Advances in Fruit Aroma Volatile Research
Abstract
:1. Introduction
2. Aroma Volatile Composition and Their Biological Characteristic of Major Fruits
2.1. Classification of Volatile Compounds in Fruit Flavor
2.2. Volatile Compounds and Their Biological Characteristic of Major Fruits
3. Factors Influencing Volatile Composition
3.1. Genetics
3.2. Maturity
3.3. Pre-Harvest Factors
3.4. Postharvest Handling
3.4.1. Temperature
3.4.2. Storage Atmosphere
3.4.3. Chemical Application
4. Volatile Aroma Compounds Biosynthestic Pathways and Related Enzymes
4.1. Fatty Acids Pathway
4.1.1. β-Oxidation
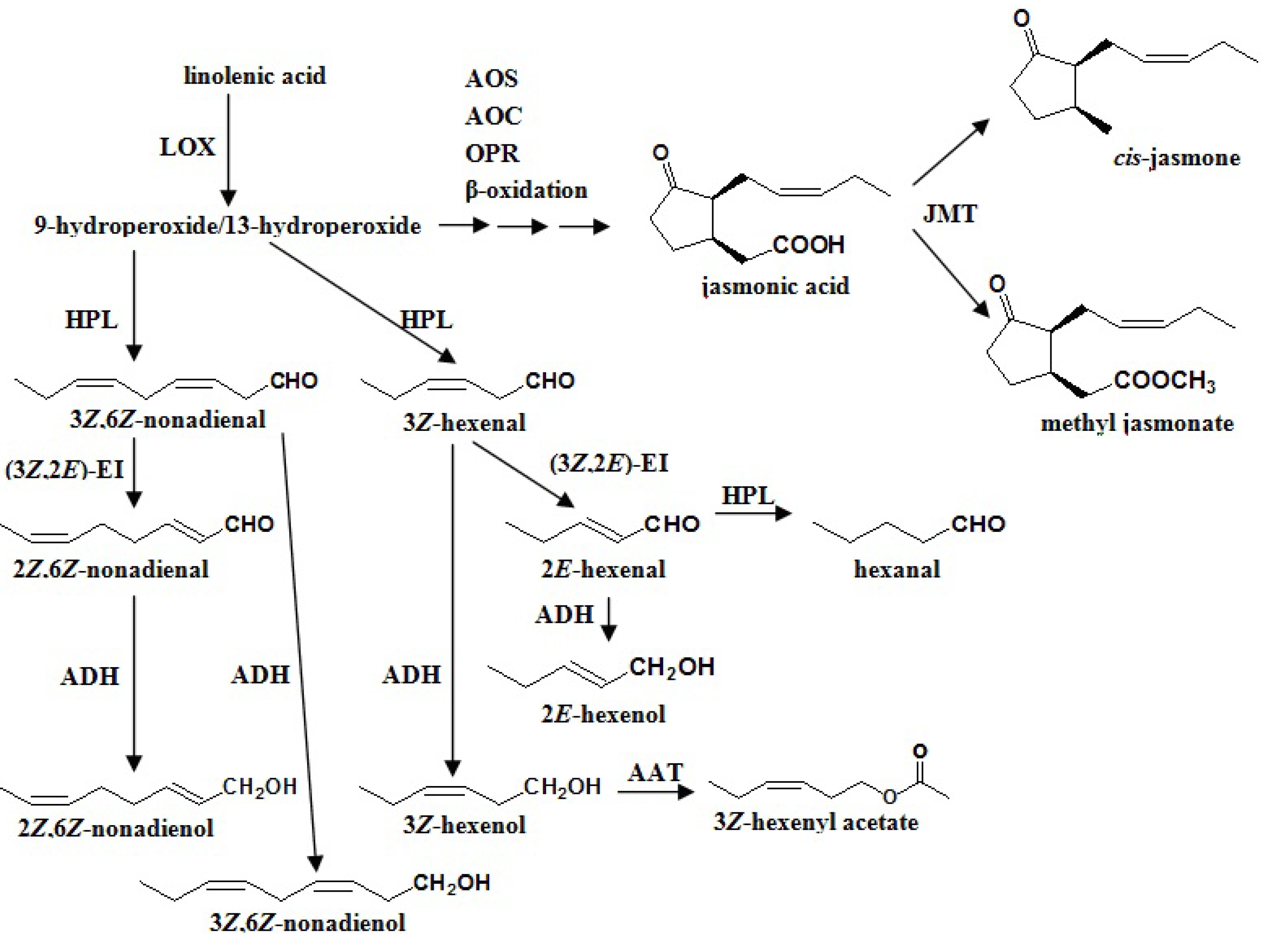
4.1.2. Lipoxygenase (LOX)
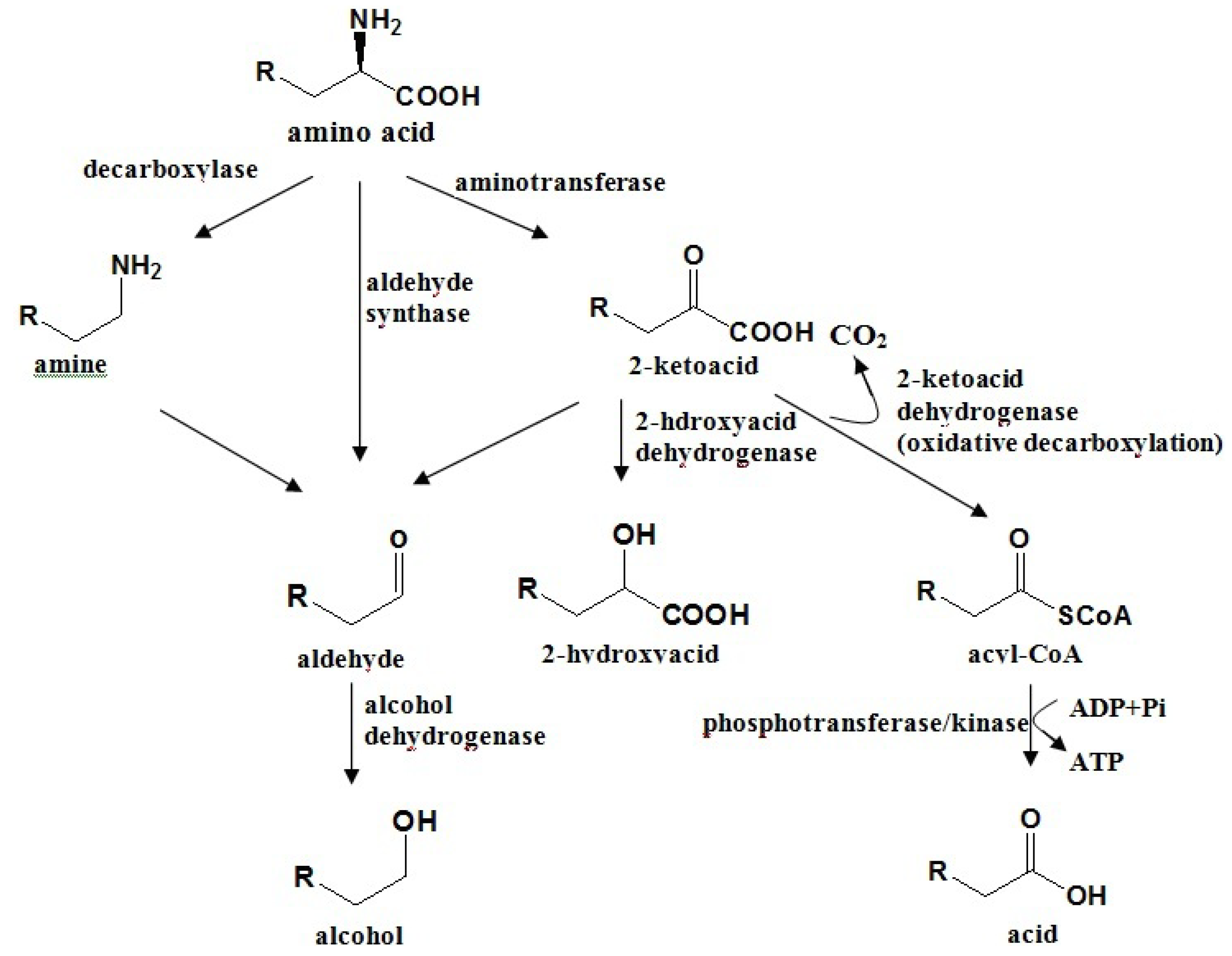
4.2. Amino Acid Pathway
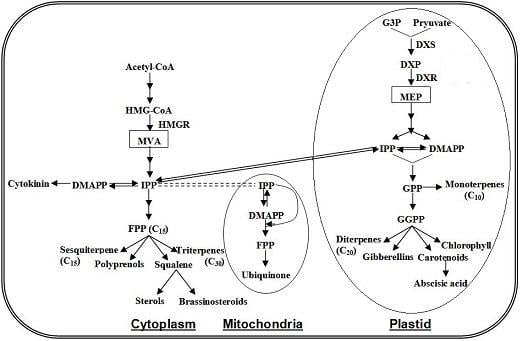
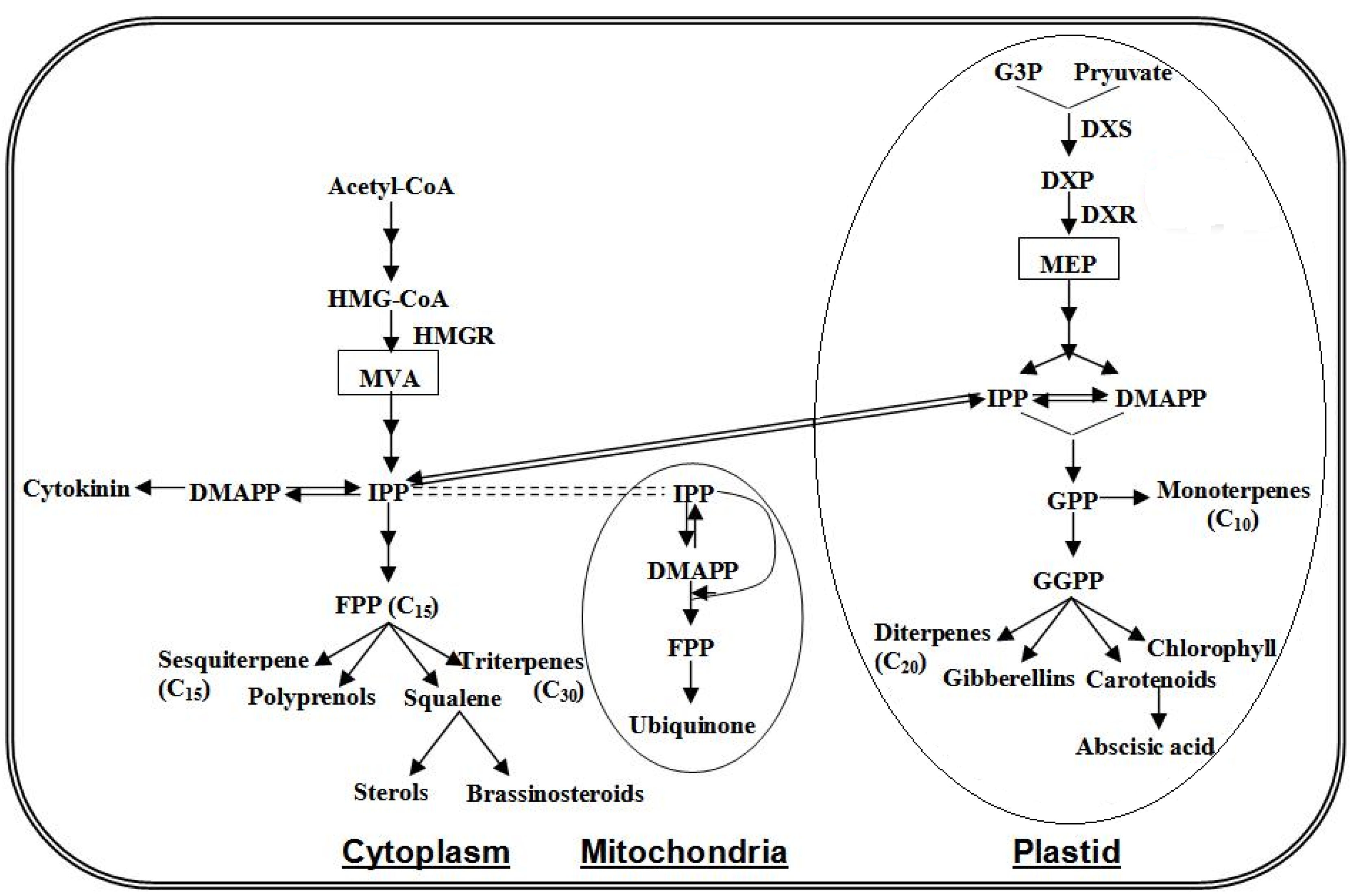
4.3. Terpenoids Pathway
4.4. Carotenoid Pathway
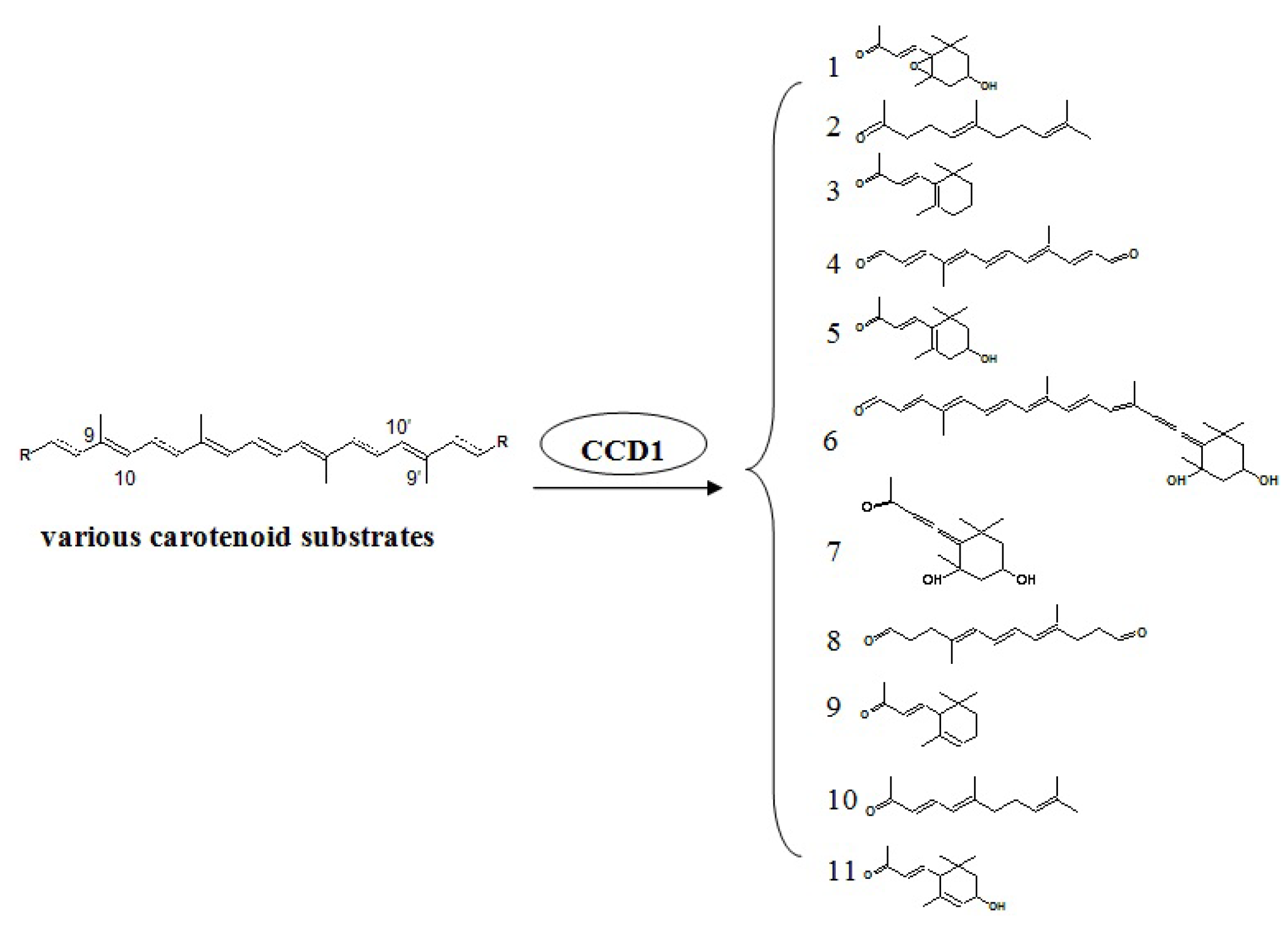
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ogundiwin, E.A.; Peace, C.P.; Gradziel, T.M.; Parfitt, D.E.; Bliss, F.A.I.; Crisosto, C.H.A. Fruit quality gene map of Prunus. BMC Genomics 2009, 10, 587. [Google Scholar] [CrossRef]
- Maffei, M.E. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of “Pink Lady” apples. South Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Cheong, K.W.; Tan, C.P.; Mirhosseini, H.; Hamid, N.S.A.; Osman, A.; Basri, M. Equilibrium headspace analysis of volatile flavour compounds extracted from soursop (Anoan muricata) using solid phase microextraction. Food Res. Int. 2010, 43, 1267–1276. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruit and Vegetables; Oxford University Press Inc.: New York, NY, USA, 1997; pp. 125–155. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Tucker, G.A. Introduction. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylo, R.J.E., Tucker, G.A., Eds.; Chapman & Hall: London, UK, 1993; pp. 1–51. [Google Scholar]
- Ortiz, A.; Graell, J.; Lara, I. Volatile ester-synthesising capacity throughout on-tree maturation of “Golden Reinders” apples. Sci. Hortic. 2011, 131, 6–14. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; López, M.L.; Echeverría, G.; Lara, I. Volatile ester-synthesising capacity in “Tardibelle” peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 2010, 123, 698–704. [Google Scholar] [CrossRef]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value. Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Drawert, F.; Heimann, W.; Emberger, R.; Tressl, R. Über die Biogenese von Aromastoffen bei Pflanzen und Frü chten. IV. Mitt Bildung der Aromamstoffe des Apfels im Verlauf des Wachstums und bei der Largerung. Zeit. Lebens. Unters. Forsch 1969, 140, 65–87. [Google Scholar] [CrossRef]
- Berger, R.G.; Drawert, F.; Kollmannsberger, H. Geruchsaktive spurenkomponenten des bananen aromas. Chem. Mikrobiol. Technol. Lebensm. 1986, 10, 120–124. [Google Scholar]
- Bruckner, B. Fruit and Vegetable Flavour: Recent Advances and Future Prospects; Woodhead Publishing Limited: Abington Hall, Cambridge, UK, 2008. [Google Scholar]
- Buttery, R.G. Quantitative and sensory aspects of flavor of tomato and other vegetable and fruits. In Flavor Science: Sensible Principles and Techniques; Acree, T.E., Teranishi, R., Eds.; ACS: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Sarry, J.E.; Gunata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Reineccius, G. Flavor Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Carcia, C.V.; Stevenson, R.J.; Atkinson, R.G.; Winz, R.A.; Yong Quik, S. Changes in the bound aroma profiles of “Hayward” and “Hort16A” kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis. Food Chem. 2013, 137, 45–54. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Leach, D.N.; Wang, Y.; Shewfelt, R.L. Key aroma compounds in melons: Their development and cultivar dependence. In Fruit Flavors: Biogenesis, Characterization, and Authentication; Rouseff, R.L., Leahy, M.M., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 248–257. [Google Scholar]
- Zabetakis, I.; Holden, M.A. Strawberry flavour: Analysis and biochemistry. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Ninio, R.; Lewinsohn, E.; Mizrahi, Y.; Sitrit, Y. Quality attributes of storage koubo (Cereus peruvianus (L.) Miller) fruit. Postharvest Biol. Technol. 2003, 30, 273–280. [Google Scholar] [CrossRef]
- Akakabe, Y.; Sakamoto, M.; Ikeda, Y.; Tanaka, M. Identification and characterization of volatile components of Japanese sour citrus fruit Citrus nagato-yuzukichi Tanaka. Biosci. Biotechnol. Biochem. 2008, 72, 1965–1968. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R.L. Carotenoid-derived aroma compounds: An introduction. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 1–17. [Google Scholar]
- Nijssen, L.M.; van Ingen-Visscher, C.A.; Donders, J.J.H. VCF Volatile Compounds in Food: database (Version 13.1.). Zeist (The Netherlands): TNO Triskelion Recuperato da, 2011. [Google Scholar]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N.Z.J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Rizzolo, A.; Grassi, M.; Zerbini, P.E. Influence of harvest date on ripening and volatile compounds in the scab-resistant apple cultivar “Golden Orange”. J. Hortic. Sci. Biotech. 2006, 81, 681–690. [Google Scholar]
- Holland, D.; Larkov, O.; Bar-Yaákov, I.; Bar, E.; Zax, A.; Brandeis, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances-Chemistry, Bioprocessing and Sustainability; Springer-Verlag: Berlin, Germany, 2007. [Google Scholar]
- Villatoro, C.; Altisent, R.; Echeverria, G.; Graell, J.; Lopez, M.L.; Lara, I. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of “Pink Lady” apples. Postharvest Biol.Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Rudell, D.R.; Mattinson, D.S.; Mattheis, J.P.; Wyllie, S.G.; Fellman, J.K. Investigations of aroma volatile biosynthesis under anoxic conditions and in different tissues of “Redchief Dilicious” apple fruit (Malus domestica Borkh.). J. Agric. Food Chem. 2002, 50, 2627–2632. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Bomben, J.L.; Hudson, J.S. Factors influencing the development of aroma in apple peel. J. Sci. Food Agric. 1971, 22, 110–115. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef]
- Rapparini, F.; Predieri, S. Pear fruit volatiles. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 237–324. [Google Scholar]
- Kahle, K.; Preston, C.; Richling, E.; Heckel, F.; Schreier, P. On-line gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (HRGC-C/P-IRMS) of major volatiles from pear fruit (Pyrus communis) and pear products. Food Chem. 2005, 91, 449–455. [Google Scholar] [CrossRef]
- Rizzolo, A.; Sodi, C.; Poleselllo, A. Influence of ethylene removal on the volatile development in Passa crassana pears stored in a controlled atmosphere. Food Chem. 1991, 42, 275–285. [Google Scholar] [CrossRef]
- Rizzolo, A.; Cambiaghi, P.; Grassi, M.; Zerbini, P.E. Influence of 1-methylcyclopropene and storage atmosphere on changes in volatile compounds and fruit quality of conference pears. J. Agric. Food Chem. 2005, 53, 9781–9789. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Moreno, E.; Garcıa-Mas, J.; Nicolai, B.; Lammertync, J.; Monforte, J.A.; Fernandez-Trujillo, J.P. Climacteric or non-climacteric behavior in melon fruit 1. Aroma volatiles. Postharvest Biol. Technol. 2008, 49, 27–37. [Google Scholar] [CrossRef]
- Perry, P.L.; Wang, Y.; Lin, J.M. Analysis of honeydew melon (Cucumis melo var. Inodorus) flavor and GC/MS identification of (E,Z)-2,6-nonadienyl acetate. Flav. Frag. J. 2009, 24, 341–347. [Google Scholar] [CrossRef]
- Portnoy, V.; Benyamini, Y.; Bar, E. The molecular and biochemical basis for varietal variation in sesquiterpene content in melon (Cucumis melo L.) rinds. Plant Mol. Biol. 2008, 66, 647–661. [Google Scholar] [CrossRef]
- Aubert, C.; Bourger, N. Investigation of volatiles in Charentais cantaloupe melons (Cucumis melo var. cantalupensis). Characterization of aroma constituents in some cultivars. J. Agric. Food Chem. 2004, 52, 4522–4528. [Google Scholar] [CrossRef]
- Aubert, C.; Pitrat, M. Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J. Agric. Food Chem. 2006, 54, 177–8182. [Google Scholar]
- Ibdah, M.; Azulay, Y.; Portnoy, V.; Wasserman, B.; Bar, E.; Meir, A.; Burger, Y.; Hirchberg, J.; Schaffer, A.A.; Katzir, N.; Tadmor, Y.; Lewinsohn, E. Functional characterization of CmCDD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 2006, 67, 1579–1589. [Google Scholar] [CrossRef]
- Manriquez, D.; El-Sharkawy, I.; Flores, F.B.; El-Yahyaoui, F.; Regad, F.; Bouzayen, M.; Latche, A.; Pech, J.C. Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Mol. Biol. 2006, 61, 675–685. [Google Scholar] [CrossRef]
- Burger, Y.; Sáar, U.; Paris, H.S.; Lewinsohn, E.; Katzir, N.; Tadmor, Y.; Schaffer, A.A. Genetic variability for valuable fruit quality traits in Cucumis melo. Israel J. Plant Sci. 2006, 54, 233–242. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Jordán, M.J.; Shaw, P.E.; Goodner, K.L. Volatile components in aqueous essence and fresh fruit of Cucumis melo cv. Athena (muskmelon) by GC-MS and GC-O. J. Agric. Food Chem. 2001, 49, 5929–5933. [Google Scholar] [CrossRef]
- Bood, K.G.; Zabetakis, I. The biosynthesis of strawberry flavor (II): Biosynthetic and molecular biology studies. J. Food Sci. 2002, 67, 2–8. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Schwab, W.; Schaart, J.G.; Rosati, C. molecular biology research in Fragaria. In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Jayanty, S.; Song, J.; Rubinstein, N.M.; Chong, A.; Beaudry, R.M. Temporal relationship between ester biosynthesis and ripening events in bananas. J. Am. Soc. Hort. Sci. 2002, 127, 998–1005. [Google Scholar]
- Wendakoon, S.K.; Ueda, Y.; Imahori, Y.; Ishimaru, M. Effect of short-term anaerobic conditions on the production of volatiles, activity of alcohol acetyltransferase and other quality traits of ripened bananas. J. Sci. Food Agric. 2006, 86, 1475–1480. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Giampaoli, P.; Bonazzi, C. Changes in aromatic components of banana during ripening and air-drying. Lebensm Wiss Technol. 2003, 36, 633–642. [Google Scholar] [CrossRef]
- Aurore, G.; Ginies, C.; Ganou-Parfait, B.; Renard, C.M.G.C.; Fahrasmane, L. Comparative study of free and glycoconjugated volatile compounds of three banana cultivars from French West Indies: Cavendish, Frayssinette and Plantain. Food Chem. 2011, 129, 28–34. [Google Scholar] [CrossRef]
- Gonza’lez-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Antonio, A.G. Comparative analysis of the volatile fraction of fruit juice from differentCitrus species. PLoS One 2011, 6, e22016. [Google Scholar]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Tomiyama, K.; Aoki, H.; Oikawa, T.; Sakurai, K.; Kashara, Y.; Kawakami, Y. Characteristic volatile components of Japanese sour citrus fruits: Yuzu, Sudachi and Kabosu. Flavour Fragr. J. 2012, 27, 341–355. [Google Scholar] [CrossRef]
- Phi, N.T.L.; Nishiyama, C.; Choi, H.S.; Sawamura, M. Evaluation of characteristic aroma compounds of Citrus natsudaidai Hayata (Natsudaidai) cold-pressed peel oil. Biosci. Biotechnol. Biochem. 2006, 70, 1832–1838. [Google Scholar] [CrossRef]
- Shibamoto, T.; Tang, C.S. “Minor” tropical fruit mango, papaya, passion fruit, and guava. In Food Flavours: Part C: The Flavour of Fruit; Morton, I.D., MacLeod, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 221–234. [Google Scholar]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flav. Frag. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Quijano, C.E.; Salamanca, G.; Jorge, A.; Pino, J.A. Aroma volatile consttuents of Colombian varieties of mango (Mangifera indica L.). Flav. Frag. J. 2007, 22, 401–406. [Google Scholar] [CrossRef]
- Narain, N.; Bora, P.S.; Narain, R.; Shaw, P.E. Mango. In Tropical and Subtropical Fruits; Shaw, P.E., Chan, H.T., Nagy, S., Eds.; AgScience: Auburndale, FL, USA, 1997; pp. 1–77. [Google Scholar]
- Olle, D.; Baumes, R.L.; Bayonove, C.L.; Lozano, Y.F.; Sznaper, C.; Brillouet, J.M. Comparison of free and glycosidically linked volatile components from polyembryonic and monoembryonic mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 1998, 46, 1094–1100. [Google Scholar] [CrossRef]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, C.X.; Li, S.H.; Yang, L.; Wang, Y.N.; Zhao, J.B.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Aubert, C.; Gunata, Z.; Ambid, C.; Baumes, R. Changes in physicochemical characteristics and volatile constituents of yellow- and white-fleshed nectarines during maturation and artificial ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.P.; Wei, W.W.; Shen, J.Y.; Ferguson, I.; Chen, K.S. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Nijssen, L.M.; Visscher, C.A.; Maarse, H.; Willemsens, L.C.; Boelens, M.H. Volatile Compounds in Foods Qualitative and Quantitative Data; TNO Nutrition and Food Research Institute: Zeist, The Netherlands, on-line version 9.2, 2007. [Google Scholar]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef]
- Aubert, C.; Chanforan, C. Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunus armeniaca L.). Characterization of 28 cultivars. J. Agric. Food Chem. 2007, 55, 3074–3082. [Google Scholar] [CrossRef]
- Dieguez, S.C.; Lois, L.C.; Gomez, E.F.; De Ia Pena, M.L.G. Aromatic composition of the Vitis vinifera grape Albariño. Lebensmittel-Wissenschaft und-Technologie 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Rosilllo, L.; Salinas, M.R.; Garijo, J.; Alonso, G.L. Study of volatiles in grapes by dynamic headspace analysis Application to the differentiation of some Vitis vinifera varieties. J. Chromatogr. A 1999, 847, 155–159. [Google Scholar] [CrossRef]
- Bellincontro, A.; Nicoletti, I; Valentini, M.; Tomas, A.; de Santis, D.; Corradini, D.; Mencarelli, F. Integration of nondestructive techniques with destructive analyses to study postharvest water stress of winegrapes. Am. J. Enol. Vitic. 2009, 60, 57–65. [Google Scholar]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Austra. J. Grape Wine Res. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Klesk, K.; Qian, M.; Martin, R. Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J. Agric. Food Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef]
- Malowicki, S.M.; Martin, R.; Qian, M.C. Volatile composition in raspberry cultivars grown in the Pacific Northwest determined by stir bar sorptive extraction-gas chromatography-mass spectrometry. J. Agric. Food Chem. 2008, 56, 4128–4133. [Google Scholar] [CrossRef]
- Turemis, N.; Kafkas, E.; Kafkas, S.; Kurkcuoglu, M.; Baser, K.H.C. Determination of aroma compounds in blackberry by GC/MS analysis. Chem. Nat. Comp. 2003, 39, 174–176. [Google Scholar] [CrossRef]
- Du, X.F.; Finn, C.E.; Qian, M.C. Volatile composition and odour-activity value of thornless “Black Diamond” and “Marion” blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar] [CrossRef]
- Du, X.F.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Blueberry volatile composition of four southern highbush cultivars and effect of growing location and harvest date. J. Agric. Food Chem. 2011, 59, 8347–8357. [Google Scholar] [CrossRef]
- Tokitomo, Y.; Steinhaus, M.; Buttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef]
- Zhang, X.M.; Du, L.Q.; Sun, G.M.; Wei, C.B.; Liu, S.H.; Xie, J.H. Changes of aroma components in Yellow Mauritius pineapple during fruit development (In Chinese). J. Fruit Sci. 2009, 26, 245–249. [Google Scholar]
- Liang, Y.Z.; Guang, M.S.; Yu, G.L.; Ling, L.L.; Wen, X.Y.; Wei, F.Z.; Chang, B.W. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef]
- Chang, B.W.; Sheng, H.L.; Yu, G.L.; Ling, L.L.; Wen, X.Y.; Guang, M.S. Characteristic aroma compounds from different pineapple parts. Molecules 2011, 16, 5104–5112. [Google Scholar] [CrossRef]
- Friel, E.N.; Wang, M.; Taylor, A.J.; MacRae, E.A. In vitro and in vivo release of aroma compounds from yellow-fleshed kiwifruit. J. Agric. Food Chem. 2007, 55, 6664–6673. [Google Scholar] [CrossRef]
- Gunther, C.S.; Matich, A.J.; Marsh, K.B.; Nicolau, L. (Methylsulfanyl) alkanoate ester biosynthesis in Actinidia chinensis kiwifruit and changes during cold storage. Phytochemistry 2010, 71, 742–750. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Wang, H.B.; Chen, X.S.; Xin, P.G.; Feng, T.; Shi, J.; Ci, Z.J. GC-MS analysis of volatile components in several early apple cultivars (in Chinese). J. Fruit Sci. 2007, 24, 11–15. [Google Scholar]
- Aharoni, A.; Giri, A.P.; Verstappen, F.W.A.; Bertea, C.M.; Sevenier, R.; Sun, Z.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 2004, 16, 3110–3131. [Google Scholar] [CrossRef]
- Hampel, D.; Mosandl, A.; Wust, M. Biosynthesis of mono- and sesquiterpenes in strawberry fruits and foliage: 2H labeling studies. J. Agric. Food Chem. 2006, 54, 1473–1478. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Shuch, W. Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortScience 2000, 35, 1013–1022. [Google Scholar]
- Shan, W.Y.; Zhao, C.; Fan, J.G.; Cong, H.; Liang, S.; Yu, X.Y. Antisense suppression of alcohol acetyltransferase gene in ripening melon fruit alters volatile composition. Sci. Hortic. 2012, 139, 96–101. [Google Scholar] [CrossRef]
- Lester, G. Consumer preference quality attributes of melon fruit. Acta Hortic. 2006, 712, 175–181. [Google Scholar]
- Kader, A.A. A perspective on postharvest horticulture (1978–2003). Hort. Sci. 2004, 38, 1004–1008. [Google Scholar]
- Fellman, J.K.; Rudell, D.R.; Mattinson, D.S.; Mattheis, J.P. Relationship of harvest maturity to flavor regeneration after CA storage of “Delicious” apples. Postharvest Biol. Technol. 2003, 27, 39–51. [Google Scholar] [CrossRef]
- Menager, I.; Jost, M.; Aubert, C. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef]
- Beaulieu, J.C. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. Hort. Sci. 2006, 41, 65–73. [Google Scholar]
- El-Mageed, M.A.A. Development of volatile compounds of avocado and casimiroa during fruit maturation. Arab Univ. J. Agric. Sci. 2007, 15, 89–99. [Google Scholar]
- Pereira, M.E.C. Changes in volatile compounds during ripening of West Indian-type “Simmonds” avocado treated with ethylene and aqueous 1-methylcyclopropene. In American Society for Horticultural Science Annual Meeting; American Society for Horticultural Science: Palm Desert, CA, USA, 2010; p. S93. [Google Scholar]
- Obenland, D.; Collin, S.; Sievert, J.; Negm, F.; Arpaia, M.L. Influence of maturity and ripening on aroma volatiles and flavor in “Hass” avocado. Postharvest Biol. Technol. 2012, 71, 41–50. [Google Scholar] [CrossRef]
- Fuggate, P.; Wongs-Aree, C.; Noichinda, S.; Kanlayanarat, S. Quality and volatile attributes of attached and detached “Pluk Mai Lie” papaya during fruit ripening. Sci. Hortic. 2010, 126, 120–129. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.J.; Wu, B.H.; Fang, J.B.; Li, S.H. Volatile compounds evolution of three table grapes with different flavor during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Leeuwen, C.V.; Tominaga, T.; Soyer, J.P.; Gaudillere, J.P.; Denis, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Salas, N.A.; Molina-Corral, F.J.; Gonzalez-Aguilar, G.A.; Otero, A.; Sepulveda, D.R.; Olivas, G.I. Volatile production by “Golden Delicious” apples is affected by preharvest application of aminoethoxyvinylglycine. Sci. Hortic. 2011, 130, 436–444. [Google Scholar] [CrossRef]
- Tietel, Z.; Lewinsohn, E.; Fallik, E.; Porat, R. Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biol. Technol. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Sanchez, F.D.; Zaldivar, C.P.; Cabrera, F.R.; Valadez, M.P.; Alejandre, X.A.; Fernandez, F.J.; Buendiac, H.B.E.; Flores, L.J.P. Effect of refrigerated storage on aroma and alcohol dehydrogenase activity in tomato fruit. Postharvest Biol. Technol. 2009, 54, 93–100. [Google Scholar] [CrossRef]
- Beaulieu, J.C. Volatile changes in cantaloupe during growth, maturation, and in stored fresh-cuts prepared from fruit harvested at various maturities. J. Am. Soc. Hort. Sci. 2006, 131, 127–139. [Google Scholar]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Fan, X.T.; Argenta, L.C. Interactive responses of gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J. Agric. Food Chem. 2005, 53, 4510–4516. [Google Scholar] [CrossRef]
- Ortiz, A.; Echeverria, G.; Lopez, M.L.; Graell, J.; Lara, I. Overall quality of “Rich Lady” peach fruit after air- or CA storage. The importance of volatile emission. LWT Food Sci. Technol. 2009, 42, 1520–1529. [Google Scholar] [CrossRef]
- Harb, J.; Bisharat, R.; Streif, J. Changes in volatile constituents of blackcurrants (Ribes nigrum L. cv. “Titania”) following controlled atmosphere storage. Postharvest Biol. Technol. 2008, 47, 271–279. [Google Scholar] [CrossRef]
- Cohen, E.; Shalom, Y.; Rosenberger, I. Postharvest ethanol buildup and off-flavor in Murcott tangerine fruits. J. Am. Soc. Hort. Sci. 1990, 115, 775–778. [Google Scholar]
- Dang, K.T.H.; Singh, Z.; Swinny, E.E. Edible Coatings Influence Fruit Ripening, Quality, and Aroma Biosynthesis in Mango Fruit. Agric. Food Chem. 2008, 56, 1361–1370. [Google Scholar] [CrossRef]
- Saftner, R.A.; Conway, W.S.; Sam, C.E. Postharvest calcium infiltration alone and combined with surface coating treatments influence volatile levels, respiration, ethylene production, and internal atmospheres of “Golden Delicious” apples. J. Am. Soc. Hort. Sci. 1999, 124, 553–558. [Google Scholar]
- Baldwin, E.; Burns, J.K.; Kazokas, W; Brecht, J.K.; Hagenmaier, R.D.; Bender, R.J.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharv. Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- McDonald, R.E.; McCollum, T.G.; Baldwin, E.A. Prestorage heat treatments influence free sterols and flavor volatiles of tomatoes stored at chilling temperature. J. Am. Soc. Hort. Sci. 1996, 12, 531–536. [Google Scholar]
- Ortiz, A.; Echeverría, G.; Graell, J.; Lara, I. The emission of flavour-contributing volatile esters by “Golden Reinders” apples is improved after mid-term storage by postharvest calcium treatment. Postharvest Biol. Technol. 2010, 57, 114–123. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. The role of methyl jasmonate in mango ripening and biosynthesis of aroma volatile compounds. J. Hortic. Sci. Biotechnol. 2003, 78, 470–484. [Google Scholar]
- Kondo, S.; Mattheis, J. Aroma volatile biosynthesis in apples at harvest or after harvest affected by jasmonates. Acta Hortic. 2006, 712, 381–388. [Google Scholar]
- De la Peña Moreno, F.; Blanch, G.P.; Ruiz del Castillo, M.L. Effect of (−)- and (+)-methyl jasmonate on the formation of aroma-active esters in strawberry fruit. Eur. Food Res. Technol. 2010, 231, 829–834. [Google Scholar] [CrossRef]
- De la Peña Moreno, F.; Blanch, G.P.; Flores, G.; Ruiz del Castillo, M.L. Impact of postharvest methyl jasmonate treatment on the volatile composition and flavonol content of strawberries. J. Sci. Food Agric. 2010, 90, 989–994. [Google Scholar]
- De la Peña Moreno, F.; Monagas, M.; Blanch, G.P.; Bartolomé, B.; Ruiz del Castillo, M.L. Enhancement of phenolic and aroma compounds in strawberry fruit through methyl jasmonate vapour treatment. Eur. Food Res. Technol. 2010, 230, 989–999. [Google Scholar] [CrossRef]
- Blanch, G.P.; Flores, G.; Castillo, M.L.R. Influence of methyl jasmonate in conjunction with ethanol on the formation of volatile compounds in berries belonging to the Rosaceae. Postharvest Biol. Technol. 2011, 62, 168–178. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Gang, D.R. Evolution of flavors and scents. Annu. Rev. Plant Biol. 2005, 56, 301–325. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Schwab, W.; Schreier, P. Enzymic formation of flavor volatiles from lipids. In Lipid Biotechnology; Kuo, T.M., Gardner, H.W., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 293–318. [Google Scholar]
- Schreier, P. Chromatographic Studies of Biogenesis of Plant Volatiles; Alfred Hüthig Verlag GmbH: Heidelberg, Germany, 1984. [Google Scholar]
- Chan, H.W.S. Autoxidation of Unsaturated Lipids; Academic Press: London, UK, 1987. [Google Scholar]
- Baker, A.; Graham, I.A.; Hodsworth, M.; Smith, S.M. Chewing the fat: β-Oxidation in signalling and development. Trends Plant Sci. 2006, 11, 124–132. [Google Scholar] [CrossRef]
- Goepfert, S.; Poirier, Y. β-Oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 2007, 10, 245–251. [Google Scholar] [CrossRef]
- Bartley, I.M.; Stoker, P.G.; Martin, A.D.E.; Hatfield, S.G.S.; Knee, M. Synthesis of aroma compounds by apples supplied with alcohols and methyl esters of fatty acids. J. Sci. Food Agric. 1985, 36, 567–574. [Google Scholar] [CrossRef]
- Paillard, N.M.M. The flavour of apples, pears and quinces. In Food Flavours, Part C: The Flavour of Fruits; Morton, L.D., MacLeod, A.J., Eds.; Elsevier Science Publishing Company Inc.: Amsterdam, The Netherlands, 1990; pp. 1–41. [Google Scholar]
- Tressl, R.; Albrecht, W. Biogenesis of aroma compounds through acyl pathways. In Biogeneration of Aromas; Parliament, T.H., Croteau, R., Eds.; ACS: Washington, DC, USA, 1986; pp. 114–133. [Google Scholar]
- Perez, A.G.; Sanz, C. Formation of fruit flavor. In Fruit and Vegetable Flavour; Bruckner, B., Wyllie, S.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 71–102. [Google Scholar]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Stumpe, M.; Feussner, I. Formation of oxylipins by CYP74 enzymes. Phytochem. Rev. 2006, 5, 347–357. [Google Scholar] [CrossRef]
- Lea, A.G.H. Apple juice. In Production and Packaging of Non-Carbonated Fruit Juices and Fruit Beverages, 3rd ed.; Ashurts, P.R., Ed.; Springer: Berlin, Germany, 1995; pp. 153–196. [Google Scholar]
- De Pooter, H.L.; Montens, J.P.; Dirinck, J.; Willaert, G.A.; Schamp, N.M. Treatment of Golden Delicious apple with aldehydes and carboxylic acids: Effect on the headspace composition. J. Agric. Food Chem. 1983, 37, 813–818. [Google Scholar]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Song, M.S.; Kim, D.G.; Lee, S.H. Isolation and characterization of a jasmonic acid carboxyl methyltransferase gene from hot pepper (Capsicum annuum L.). J. Plant Biol. 2005, 48, 292–297. [Google Scholar] [CrossRef]
- Akacha, N.B.; Boubaker, O.; Gargouri, M. Production of hexenol in a two-enzyme system: Kinetic study and modelling. Biotechnol. Lett. 2005, 27, 1875–1878. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Kessler, A.; Halitschke, R. Volatile signaling in plantplant-herbivore interactions: What is real. J. Curr. Opin. Plant Biol. 2002, 5, 351–354. [Google Scholar] [CrossRef]
- Beck, H.C.; Hansen, A.M.; Lauritsen, F.R. Metabolite production and kinetics of branched-chain aldehyde oxidation in Staphylococcus xylosus. Enzyme Microb. Technol. 2002, 31, 94–101. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Dahl, S.; Carballo, F.J.; Malcata, F.X. Amino acid catabolism and generation of volatiles by lactic acid bacteria. J. Dairy Sci. 2002, 85, 2462–2470. [Google Scholar] [CrossRef]
- Perez, A.G.; Olias, R.; Luaces, P.; Sanz, C. Biosynthesis of Strawberry Aroma Compounds through Amino Acid Metabolism. J. Agric. Food Chem. 2002, 50, 4037–4042. [Google Scholar] [CrossRef]
- Rowan, D.D.; Lane, H.P.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of 2-methylbutyl, 2-methyl-2-butenyl, and 2-methylbutanoate esters in red delicious and granny smith apples using deuterium-labeled substrates. J. Agric. Food Chem. 1996, 44, 3276–3285. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Fellman, J.K. Formation of volatile branched chain esters in bananas (Musa sapientum L.). J. Agric. Food Chem. 2000, 48, 3493–3496. [Google Scholar] [CrossRef]
- Perez, A.G.; Rios, J.J.; Sanz, C.; Olias, J.M. Aroma components and free amino acids in strawberry variety Chandler during ripening. J. Agric.Food Chem. 1992, 40, 2232–2235. [Google Scholar] [CrossRef]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Substrate usage by recombinant alcohol acyltransferases from various fruit species. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef]
- Newman, J.D.; Chappell, J. Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar]
- Laule, O.; Furholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef]
- Miller, B.; Oschinski, C.; Zimmer, W. First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 2001, 213, 483–487. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar]
- Ogura, K.; Koyama, T. Enzymatic aspects of isoprenoid chain elongation. Chem. Rev. 1998, 98, 1263–1276. [Google Scholar] [CrossRef]
- Koyama, T.; Ogura, K. Isopentenyl diphosphate isomerase and prenyltransferases. In Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids; Cane, D.E., Ed.; Pergamon Press, Oxford, UK, 1999; Volume 2, pp. 69–96. [Google Scholar]
- Cane, D.E. Sesquiterpene biosynthesis: Cyclization mechanisms. In Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids; Cane, D.E., Ed.; Pergamon Press: Oxford, UK, 1999; Volume 2, pp. 155–200. [Google Scholar]
- Wise, M.L.; Croteau, R. Monoterpene biosynthesis. In Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids; Cane, D.E., Ed.; USA: Pergamon Press: Oxford, UK, 1999; Volume 2, pp. 97–153. [Google Scholar]
- Martin, D.; Faldt, J.; Bohlmann, J. Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol. 2004, 135, 1908–1927. [Google Scholar] [CrossRef]
- Lupien, S.; Karp, F.; Wildung, M.; Croteau, R. Regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha) species: Cdna isolation, characterization, and functional expression of (−)-4S-limonene-3-hydroxylase and (−)-4S-limonene-6-hydroxylase. Arch. Biochem. Biophys. 1999, 368, 181–192. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Gershenzon, J.; Konings, M.C.J.M.; Croteau, R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway—I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998, 117, 901–912. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Konings, M.C.J.M.; Gershenzon, J.; Karp., F.; Croteau, R. Cytochrome P-450 dependent (+)-limonene-6-hydroxylation in fruits of caraway (Carum carvi). Phytochemistry 1999, 50, 243–248. [Google Scholar]
- Iijima, Y.; Wang, G.; Fridman, E.; Pichersky, E. Analysis of the enzymatic formation of citral in the glands of sweet basil. Arch. Biochem. Biophys. 2006, 448, 141–149. [Google Scholar] [CrossRef]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses: Identification of an acetyl-CoA: Geraniol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials; Wiley-VCH Velagsgesellschaft mbH: Weinheim, Germany, 2001. [Google Scholar]
- Luan, F.; Mosandl, A.; Munch, A.; Wust, M. Metabolism of geraniol in grape berry mesocarp of Vitis vinifera L. cv. Scheurebe: Demonstration of stereoselective reduction, E/Z-isomerization, oxidation and glycosylation. Phytochemistry 2005, 66, 295–303. [Google Scholar]
- Degenhardt, J.; Gershenzon, J. Demonstration and characterization of (E)-nerolidol synthase from maize: A herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 2000, 210, 815–822. [Google Scholar] [CrossRef]
- Rosati, C.; Diretto, G.; Giuliano, G. Biosynthesis and Engineering of Carotenoids and Apocarotenoids in Plants: State of the Art and Future Prospects. Biotech. Genet. Eng. Rev. 2009, 26, 151–174. [Google Scholar]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gomez, M.D.; Orzaez, D.; Granell, A.; Gomez-Gomez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in betai onone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.J.; Leyser, O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Loewen, M.C. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J. Biol. Chem. 2004, 279, 46940–46945. [Google Scholar] [CrossRef]
- Bouvier, F.; Dogbo, O.; Camara, B. Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 2003, 300, 2089–2091. [Google Scholar] [CrossRef]
- Bouvier, F.; Suire, C.; Mutterer, J.; Camara, B. Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 2003, 15, 47–62. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200-8229. https://doi.org/10.3390/molecules18078200
El Hadi MAM, Zhang F-J, Wu F-F, Zhou C-H, Tao J. Advances in Fruit Aroma Volatile Research. Molecules. 2013; 18(7):8200-8229. https://doi.org/10.3390/molecules18078200
Chicago/Turabian StyleEl Hadi, Muna Ahmed Mohamed, Feng-Jie Zhang, Fei-Fei Wu, Chun-Hua Zhou, and Jun Tao. 2013. "Advances in Fruit Aroma Volatile Research" Molecules 18, no. 7: 8200-8229. https://doi.org/10.3390/molecules18078200
APA StyleEl Hadi, M. A. M., Zhang, F.-J., Wu, F.-F., Zhou, C.-H., & Tao, J. (2013). Advances in Fruit Aroma Volatile Research. Molecules, 18(7), 8200-8229. https://doi.org/10.3390/molecules18078200