1. Introduction
Carotenoids are the most important pigments in Nature that are responsible for the various colours of different photosynthetic organisms [
1,
2,
3]. Because of carotenoids’ general inability to be synthesised in animal cells, they must be obtained from the diet [
1,
4]. Recent studies have considered carotenoids with no pro-vitamin A activity (such as astaxanthin) because of their importance to cell survival, growth, reproduction, pigmentation and protection against light sensitisation [
5]. Astaxanthin, which can be found in different aquatic animals (and, therefore, seafoods), including lobster, salmon, red sea bream, trout, shrimp, and fish eggs, has superior antioxidant activity due to existence of both hydroxyl and ketonic end groups on each ionone ring of its chemical structure [
2]. It can react with a scavenger radical either by removing one of the latter’s electrons or by adding itself to another molecule to pair its own unpaired electron, thereby forming an adduct [
6].
However, like other carotenoids, the low water solubility of this functional lipid compound has made its use in water based food formulations problematic. Furthermore, these compounds may be prone to reduced bioavailability [
7]. Therefore, it is important to find solutions to this problem. One of the most important applications of nanotechnology in the food and nutrition industries is in the design and development of novel functional food ingredients with improved water solubility, thermal stability, oral bioavailability, sensory attributes and physiological performances. Nanodispersion systems are a few of the nanoscale systems currently being investigated for commercial applications in this regard [
3,
8].
It is noteworthy that like other carotenoids, astaxanthin is unstable because of its susceptibility to light, oxygen, and autooxidation [
9,
10]. Generally the combination of different antioxidants may act additively or even synergistically. The synergistic antioxidant activities of carotenoids with α-tocopherol and ascorbic acid have already been confirmed by various previous researches [
10,
11,
12]. It was shown that α-tocopherol, that is an important natural antioxidant in living cells and the most important lipid-soluble radical scavenger in membranes and plasma, can protect carotenoids from autooxidation [
10,
13,
14]. Although water-soluble ascorbic acid was less reactive with lipid radicals than hydrophobic astaxanthin, it was able to interact with the oxidized forms of astaxanthin to regenerate astaxanthin in a time-course reaction [
15].
Therefore, in this research astaxanthin was first incorporated in nanodispersion system to produce water dispersible astaxanthin nanoparticles through a solvent diffusion technique. A mixture of a small molecular emulsifier (Polysorbate 20), protein (sodium caseinate) and polysaccharide (gum Arabic) with optimized proportions was used as a three-component stabilizer system in production of astaxanthin nanoparticles [
16]. Then the effects of additional α-tocopherol and ascorbic acid on astaxanthin stability of produced nanodispersions were investigated and at last, the concentrations of these additional antioxidant compounds were optimized using response surface methodology.
2. Results and Discussion
Astaxanthin nanodispersions were produced using emulsification solvent-diffusion method. For this method, the first step of particle preparation is organic phase dispersion of globules in aqueous phase at high stirring speed. Once the emulsion is formed, the submicron droplets are then diluted in water by removing the organic phase, and the interaction between the emulsion droplets and the dilution phase is referred to as a modification of phase equilibrium and solvent diffusion, which leads to precipitation of astaxanthin and production of astaxanthin particles. Since the particle size of produced astaxanthin nanodispersions (measured using a Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK), was in nanometre ranges (98.3 ± 4.27 nm), it can be concluded that the high shear stress due to the emulsification step have guaranteed the submicron droplet formation of astaxanthin in studied dispersion system. The particle size distribution of freshly produced astaxanthin nanodispersions was shown in
Figure 1.
Figure 1.
Particle size distribution of freshly produced astaxanthin nanodispersion.
Figure 1.
Particle size distribution of freshly produced astaxanthin nanodispersion.
The astaxanthin degradation rates in all studied samples can be fit by first-order kinetic equations with coefficients of determination (
R2) higher than 0.8 (
Table 1). Therefore, the degradation rate of astaxanthin (r) in nanodispersions can be written as:
where C is the concentration of astaxanthin at time t, C
0 is initial concentration of astaxanthin (100 mg/L) and k is the rate constant.
In autooxidation of astaxanthin through a free radical chain mechanism, the astaxanthin radical is formed by an attack of radicals, in the initiation step, then the carbon-centered, carotenoid radical reacts with oxygen to give astaxanthin peroxyl radical, which attacks another astaxanthin molecule and abstracts a hydrogen atom to give astaxanthin hydroperoxide and at the same time a new astaxanthin radical starts the propagation sequence over again. Consequently, many molecules of astaxanthin can be oxidized to astaxanthin hydroperoxides for every initiation event.
The propagation cycle can be broken by termination reactions, which result in the destruction of free radicals. The autooxidation of astaxanthin can be suppressed by inhibiting the initiation step or accelerating the termination step, which can be provided by addition of antioxidants that are divided into preventive antioxidants and chain-breaking antioxidants categories. Whereas preventive antioxidants neutralize the active species and possible precursors of free radicals and thereby suppress the generation of free radicals and reduce the rate of chain initiation, the chain-breaking antioxidants can scavenge oxygen-centred radicals and suppress the autooxidation [
17,
18].
Table 1.
Rate constant and coefficient of determinations for astaxanthin degradation of optimum astaxanthin nanodispersions diluted in deionized water with different concentrations of additional antioxidant compounds (followed a first order kinetic equation).
Table 1.
Rate constant and coefficient of determinations for astaxanthin degradation of optimum astaxanthin nanodispersions diluted in deionized water with different concentrations of additional antioxidant compounds (followed a first order kinetic equation).
| Antioxidant | Antioxidant concentrations (mg/L) |
|---|
| 0 | 5 | 10 | 50 | 100 |
|---|
| α-tocopherol | Rate constant (week−1) | 0.3685 aA | 0.0926 bB | 0.0140 cB | 0.0190 dB | 0.0125 eB |
| | SE Coef. (n = 3) 1 | 0.0042 | 0.0065 | 0.0010 | 0.0000 | 0.0008 |
| | R2 | 0.9915 | 0.9857 | 0.8977 | 0.9526 | 0.8139 |
| Ascorbic acid | Rate constant (week−1) | 0.3685 aA | 0.2528 cA | 0.2106 dA | 0.2028 eA | 0.2854 bA |
| | SE Coef. (n = 3) 1 | 0.0551 | 0.0004 | 0.0043 | 0.0029 | 0.0000 |
| | R2 | 0.9915 | 0.9506 | 0.9822 | 0.9793 | 0.9856 |
Ascorbic acid and α-tocopherol are two common antioxidant molecules used in different food and beverage formulations. The effect of water-soluble ascorbic acid as an antioxidant is complicated because of its multiple functions. Ascorbic acid is oxidized by oxygen to give the ascorbate radical anion and hydrogen peroxide, although the precise molecular mechanism remains controversial. Ascorbic acid also can react with iron and reduce ferric ion to ferrous ion which lead to decomposition of hydroperoxide and hydrogen peroxide to give alkoxyl and hydroxyl radicals respectively. Accordingly, ascorbic acid may function as a prooxidant in the presence of iron and hydrogen peroxide or hydroperoxide. On the other hand, α-tocopherol is a fat-soluble phenolic compound that can scavenge the chain-carrying peroxyl radicals rapidly and interrupts the chain propagation [
19]. The high activity of α-tocopherol as an antioxidant stems from the high reactivity of α-tocopherol toward oxygen-centred radicals. In fact, α-tocopherol has much higher rate constant than other synthetic phenolic antioxidants [
18,
20].
Table 1 shows the astaxanthin loss rate in nanodispersions with deionized water containing α-tocopherol and acid ascorbic in different concentrations. The astaxanthin degradation rate in all studied diluted nanodispersion systems in this part were also followed first order kinetic equations. As can be seen in
Table 1, the addition of α-tocopherol was more efficient than ascorbic acid in prevention of astaxanthin degradation of diluted optimum formulated nanodispersions. Therefore, as also reported in previous researchers, the presence of lipophilic antioxidants in carotenoid nanodispersion systems can inhibit carotenoid oxidation more effectively than hydrophilic antioxidants [
21]. They can react with free radical intermediates of carotenoid peroxidation and with the peroxides and consequently make the carotenoids inactive against these active molecules. They can also act as a reducing agent for transition metals. The major biochemical function of α-tocopherol in these systems is its reactivity with possible presented organic peroxyl radicals in environment. Additionally, tocopherols are the most effective antioxidant at high oxygen tensions [
21,
22]. Additionally,
Table 1 illustrated that in the systems containing antioxidants, astaxanthin degradation could also be well explained by first-order kinetics (high
R2 values). Increasing the concentrations of added antioxidants up to certain concentrations could caused a decrease in astaxanthin degradation of systems, but further increase in their concentrations did not affect the astaxanthin loss rates considerably or affect it inversely. Due to synergistic antioxidant effect of ascorbic acid and α-tocopherol, which was reported in previous reports [
23,
24,
25], a CCD was conducted to study the effects of their combinational effects on retarding of astaxanthin loss of diluted optimum formulated nanodispersions in deionized water.
Response-surface analysis provided empirically significant (p < 0.05) models for estimating the rate constant of astaxanthin degradation during the storage at 5 °C as function of the ascorbic acid and α-tocopherol concentrations in the prepared nanodispersions. As a result, the optimization process predicted an optimum level for these independent variables that resulted in the desirable goals. The interaction term were dropped because of its insignificant effect on studied response value (p > 0.05). However, the linear term of ascorbic acid concentration were included in the final reduced despite of its insignificancy (p > 0.05), because its quadratic terms were found to be significant (p < 0.05).
Table 2 contains the corresponding
R2, adjusted
R2 values of the regression equations and significance of each term in final reduced model which were determined using the F-ratio and p-values. The relationship between astaxanthin degradation rate constant (week
−1) and concentrations of ascorbic acid and α-tocopherol could be explained significantly (
p < 0.05) by a second-order polynomial regression model. The main and quadratic effect of α-tocopherol concentration had a highest significant effect (highest F-ratio) on studied response than of ascorbic acid. Therefore, α-tocopherol concentration was considered as critical parameter to control astaxanthin degradation of nanodispersions with additional antioxidants, diluted in deionized water during storage. The final reduced model fitted to the experimental data, was a valid statistical empirical model only in the selected ranges [
26].
Table 2.
Regression coefficients, R2, adjusted R2 and probability values for the final reduced models.
Table 2.
Regression coefficients, R2, adjusted R2 and probability values for the final reduced models.
| Regression coefficients | Coefficients | F-ratio | P-value |
|---|
| β0 | 20.6923 | 44.42 | 0.000b |
| β1 | −0.1490 | 2.84 | 0.138 |
| β2 | −0.6854 | 57.35 | 0.000b |
| β11 | 0.0028 | 10.89 | 0.011b |
| β22 | 0.0059 | 47.83 | 0.000b |
| β12 | - | - | - |
| R2 | 91.5% | 21.64 (Regression) | 0.000 (Regression) |
| R2-adj | 87.3% |
Generally, the results demonstrated that the addition of antioxidants, either individually or in combination, could decrease the astaxanthin degradation rate constant in all prepared astaxanthin nanodispersions. The astaxanthin degradation rate constant in all samples were less than astaxanthin degradation rate constant of optimum formulated nanodispersion without addition of any antioxidant agents. The astaxanthin degradation rate constant was negatively associated with the linear effects of α-tocopherol, and positively associated with the quadratic effects of both ascorbic acid and α-tocopherol concentrations.
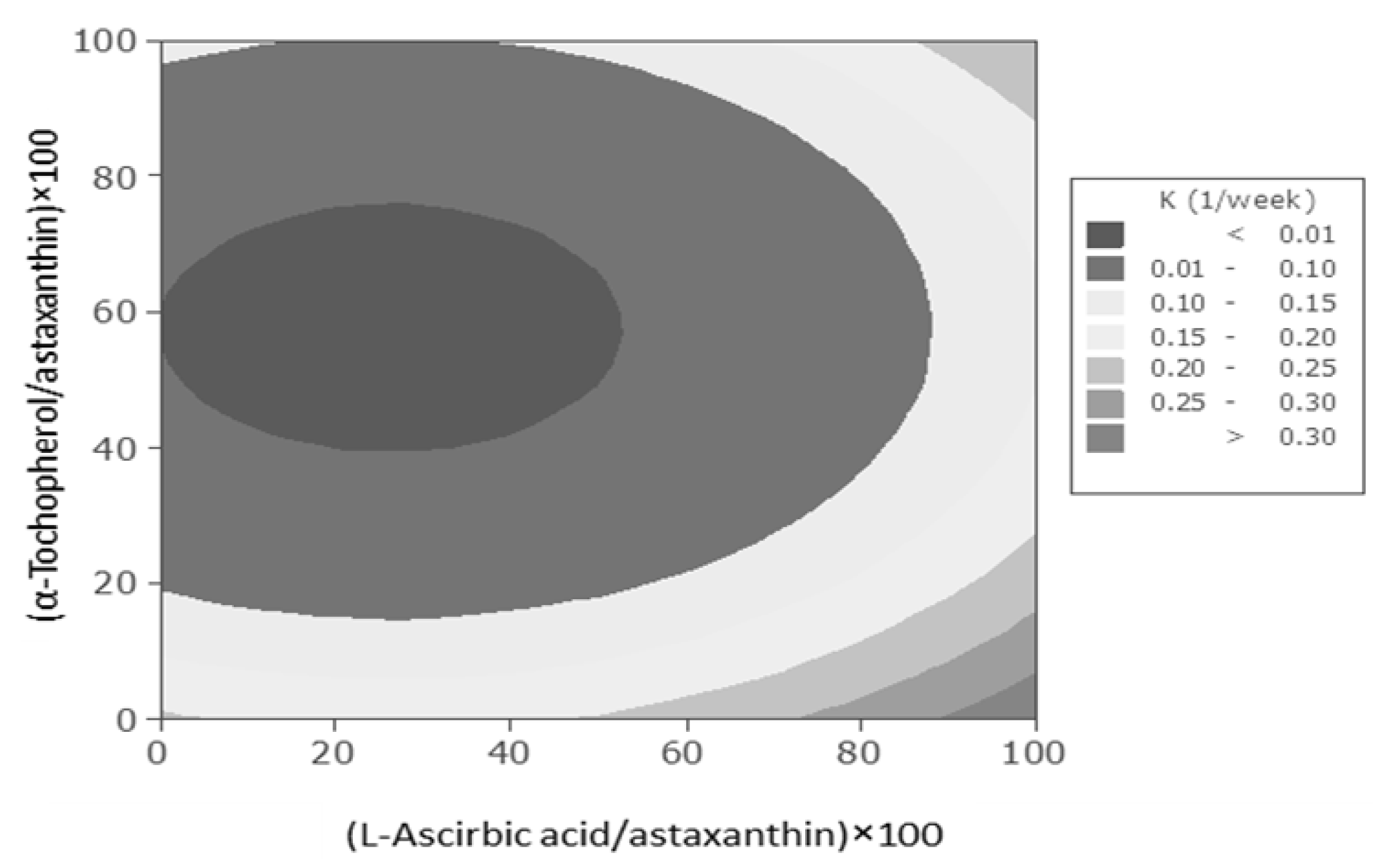
As shown in
Figure 2, the combination of the highest levels of ascorbic acid and the least levels of α-tocopherol or
vice versa led to highest astaxanthin degradation rates in these systems. In contrast, the considerable decrease in astaxanthin degradation rate took place due to combination of middle levels of α-tocopherol concentration with low levels of ascorbic acid concentrations. Despite of relatively low antioxidant activity of ascorbic acid when it was added individually to diluted astaxanthin nanodispersions, it could drastically enhance the antioxidant activity when it was combined with α-tocopherol. In combination of low concentrations of ascorbic acid with relatively middle concentrations of α-tocopherol, the ascorbic acid might reduce the tocopheroxyl radical formed from tocopherol during the scavenging of free radicals
in vivo, which permits a single molecule of α-tocopherol to scavenge many radicals and also links ascorbic acid to the protection of astaxanthin against free-radical damage. It was also reported that in these systems, both α-tocopherol and ascorbic acid can scavenge the peroxyl radicals, but α-tocopherol scavenges the radicals faster than ascorbic acid. However, the α-tocopherol radical formed is reduced by ascorbic acid to regenerate α-tocopherol. Thus, α-tocopherol is not consumed at first and only ascorbic acid disappears [
18].
Figure 2.
.Contour plot for astaxanthin degradation rate constant (week−1) in astaxanthin nanodispersions as a function of added ascorbic acid and α-tocopherol concentrations.
Figure 2.
.Contour plot for astaxanthin degradation rate constant (week−1) in astaxanthin nanodispersions as a function of added ascorbic acid and α-tocopherol concentrations.
Astaxanthin might scavenge the existed radicals in the system and produce radicals which may undergo following competing reactions; it may react with another peroxyl radical to give a stable adduct, react with another astaxanthin to give a dimmer or react with α-tocopherol or ascorbic acid to regenerate astaxanthin and produce α-tocopherol or ascorbic acid radicals. The reduction of astaxanthin with α-tocopherol was more preferable than ascorbic acid. The same procedure would be happened to α-tocopherol radicals. The mentioned path reactions are determined by the reactivities, concentrations and accessibilities components. It could be suggested that in studied binary mixture of ascorbic acid and α-tocopherol, high concentrations of ascorbic acid would interrupt the reduction of astaxanthin molecules by α-tocopherol in regeneration process of astaxanthin. Therefore, the synergistic effect of ascorbic acid and α-tocopherol in studied system were found to be dependant of their concentrations [
18,
24,
27,
28].
The results also showed that high concentrations of ascorbic acid (higher than 60 mg/L), led to physical instability in diluted nanodispersions due to decreasing in pH of system that approached to isoelectric pH of used stabilizer system. The regression equations showed that, at the lower levels of studied factors, increasing the concentrations of both antioxidants caused a decrease in astaxanthin autooxidation rate constant, but further increasing in concentrations affected the astaxanthin degradation inversely and led to increase in astaxanthin loss of diluted nanodispersions. Therefore, the optimum values for antioxidant concentrations to produce diluted astaxanthin nanodispersions with minimum autooxidation of astaxanthin and maximum chemical stability, fell between the low concentrations for ascorbic acid and middle concentrations for α-tocopherol. The same results had also been obtained in previous researchers which were done on synergic effect of different groups of water soluble and fat soluble antioxidants [
24,
27].
In this study, concentrations of antioxidants in optimum astaxanthin nanodispersions that led to considerable chemical stability were considered as most favourable, if they led to a considerable increase in chemical stability of system without any changes in their physical stability. A numerical optimization was also carried out to check the exact optimum levels of the independent variables leading to the desired response goal. It showed that the diluted astaxanthin nanodispersion with the highest chemical stability was predicted to be obtained by using 40 mg/L ascorbic acid and 60 mg/L α-tocopherol as additives during the preparation of astaxanthin nanodispersions. Under the optimum conditions, the corresponding predicted response value for astaxanthin degradation rate constant was found to be less than 0.00001 (week
−1). No significant (
p > 0.05) difference that were observed between the initial and after storage (four weeks at 5 °C) astaxanthin concentrations of nanodispersions containing optimized antioxidants additives, confirmed the high chemical stability of produced astaxanthin nanodispersions. The experimental and predicted values of astaxanthin loss rate constants of diluted optimum nanodispersions in deionized water were shown in
Table 3.
Table 3.
Experimental and predicted values of astaxanthin loss rate of diluted optimum nanodispersions in deionized water obtained from the applied CCD for optimization the concentrations of additional antioxidants.
Table 3.
Experimental and predicted values of astaxanthin loss rate of diluted optimum nanodispersions in deionized water obtained from the applied CCD for optimization the concentrations of additional antioxidants.
| Treatment runs | Y exp | Y pre | Y exp- Y pre |
|---|
| 1 | 0.0092 | 0.0093 | −0.0001 |
| 2 | 0.0095 | 0.0093 | 0.0002 |
| 3 | 0.1143 | 0.1136 | 0.0007 |
| 4 | 0.2028 | 0.2033 | −0.0005 |
| 5 | 0.0091 | 0.0093 | −0.0002 |
| 6 | 0.0937 | 0.0945 | −0.0008 |
| 7 | 0.0093 | 0.0093 | 0.0000 |
| 8 | 0.0075 | 0.0073 | 0.0002 |
| 9 | 0.1550 | 0.1524 | 0.0026 |
| 10 | 0.0190 | 0.0188 | 0.0002 |
| 11 | 0.0092 | 0.0093 | −0.0001 |
| 12 | 0.1834 | 0.1875 | −0.0041 |
| 13 | 0.1765 | 0.1888 | −0.0123 |
No significant difference (p > 0.05) between experimental and predicted value of astaxanthin degradation rate constants confirmed the sufficiency of the corresponding regression model used for predicting the variation of studied response.