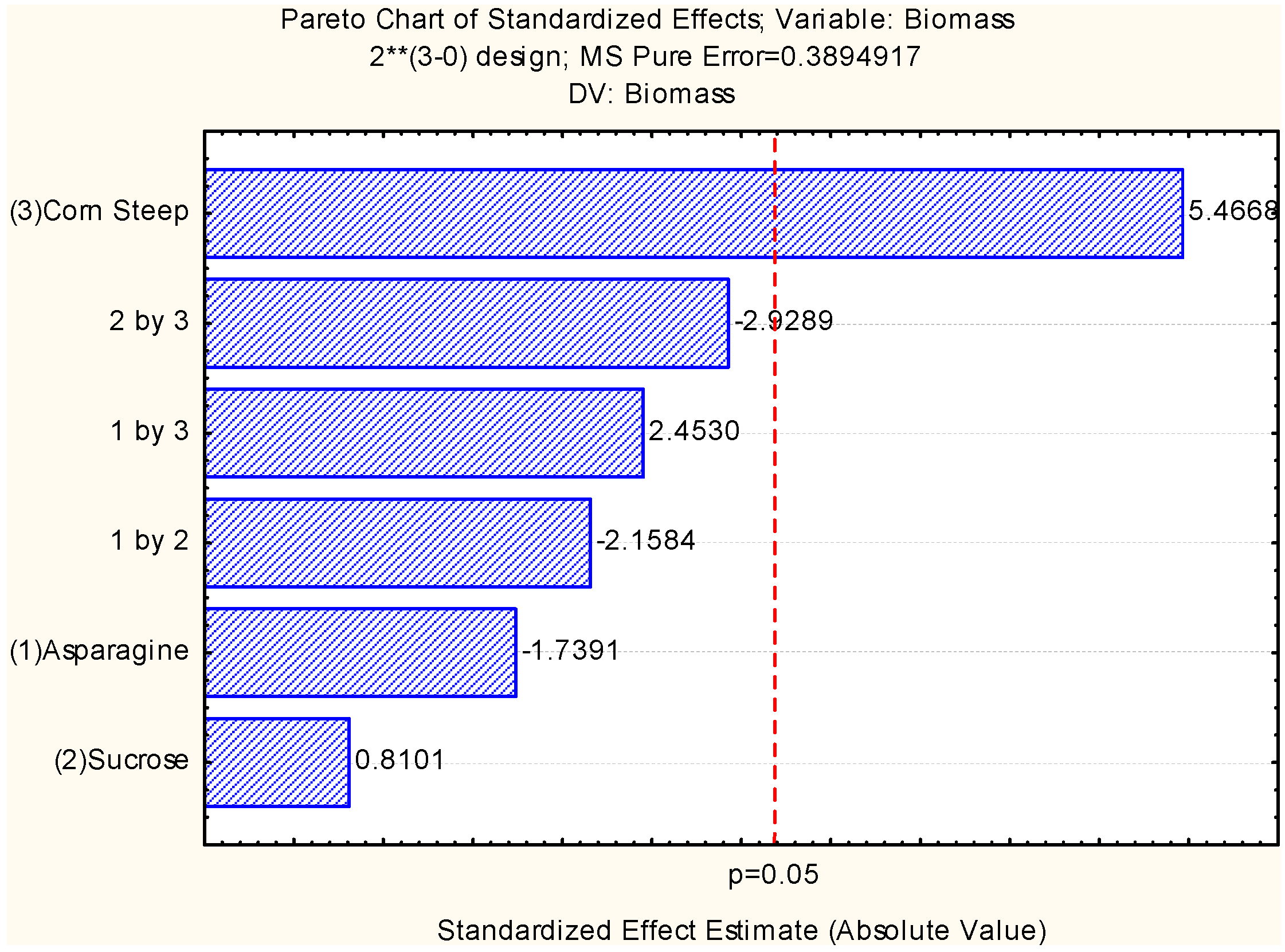
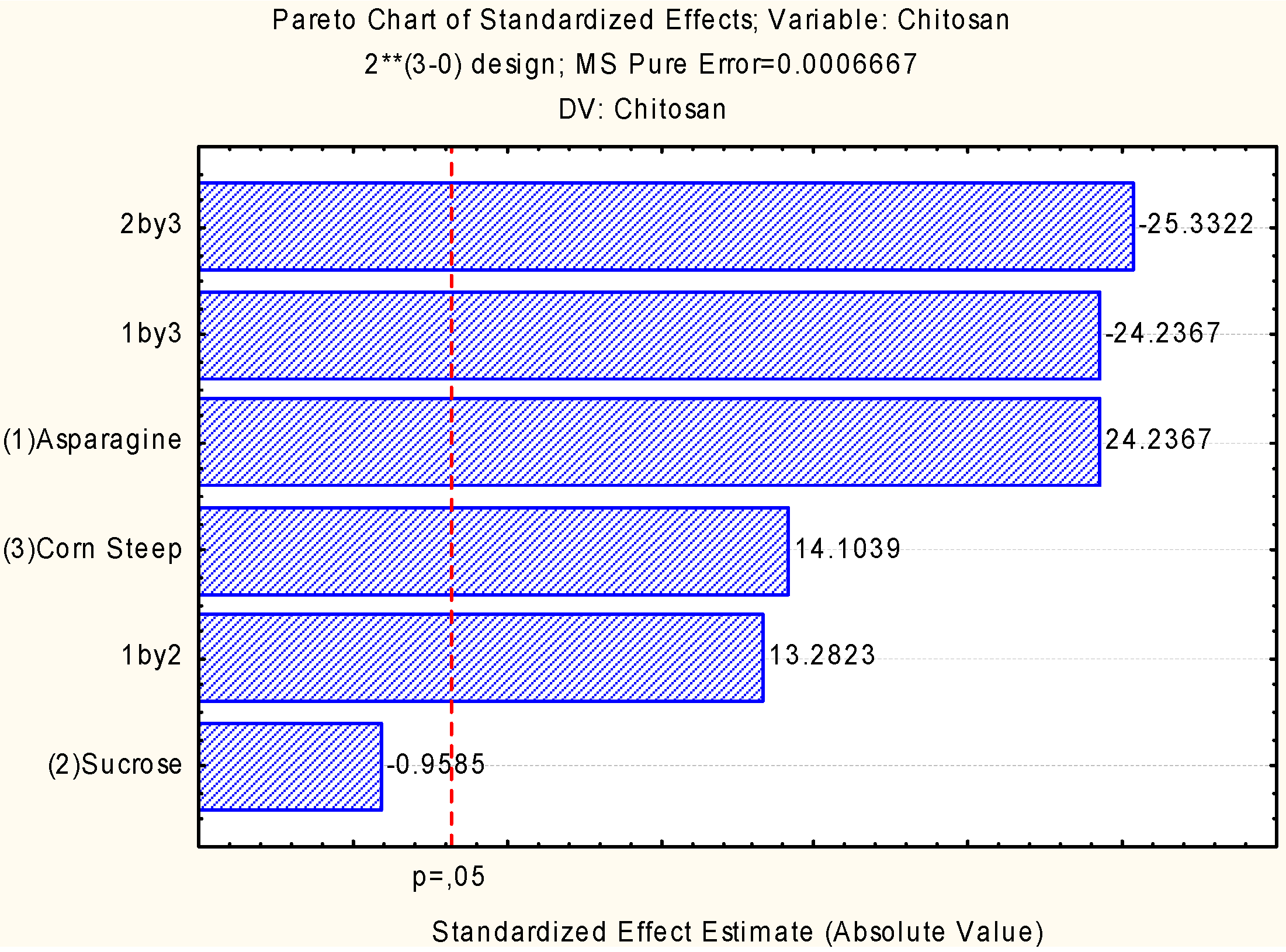
2.3. Chitosan Characterization

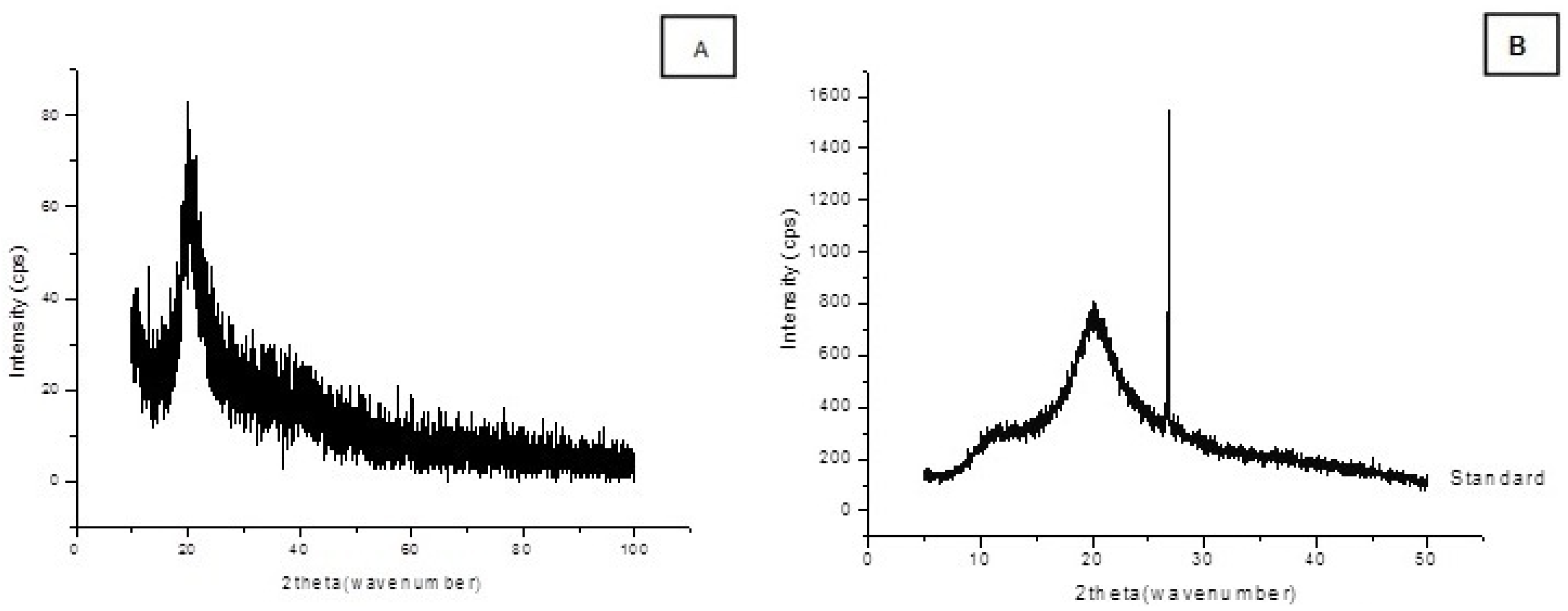
3. Experimental
3.1. Microorganism and Maintenance
3.2. Chemicals and Corn Steep Liquor
3.3. Biomass Production by C. Elegans
| Independent Variable | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Asparagine % (w/v) | 0 | 0.25 | 0.50 |
| Sucrose % (w/v) | 0.10 | 0.15 | 0.20 |
| Corn steep liquor % (v/v) | 0.30 | 0.45 | 0.60 |
3.4. Extraction of Chitosan
3.5. Determining Total Carbon and Nitrogen
3.6. Determination of pH
3.7. Physical-Chemistry Methods
3.8. Factorial design
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Stamford, T.C.M.; Stamford, T.L.M.; Stamford, N.P.; Barros Neto, B.; Campos-Takaki, G.M. Growth of Cunninghamella elegans UCP 542 and production of chitin and chitosan using yam bean medium. Electron. J. Biotechnol. 2007, 10, 61–68. [Google Scholar]
- Ambrósio, S.T.; Campos-Takaki, G.M. Decolorization of reactive azo dyes by Cunninghamella elegans UCP 542 under co-metabolic conditions. Bioresour. Technol. 2004, 91, 69–75. [Google Scholar] [CrossRef]
- Ambrósio, S.T.; Vilar Júnior, J.C.; Alves da Silva, C.A.; Okada, K.; Nascimento, A.E.; Longo, R.L.; Campos-Takaki, G.M. Biosorption Isotherm Model for the Removal of Reactive Azo Dyes by Inactivated Mycelia of Cunninghamella elegans UCP542. Molecules 2012, 17, 452–462. [Google Scholar] [CrossRef]
- Lima e Silva, T.A.L.; Tambourgi, E.B.; Campos-Takaki, G.M. Inorganic polyphosphate accumulation by Cunninghamella elegans (UCP 542) and its influence in the decolorization of textile azo dye Orange II. Clean Technol. Environ. Policy 2013, 15, 179–184. [Google Scholar] [CrossRef]
- Lima, M.A.; Souza, P.M.; Marinho, P.H.; Stamford, T.C.M.; Nascimento, A.E.; Campos-Takaki, G.M. Cadmium toxicity on Cunninghamella elegans: Ultrastructural damage and actin cytoskeleton alterations. In Current Research Topics in Applied Microbiology and Microbial Biotechnology, 1st ed; Antonio Mendez-Vilas: Seville, Spain, 2009; Volume 2, pp. 184–189. [Google Scholar]
- Lima, M.A.B.; Franco, L.O.; Souza, P.M.; Nascimento, A.E.; Silva, C.A.A.; Maia, R.C.C.; Rolim, H.M.L.R.; Campos-Takaki, G.M. Cadmium Tolerance and Removal from Cunninghamella elegans Related to the Polyphosphate Metabolism. Int. J. Mol. Sci. 2013, 14, 7180–7192. [Google Scholar] [CrossRef]
- Souza, P.M.; Marinho, P.H.C.; Lima, M.A.; Nascimento, A.E.; Campos-Takaki, G.M.C. Effect of Dibenzothiophene on the Growth, on the Morphology and on the Ultrastructure of Cunninghamella elegans. In Current Research Topics in Applied Microbiology and Microbial Biotechnology, 1st ed; Antonio Mendez-Vilas: Seville, Spain, 2009; Volume 2, pp. 645–650. [Google Scholar]
- Bernat, P.; Szewczyk, R.; Krupiński, M.; Długoński, J. Butytins degradation by C. elegans and Cochliobolus lunatus co-culture. J. Hazard Mater. 2013, 246–247, 277–282. [Google Scholar] [CrossRef]
- Cardoso, A. Chitin and chitosan: versatile polysaccharides produced by Rhizopus arrhizus. Asian Chitin J. 2008, 4, 1–8. [Google Scholar]
- Makkar, R.S.; Cameotra, S.S. Biosurfactant production by microorganisms on unconventional carbon sources. J. Surfact. Deterg. 1999, 2, 237–242. [Google Scholar] [CrossRef]
- Amorim, S.R.V.; Pedrosa, R.P.; Fukushima, K.; Martínez, C.R.; Ledingham, W.M.; Campos-Takaki, G.M. Alternative carbon sources from sugar cane process for submerged cultivation of Cunninghamella bertholletiae to produce chitosan. Food Technol. Biotechnol. 2006, 44, 519–534. [Google Scholar]
- Freitas, S.M.C.; Stamford, T.C.M.; Franco, L.O.; Campos-Takaki, G.M. Effect of salinity and glucose on chitin and chitosan production by Cunninghamella elegans. Asian Chitin J. 2006, 2, 29–38. [Google Scholar]
- Jara, A.M.A.T.; Andrade, R.F.S.; Campos-Takaki, G.M. Physicochemical characterization of tensio-active produced by Geobacillus stearothermophilus isolated from petroleum-contaminated soil. Colloids Surf. B 2013, 101, 315–318. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B 2013, 102, 202–209. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Koniecza, E. The influence of supplemental components in nutrient medium on chitosan formation by the fungus Absidia orchidis. J. Appl. Microbiol. Biotechnol. 2001, 56, 220–224. [Google Scholar] [CrossRef]
- Khalaf, S.A. Purification and characterization of fungal chitosan under solid-state fermentation conditions. Int. J. Agric. Biol. 2004, 6, 1033–1036. [Google Scholar]
- West, T.P.; Strohfus, B. Effect of nitrogen source on pullulan production by Aureobasidium pullulans grown in a batch bioreactor. Microbios 1999, 99, 147–159. [Google Scholar]
- Cardoso, A.; Lins, C.I.M.; Santos, E.R.; Freitas da Silva, M.C.; Campos-Takaki, G.M. Microbial enhance of chitosan production by Rhizopus arrhizus using agroindustrial substrates. Molecules 2012, 17, 4904–4914. [Google Scholar] [CrossRef]
- Obayori, O.S.; Adebusoye, S.A.; Ilori, M.O.; Oyetibo, G.O.; Omotayo, A.E.; Amund, O.O. Effects of corn steep liquor on growth rate and pyrene degradation by Pseudomonas strains. Curr. Microbiol. 2010, 60, 407–411. [Google Scholar] [CrossRef]
- Batista, A.C.L.; Cardoso, A.; Santos, E.; Freitas, S.M.C.; Alves, S.C.A.; Campos-Takaki, G.M. Influence of simultaneous factors on chitosan production by Syncephalastrum racemosum (UCP/WFCC 0148) in corn steep liquor culture media. Asian Chitin J. 2011, 7, 63–67. [Google Scholar]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress Polymer Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lower, S.E. Polymers from the Sea Chitin and Chitosan. Manuf. Chem. 1984, 55, 73–75. [Google Scholar]
- Campana-Filho, S.P.; Britto, D.; Curti, E.; Abreu, F.R.; Cardoso, M.B.; Battisti, M.V.; Sim, P.C.; Goy, R.C.; Signini, R.; Lavall, R.L. Extração, estruturas e propriedades de alfa e beta-quitina. Quím. Nova 2007, 30, 644–650. [Google Scholar]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. Production, properties and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fifiber formation. Progress Polymer Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Cao, W.L.; Jing, D.H.; Li, J.M.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Effects of the degree of deacetylation on the physicochemical properties and Schwann cell affinity of chitosan films. J. Biomater. Appl. 2005, 20, 157–177. [Google Scholar] [CrossRef]
- Uzun, I.; Topal, G. Synthesis and Physicochemical Characterization of Chitin Derivatives. J. Chem. 2013, 2013, 1–8. [Google Scholar]
- Gavhane, Y.N.; Gurav, A.S.; Yadav, A.V. Chitosan and Its Applications: A Review of Literature. Int. J. Res. Pharma. Biomedical Sci. 2013, 4, 311–331. [Google Scholar]
- Muzzarelli, R.A.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar Drugs. 2010, 8, 292–312. [Google Scholar] [CrossRef]
- Park, J.P.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Optimization of submerged culture conditions for mycelial growth and exobiopolymer production by Cordyceps militares. Lett. Appl. Microbiol. 2001, 33, 76–84. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P. Influence of chitosan characteristics on polymer properties. Polymers Internat. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Guerra-Sánchez, M.G.; Vega-Pérez, J.; Velázquez-Del, Valle M.G.; Hernández-Lauzardo, N.A. Antifungal activity and release of compounds on Rhizopus stolonifer by effect of chitosan with different molecular weights. Pestic. Biochem. Physiol. 2009, 93, 18–22. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. Chitosan from aquatic and terrestrial organisms and 27 microorganisms: Production, properties and applications. In Biodegradable Materials: 28 Production, Properties and Applications; Johnson, B.M., Berkel, Z.E., Eds.; Nova Science publishers: New York, NY, USA, 2011; pp. 29–50. [Google Scholar]
- Roberts, G.A.F. Thirty years of progress in chitin and chitosan. In Progress on Chemistry and Application of Chitin and Its Derivatives; Jaworska, M.M., Ed.; Polish Chitin Soc.: Lodz, Poland, 2008; Volume XIII, pp. 7–15. [Google Scholar]
- Campos-Takaki, G.M. The versatility on copolymers chitin and chitosan production. In Chitin and Chitosan Opportunities and Challenges; SMM International Publication: Contai, Midnapore, India, 2005; pp. 69–94. [Google Scholar]
- Kafetzopoulos, D.; Aggeliki Martinou, A.; Bouriotis, V. Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Natl. Acad. Sci. USA 1993, 90, 2564–2568. [Google Scholar] [CrossRef]
- Bento, R.A.; Stamford, T.L.; Campos-Takaki, G.M.; Stamford, T.; Souza, E.L.D. Potential of chitosan from Mucor rouxxi UCP064 as alternative natural compound to inhibit Listeria monocytogenes. Braz. J. Microbiol. 2009, 40, 583–589. [Google Scholar] [CrossRef]
- Streit, F.; Koch, F.; Laranjeira, M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef]
- Fai, A.E.C.; Stamford, T.C.M.; Stamford-Arnaud, T.M.; Santa-Cruz, P.D.A.; Silva, M.C.F.; Campos-Takaki, G.M.; Stamford, T.L.M. Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucor circinelloides using yam bean as substrate. Molecules 2011, 16, 7143–7154. [Google Scholar] [CrossRef]
- Hu, K.J.; Hu, J.L.; Ho, K.P. Screening of fungi for chitosan producers, and copper adsorption capacity of fungal chitosan and chitosanaceous materials. Carbohyd. Polymers. 2004, 58, 45–56. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; MacMillan Press: London, UK, 1992. [Google Scholar]
- Baumgarten, M.G.Z.; Rocha, J.M.B.; Niencheski, L.F.H. Manual de análises em oceanografia química. Editora da FURG: Rio Grande, Brasil, 1996; p. 132. [Google Scholar]
- Yasuhara, T.; Nokihara, K. High-throughput analysis of total nitrogen content that replaces the classic Kjeldahl method. J. Agric. Food Chem. 2001, 49, 4581–4583. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos, E.R.; Da Silva, M.C.F.; De Souza, P.M.; Da Silva, A.C.; De Paiva, S.C.; Albuquerque, C.D.C.; Nascimento, A.E.; Okada, K.; Campos-Takaki, G.M. Enhancement of Cunninghamella elegans UCP/WFCC 0542 Biomass and Chitosan with Amino Acid Supply. Molecules 2013, 18, 10095-10107. https://doi.org/10.3390/molecules180910095
Dos Santos ER, Da Silva MCF, De Souza PM, Da Silva AC, De Paiva SC, Albuquerque CDC, Nascimento AE, Okada K, Campos-Takaki GM. Enhancement of Cunninghamella elegans UCP/WFCC 0542 Biomass and Chitosan with Amino Acid Supply. Molecules. 2013; 18(9):10095-10107. https://doi.org/10.3390/molecules180910095
Chicago/Turabian StyleDos Santos, Ednaldo Ramos, Marta Cristina Freitas Da Silva, Patrícia Mendes De Souza, Antonio Cardoso Da Silva, Sergio Carvalho De Paiva, Clarissa D. C. Albuquerque, Aline E. Nascimento, Kaoru Okada, and Galba M. Campos-Takaki. 2013. "Enhancement of Cunninghamella elegans UCP/WFCC 0542 Biomass and Chitosan with Amino Acid Supply" Molecules 18, no. 9: 10095-10107. https://doi.org/10.3390/molecules180910095
APA StyleDos Santos, E. R., Da Silva, M. C. F., De Souza, P. M., Da Silva, A. C., De Paiva, S. C., Albuquerque, C. D. C., Nascimento, A. E., Okada, K., & Campos-Takaki, G. M. (2013). Enhancement of Cunninghamella elegans UCP/WFCC 0542 Biomass and Chitosan with Amino Acid Supply. Molecules, 18(9), 10095-10107. https://doi.org/10.3390/molecules180910095




