Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology
Abstract
:1. Introduction
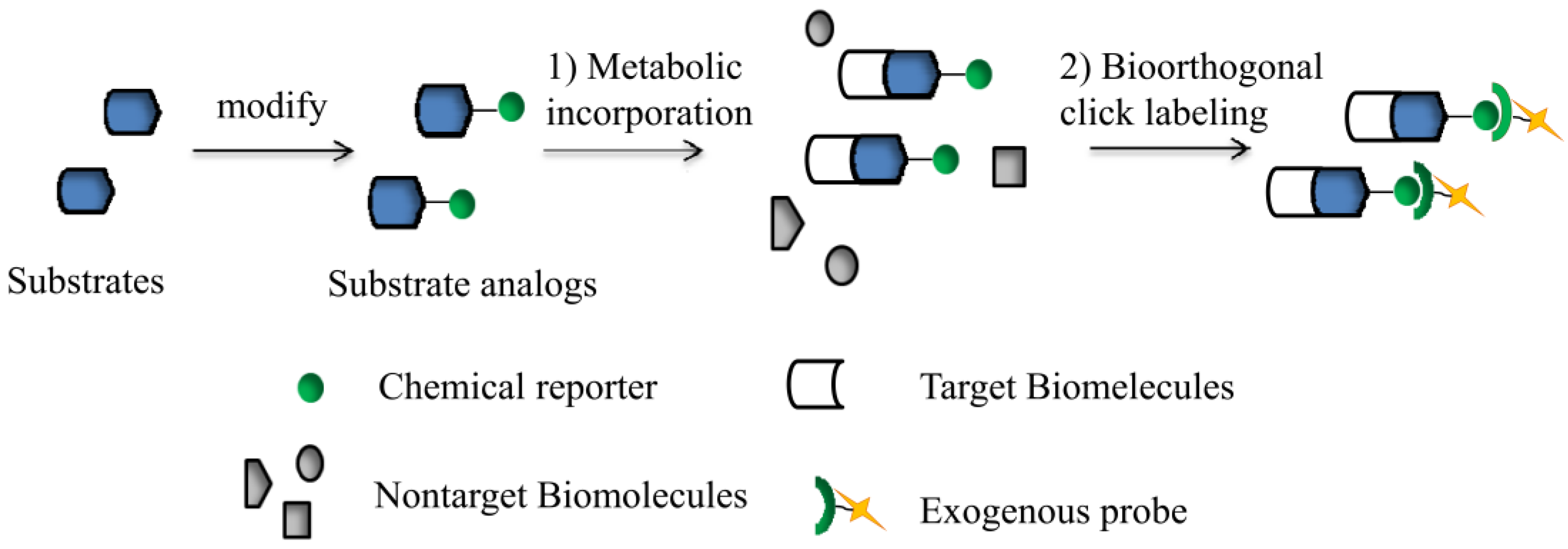
1.1. Azide-Based Click Chemistry in Glycobiology
1.1.1. Azide-Alkyne Cycloaddition
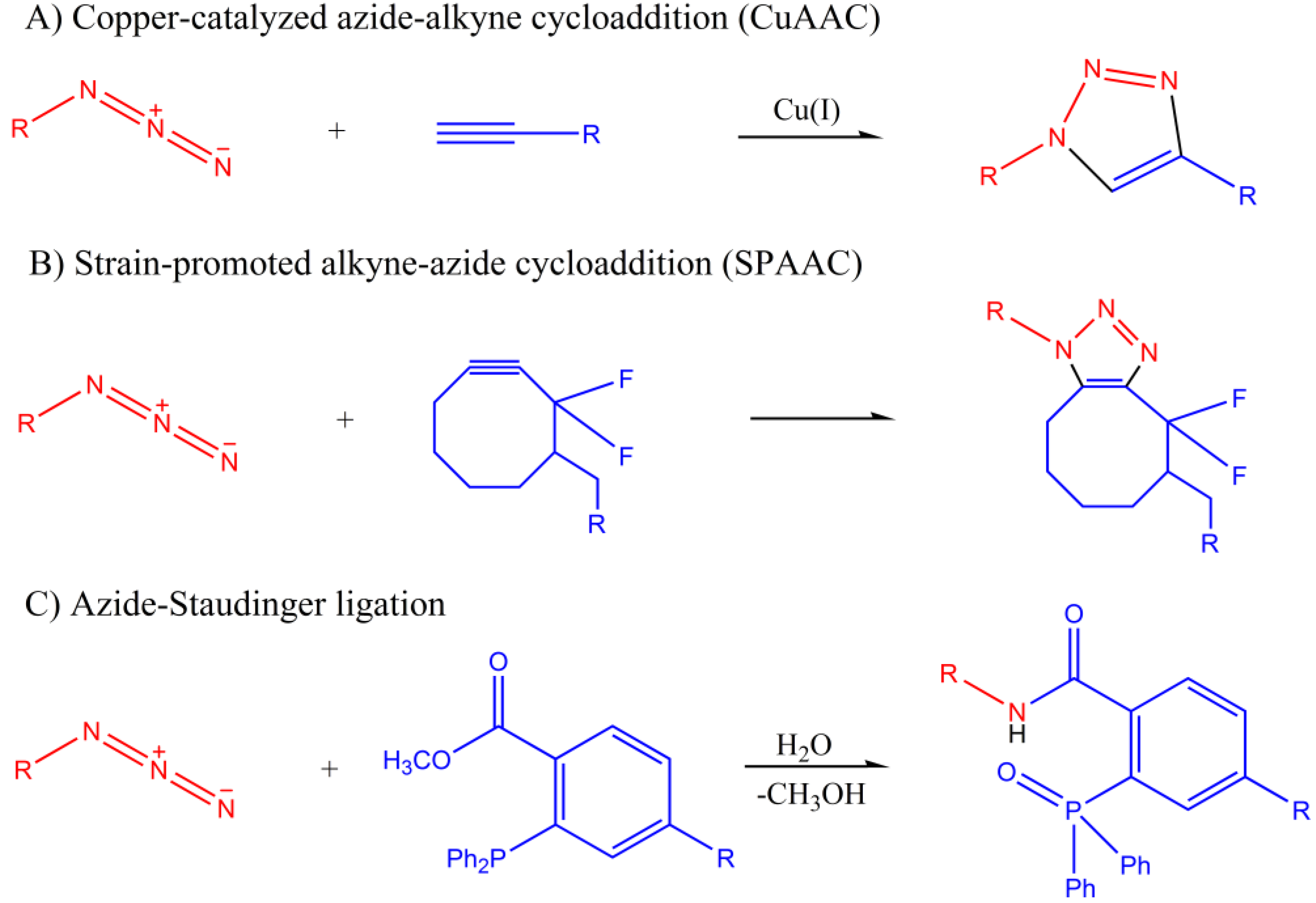
1.1.2. Azide-Staudinger Ligation
2. Applications of Azide-Based Click Chemistry in Glycan Research
2.1. Glycan Metabolic Engineering
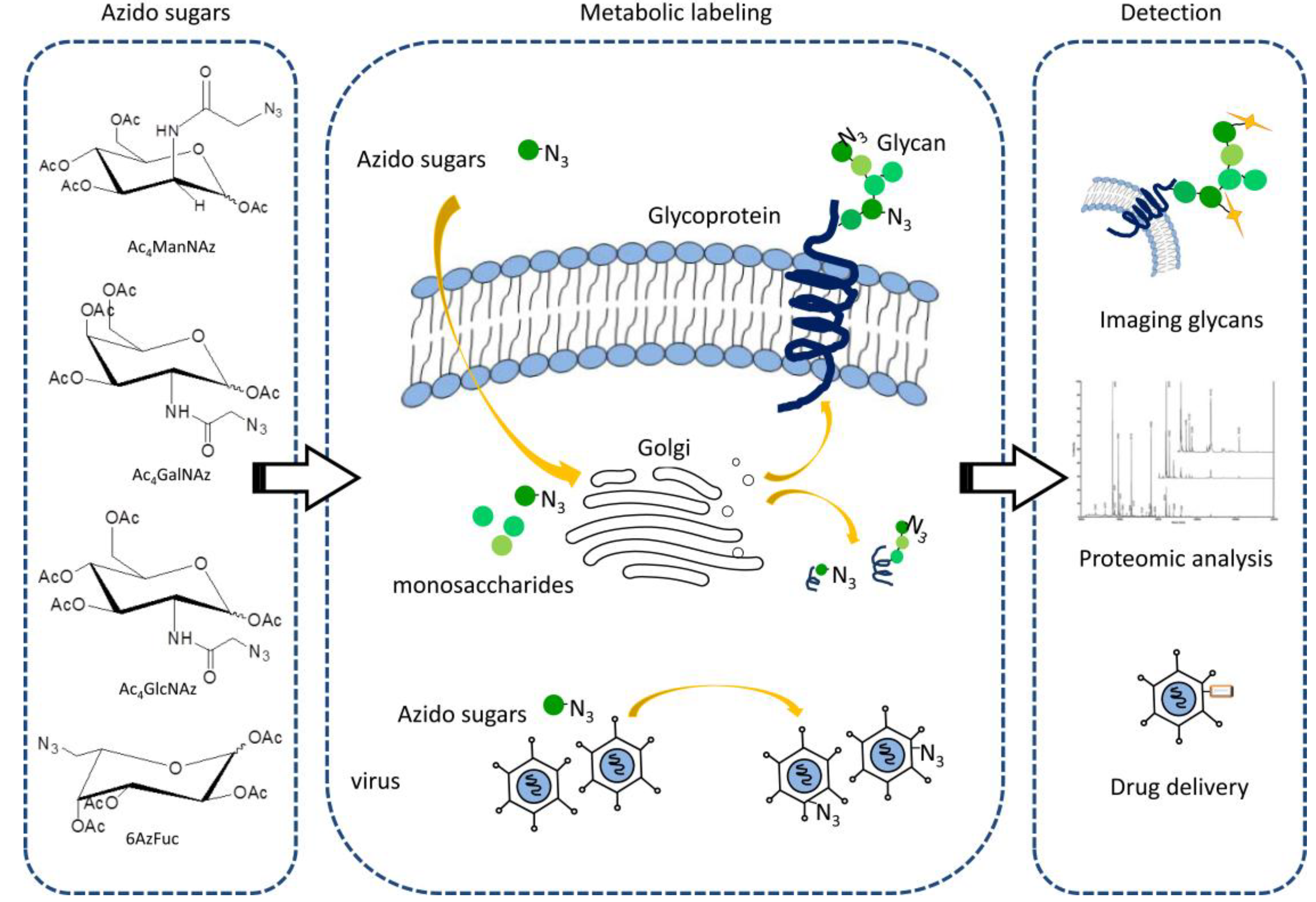
2.1.1. Metabolic Labeling for in Vivo Imaging of Glycans
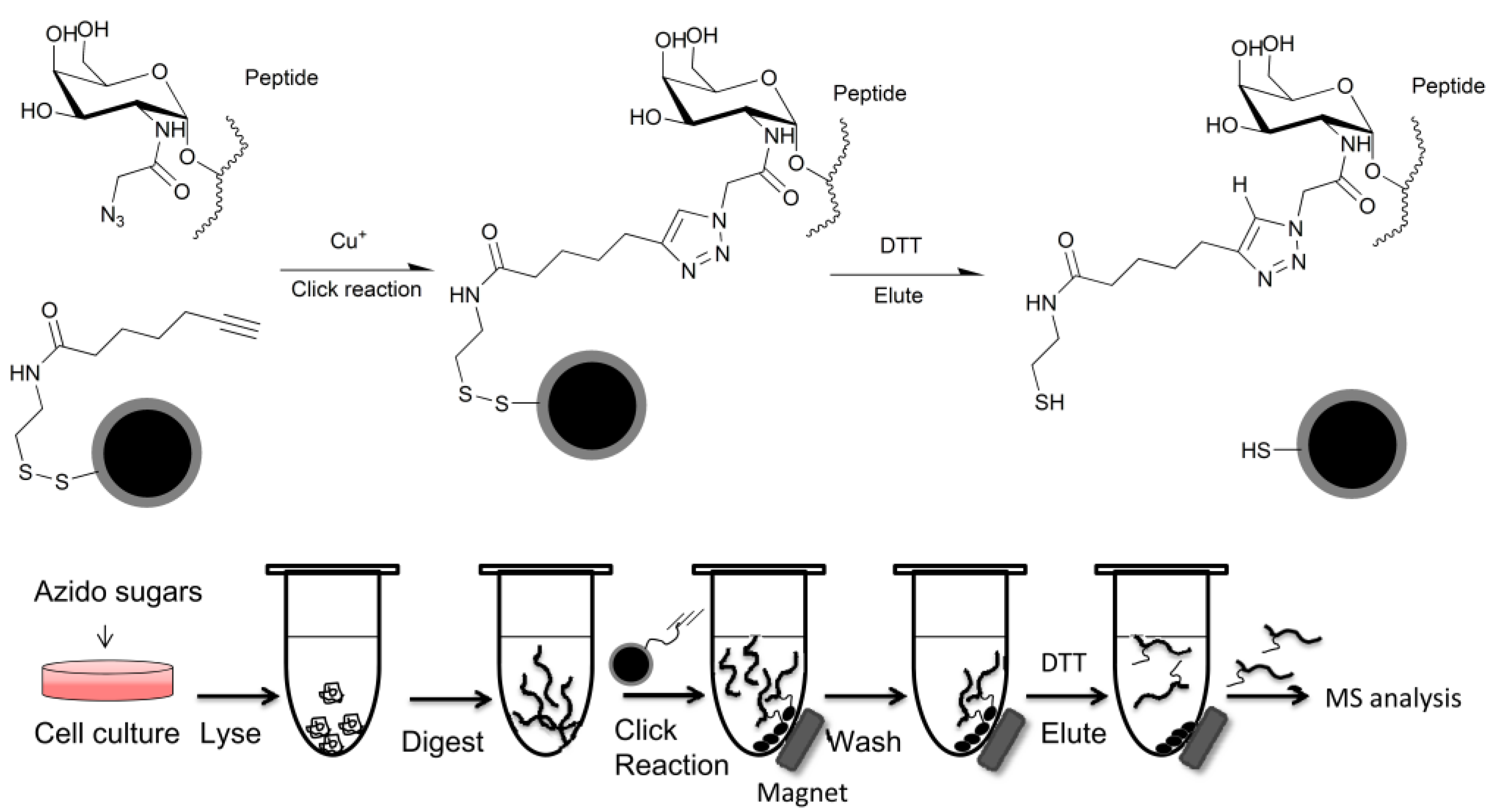
2.1.2. Glycan Enrichment and Glycomics Analysis
2.1.3. Viral Surface Engineering and Drug Delivery
2.2. Other Applications
2.2.1. Click Chemistry-Based Activity Based Protein Profiling (CC-ABPP)
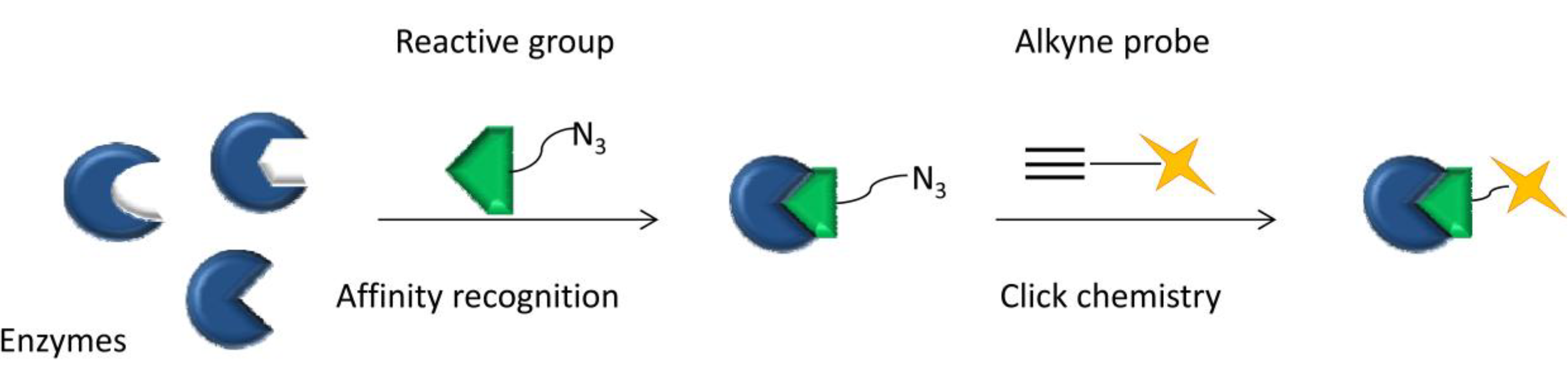
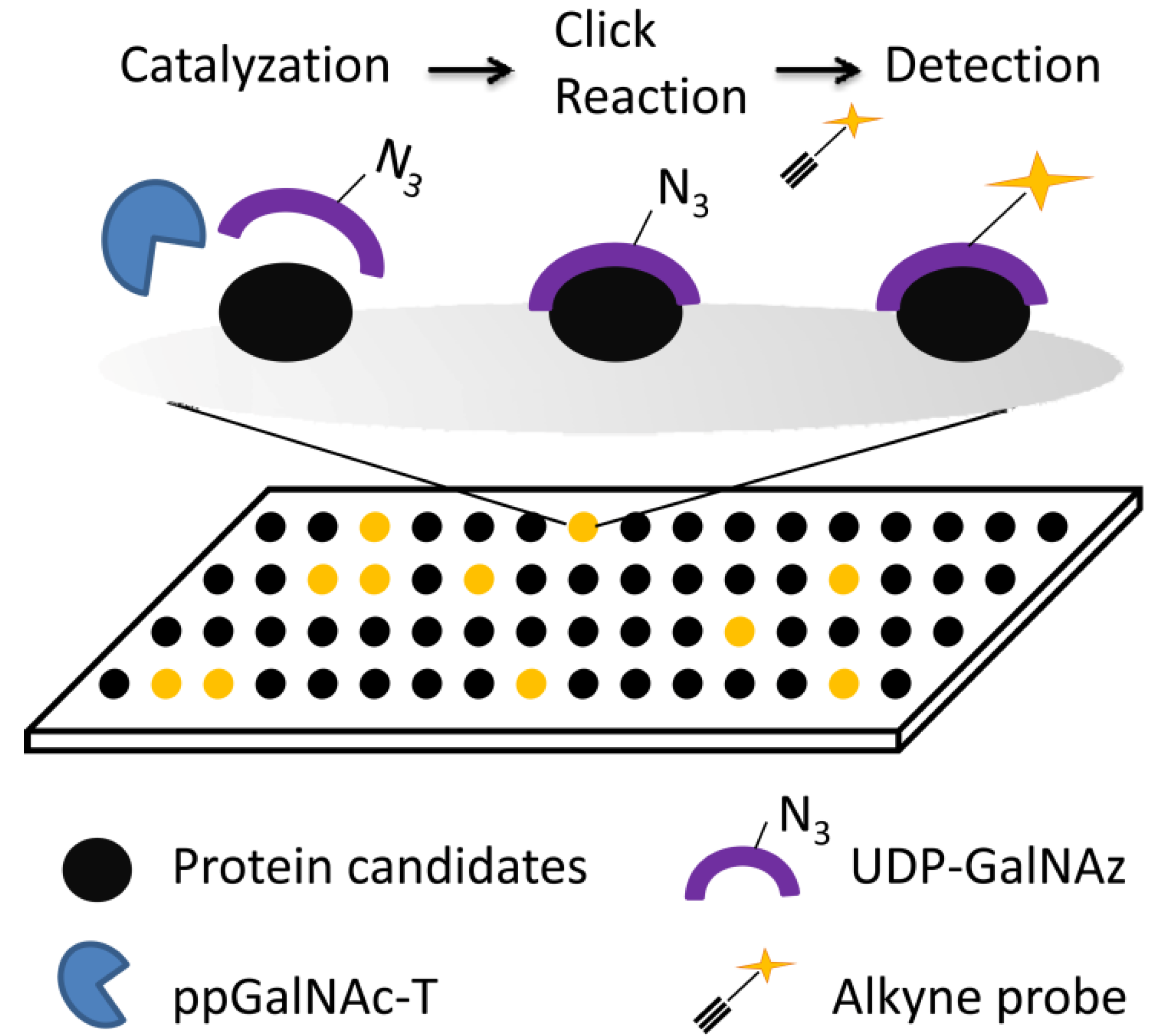
2.2.2. Glycan Microarrays
3. Conclusion and Future Directions
Acknowledgments
Conflicts of Interest
References
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar]
- Rademacher, T.W.; Parekh, R.B.; Dwek, R.A. Glycobiology. Annu. Rev. Biochem. 1988, 57, 785–838. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef]
- Baskin, J.M.; Bertozzi, C.R. Bioorthogonal click chemistry: Covalent labeling in living systems. Qsar Comb. Sci. 2007, 26, 1211–1219. [Google Scholar] [CrossRef]
- Zeng, D.; Zeglis, B.M.; Lewis, J.S.; Anderson, C.J. The Growing Impact of Bioorthogonal Click Chemistry on the Development of Radiopharmaceuticals. J. Nucl. Med. 2013, 54, 829–832. [Google Scholar] [CrossRef]
- Rouhanifard, S.H.; Nordstrom, L.U.; Zheng, T.; Wu, P. Chemical probing of glycans in cells and organisms. Chem. Soc. Rev. 2013, 42, 4284–4296. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: synthesis of 5-acyltetrazoles from azides and acyl cyanides. Angew. Chem. Int. Ed. 2002, 41, 2113–2116. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling live cells by copper-catalyzed alkyne-azide click chemistry. Bioconjug. Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef]
- Soriano Del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A.C.; Levy, M.; Liu, Y.; Marlow, F.L.; Wu, P. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [Google Scholar] [CrossRef]
- Besanceney-Webler, C.; Jiang, H.; Zheng, T.; Feng, L.; Soriano del Amo, D.; Wang, W.; Klivansky, L.M.; Marlow, F.L.; Liu, Y.; Wu, P. Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study. Angew. Chem. Int. Ed. 2011, 50, 8051–8056. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Agard, N.J.; Baskin, J.M.; Prescher, J.A.; Lo, A.; Bertozzi, C.R. A comparative study of bioorthogonal reactions with azides. ACS Chem. Biol. 2006, 1, 644–648. [Google Scholar] [CrossRef]
- Chang, P.V.; Prescher, J.A.; Sletten, E.M.; Baskin, J.M.; Miller, I.A.; Agard, N.J.; Lo, A.; Bertozzi, C.R. Copper-free click chemistry in living animals. Proc. Natl. Acad. Sci. USA 2010, 107, 1821–1826. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef]
- Kiick, K.L.; Saxon, E.; Tirrell, D.A.; Bertozzi, C.R. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl. Acad. Sci. USA 2002, 99, 19–24. [Google Scholar] [CrossRef]
- Lemieux, G.A.; De Graffenried, C.L.; Bertozzi, C.R. A fluorogenic dye activated by the staudinger ligation. J. Am. Chem. Soc. 2003, 125, 4708–4709. [Google Scholar]
- Mahal, L.K.; Yarema, K.J.; Bertozzi, C.R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 1997, 276, 1125–1128. [Google Scholar] [CrossRef]
- Luchansky, S.J.; Argade, S.; Hayes, B.K.; Bertozzi, C.R. Metabolic functionalization of recombinant glycoproteins. Biochemistry 2004, 43, 12358–12366. [Google Scholar] [CrossRef]
- Prescher, J.A.; Dube, D.H.; Bertozzi, C.R. Chemical remodelling of cell surfaces in living animals. Nature 2004, 430, 873–877. [Google Scholar] [CrossRef]
- Dube, D.H.; Prescher, J.A.; Quang, C.N.; Bertozzi, C.R. Probing mucin-type O-linked glycosylation in living animals. Proc. Natl. Acad. Sci. USA 2006, 103, 4819–4824. [Google Scholar] [CrossRef]
- Dehnert, K.W.; Baskin, J.M.; Laughlin, S.T.; Beahm, B.J.; Naidu, N.N.; Amacher, S.L.; Bertozzi, C.R. Imaging the sialome during zebrafish development with copper-free click chemistry. Chembiochem 2012, 13, 353–357. [Google Scholar] [CrossRef]
- Laughlin, S.T.; Baskin, J.M.; Amacher, S.L.; Bertozzi, C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 2008, 320, 664–667. [Google Scholar] [CrossRef]
- Baskin, J.M.; Dehnert, K.W.; Laughlin, S.T.; Amacher, S.L.; Bertozzi, C.R. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 10360–10365. [Google Scholar]
- Vocadlo, D.J.; Hang, H.C.; Kim, E.J.; Hanover, J.A.; Bertozzi, C.R. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9116–9121. [Google Scholar]
- Boyce, M.; Carrico, I.S.; Ganguli, A.S.; Yu, S.H.; Hangauer, M.J.; Hubbard, S.C.; Kohler, J.J.; Bertozzi, C.R. Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 3141–3146. [Google Scholar] [CrossRef]
- Rabuka, D.; Hubbard, S.C.; Laughlin, S.T.; Argade, S.P.; Bertozzi, C.R. A chemical reporter strategy to probe glycoprotein fucosylation. J. Am. Chem. Soc. 2006, 128, 12078–12079. [Google Scholar]
- Sawa, M.; Hsu, T.L.; Itoh, T.; Sugiyama, M.; Hanson, S.R.; Vogt, P.K.; Wong, C.H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12371–12376. [Google Scholar]
- Liu, T.W.; Kaji, H.; Togayachi, A.; Ito, H.; Sato, T.; Narimatsu, H. A chemoenzymatic approach toward the identification of fucosylated glycoproteins and mapping of N-glycan sites. Glycobiology 2012, 22, 630–637. [Google Scholar] [CrossRef]
- Dehnert, K.W.; Beahm, B.J.; Huynh, T.T.; Baskin, J.M.; Laughlin, S.T.; Wang, W.; Wu, P.; Amacher, S.L.; Bertozzi, C.R. Metabolic labeling of fucosylated glycans in developing zebrafish. ACS Chem. Biol. 2011, 6, 547–552. [Google Scholar] [CrossRef]
- Hang, H.C.; Yu, C.; Pratt, M.R.; Bertozzi, C.R. Probing glycosyltransferase activities with the Staudinger ligation. J. Am. Chem. Soc. 2004, 126, 6–7. [Google Scholar] [CrossRef]
- Hang, H.C.; Yu, C.; Kato, D.L.; Bertozzi, C.R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA 2003, 100, 14846–14851. [Google Scholar] [CrossRef]
- Sprung, R.; Nandi, A.; Chen, Y.; Kim, S.C.; Barma, D.; Falck, J.R.; Zhao, Y. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J. Proteome Res. 2005, 4, 950–957. [Google Scholar] [CrossRef]
- Tsai, C.S.; Liu, P.Y.; Yen, H.Y.; Hsu, T.L.; Wong, C.H. Development of trifunctional probes for glycoproteomic analysis. Chem. Commun .(Camb) 2010, 46, 5575–5577. [Google Scholar] [CrossRef]
- Clark, P.M.; Dweck, J.F.; Mason, D.E.; Hart, C.R.; Buck, S.B.; Peters, E.C.; Agnew, B.J.; Hsieh-Wilson, L.C. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 2008, 130, 11576–11577. [Google Scholar] [CrossRef]
- Wang, Z.; Udeshi, N.D.; O'Malley, M.; Shabanowitz, J.; Hunt, D.F.; Hart, G.W. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 2010, 9, 153–160. [Google Scholar] [CrossRef]
- Wang, S.; Xie, W.; Zhang, X.; Zou, X.; Zhang, Y. Disulfide- and terminal alkyne-functionalized magnetic silica particles for enrichment of azido glycopeptides. Chem. Commun. (Camb.) 2012, 48, 5907–5909. [Google Scholar]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef]
- Bruckman, M.A.; Kaur, G.; Lee, L.A.; Xie, F.; Sepulveda, J.; Breitenkamp, R.; Zhang, X.; Joralemon, M.; Russell, T.P.; Emrick, T.; Wang, Q. Surface modification of tobacco mosaic virus with "click" chemistry. ChemBioChem 2008, 9, 519–523. [Google Scholar]
- Banerjee, P.S.; Ostapchuk, P.; Hearing, P.; Carrico, I. Chemoselective attachment of small molecule effector functionality to human adenoviruses facilitates gene delivery to cancer cells. J. Am. Chem. Soc. 2010, 132, 13615–13617. [Google Scholar]
- Banerjee, P.S.; Zuniga, E.S.; Ojima, I.; Carrico, I.S. Targeted and armed oncolytic adenovirus via chemoselective modification. Bioorg. Med. Chem. Lett. 2011, 21, 4985–4988. [Google Scholar]
- Cravatt, B.F.; Sorensen, E.J. Chemical strategies for the global analysis of protein function. Curr. Opin. Chem. Biol. 2000, 4, 663–668. [Google Scholar]
- Speers, A.E.; Adam, G.C.; Cravatt, B.F. Activity-based protein profiling in vivo using a copper (I)-catalyzed azide-alkyne [3+ 2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 4686–4687. [Google Scholar] [CrossRef]
- Rempel, B.P.; Withers, S.G. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 2008, 18, 570–586. [Google Scholar] [CrossRef]
- Ovaa, H.; van Swieten, P.F.; Kessler, B.M.; Leeuwenburgh, M.A.; Fiebiger, E.; van den Nieuwendijk, A.M.; Galardy, P.J.; van der Marel, G.A.; Ploegh, H.L.; Overkleeft, H.S. Chemistry in living cells: detection of active proteasomes by a two-step labeling strategy. Angew. Chem. Int. Ed. 2003, 42, 3626–3629. [Google Scholar] [CrossRef]
- Lee, L.V.; Mitchell, M.L.; Huang, S.J.; Fokin, V.V.; Sharpless, K.B.; Wong, C.H. A potent and highly selective inhibitor of human alpha-1,3-fucosyltransferase via click chemistry. J. Am. Chem. Soc. 2003, 125, 9588–9589. [Google Scholar]
- Speers, A.E.; Cravatt, B.F. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 2004, 11, 535–546. [Google Scholar] [CrossRef]
- Tsai, C.S.; Li, Y.K.; Lo, L.C. Design and synthesis of activity probes for glycosidases. Org. Lett. 2002, 4 , 3607–3610. [Google Scholar] [CrossRef]
- Feizi, T.; Fazio, F.; Chai, W.; Wong, C.H. Carbohydrate microarrays—A new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 2003, 13, 637–645. [Google Scholar] [CrossRef]
- Song, X.; Xia, B.; Stowell, S.R.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009, 16, 36–47. [Google Scholar] [CrossRef]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef]
- Kohn, M.; Wacker, R.; Peters, C.; Schroder, H.; Soulere, L.; Breinbauer, R.; Niemeyer, C.M.; Waldmann, H. Staudinger ligation: a new immobilization strategy for the preparation of small-molecule arrays. Angew. Chem. Int. Ed. 2003, 42, 5830–5834. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, X.; Zhang, Y. Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology. Molecules 2013, 18, 7145-7159. https://doi.org/10.3390/molecules18067145
Zhang X, Zhang Y. Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology. Molecules. 2013; 18(6):7145-7159. https://doi.org/10.3390/molecules18067145
Chicago/Turabian StyleZhang, Xiu, and Yan Zhang. 2013. "Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology" Molecules 18, no. 6: 7145-7159. https://doi.org/10.3390/molecules18067145
APA StyleZhang, X., & Zhang, Y. (2013). Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology. Molecules, 18(6), 7145-7159. https://doi.org/10.3390/molecules18067145



