Isocyanate-Functionalized Chitin and Chitosan as Gelling Agents of Castor Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Isocyanate—Functionalized Chitin and Chitosan
| Starting material | HMDI | Et3N | Toluene | |
|---|---|---|---|---|
| [equiv.] * | [equiv.] * | [equiv.] * | [mL] | |
| CSAN–1 | 1.00 | 0.50 | 1.00 | 100 |
| CSAN–2 | 1.00 | 0.25 | 0.50 | 100 |
| CTIN–1 | 1.00 | 0.25 | 0.50 | 100 |
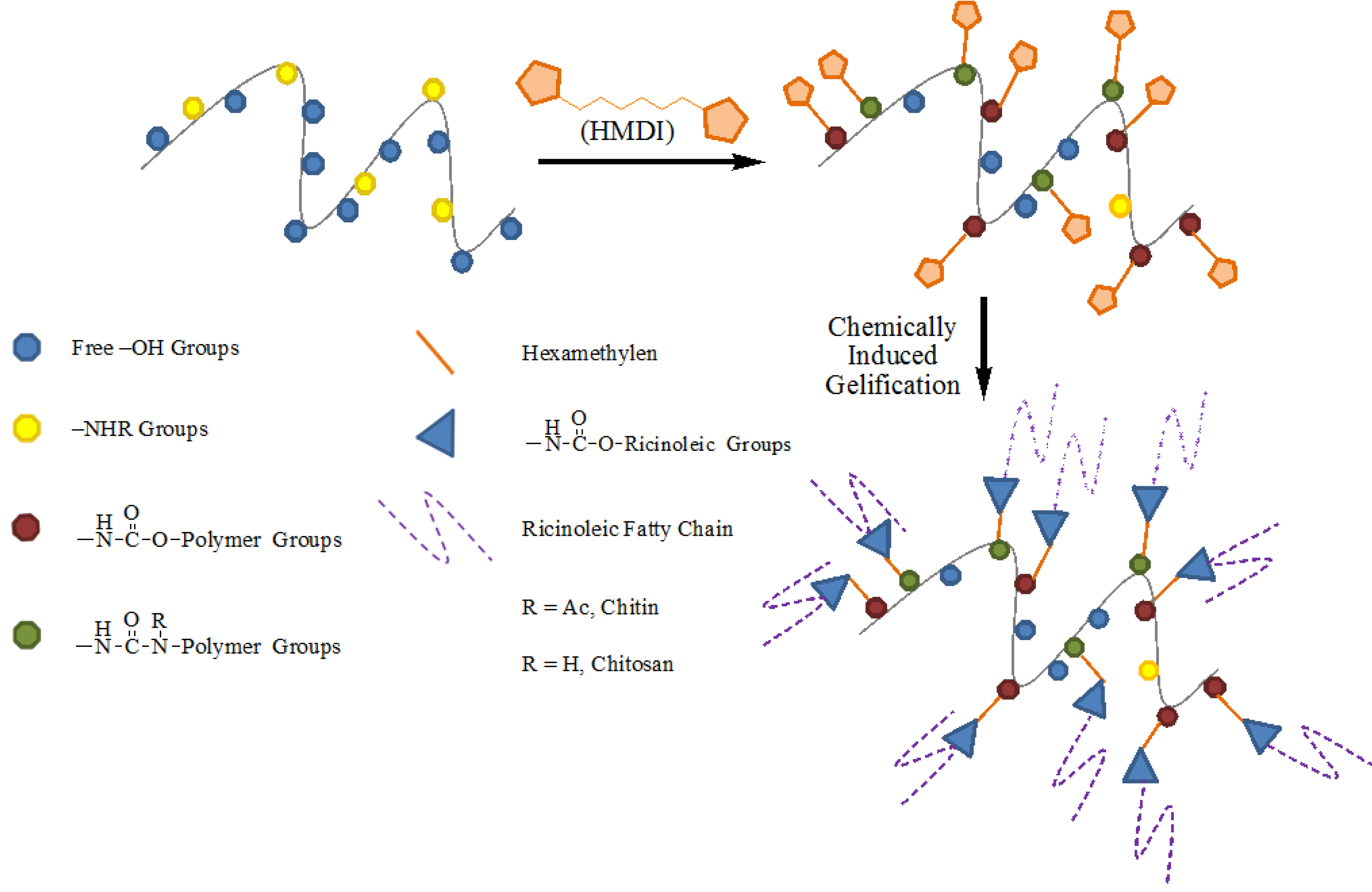

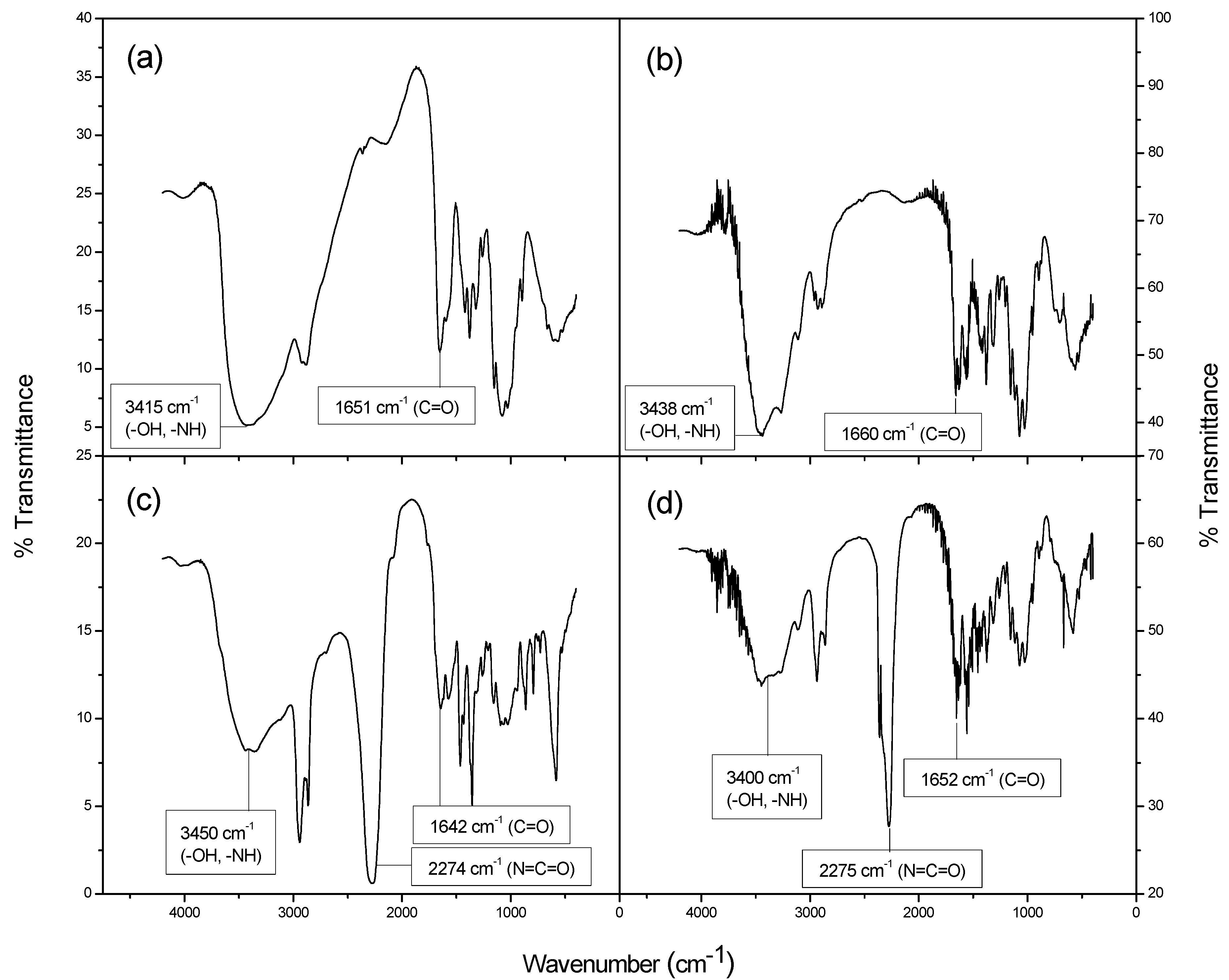

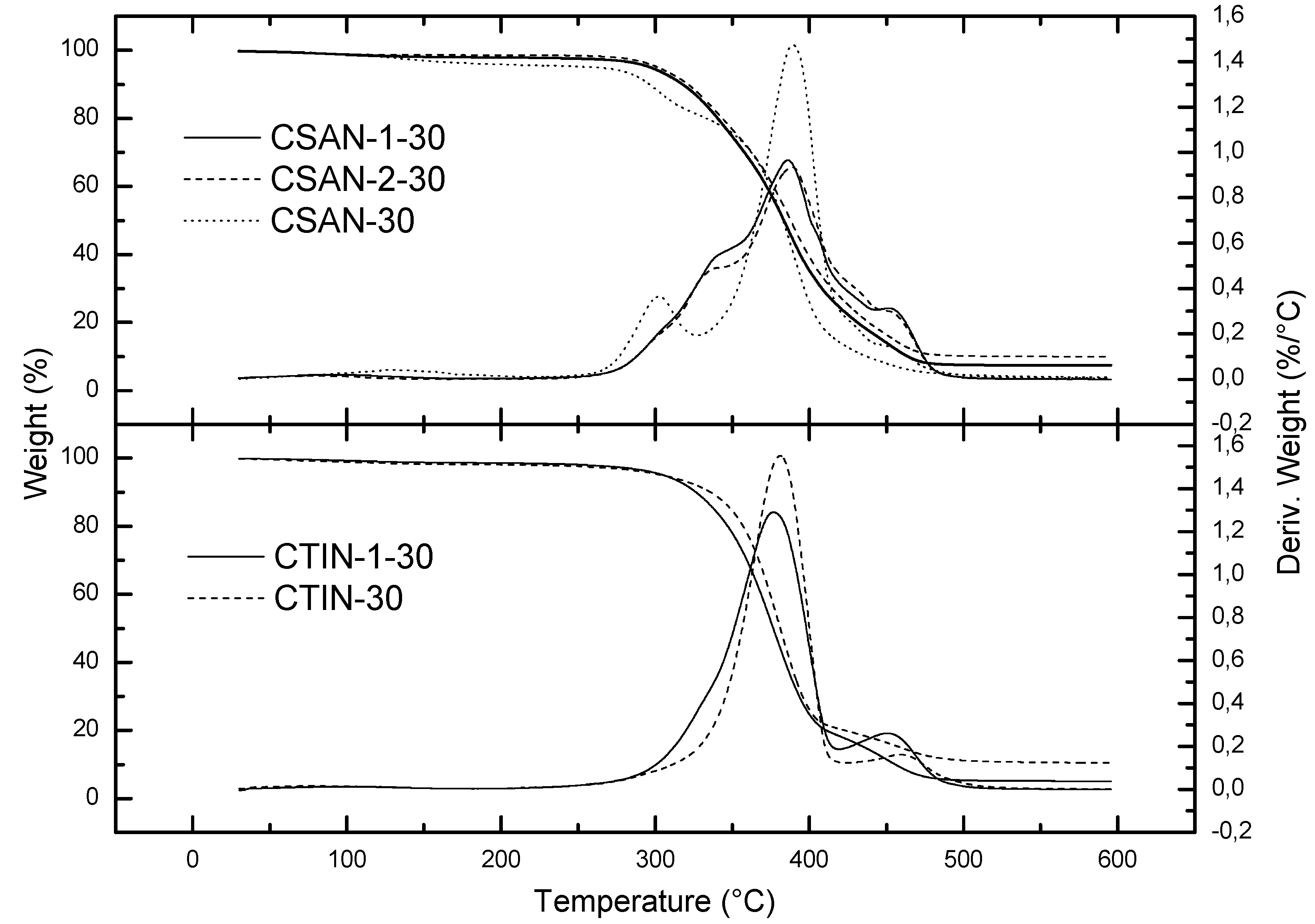
2.2. Thermogravimetric Analysis of Isocyanate-Functionalized Chitosan and Chitin

| Sample | Tonset | Tmax | Tfinal | Residue | ∆W |
|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (%) | (%) | |
| Chitosan | 43/281 | 51/301 | 77/472 | 34 | 9/57 |
| Chitin | 40/343 | 49/389 | 79/405 | 23 | 4/72 |
| HMDI | 137 | 172 | 178 | 1 | 99 |
| CSAN-1 | 43/122/240/292/429 | 50/153/258/306/450 | 60/179/266/340/483 | 11 | 4/44/6/26/9 |
| CSAN-2 | 40/107/283/431 | 43/133/306/448 | 50/150/335/486 | 15 | 2/30/43/9 |
| CTIN-1 | 106/275/363/439 | 130/302/389/454 | 140/309/403/494 | 16 | 34/10/36/4 |
2.3. Thermal and Spectroscopic Characterization of Isocyanate-Functionalized Chitosan and Chitin Gel-Like Dispersions in Castor Oil
| Sample | Tonset | Tmax | Tfinal | Residue | ∆W |
|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (%) | (%) | |
| CSAN-1-30 | 297 | 344/386/452 | 481 | 7.40 | 92 |
| CSAN-2-30 | 301 | 339/389/451 | 481 | 9.97 | 90 |
| CTIN-1-30 | 327/444 | 376/450 | 410/485 | 5.07 | 95 |
| CSAN-30 | 278/357 | 302/389/449 | 319/482 | 3.89 | 96 |
| CTIN-30 | 342/425 | 381/459 | 409/595 | 10.46 | 89 |
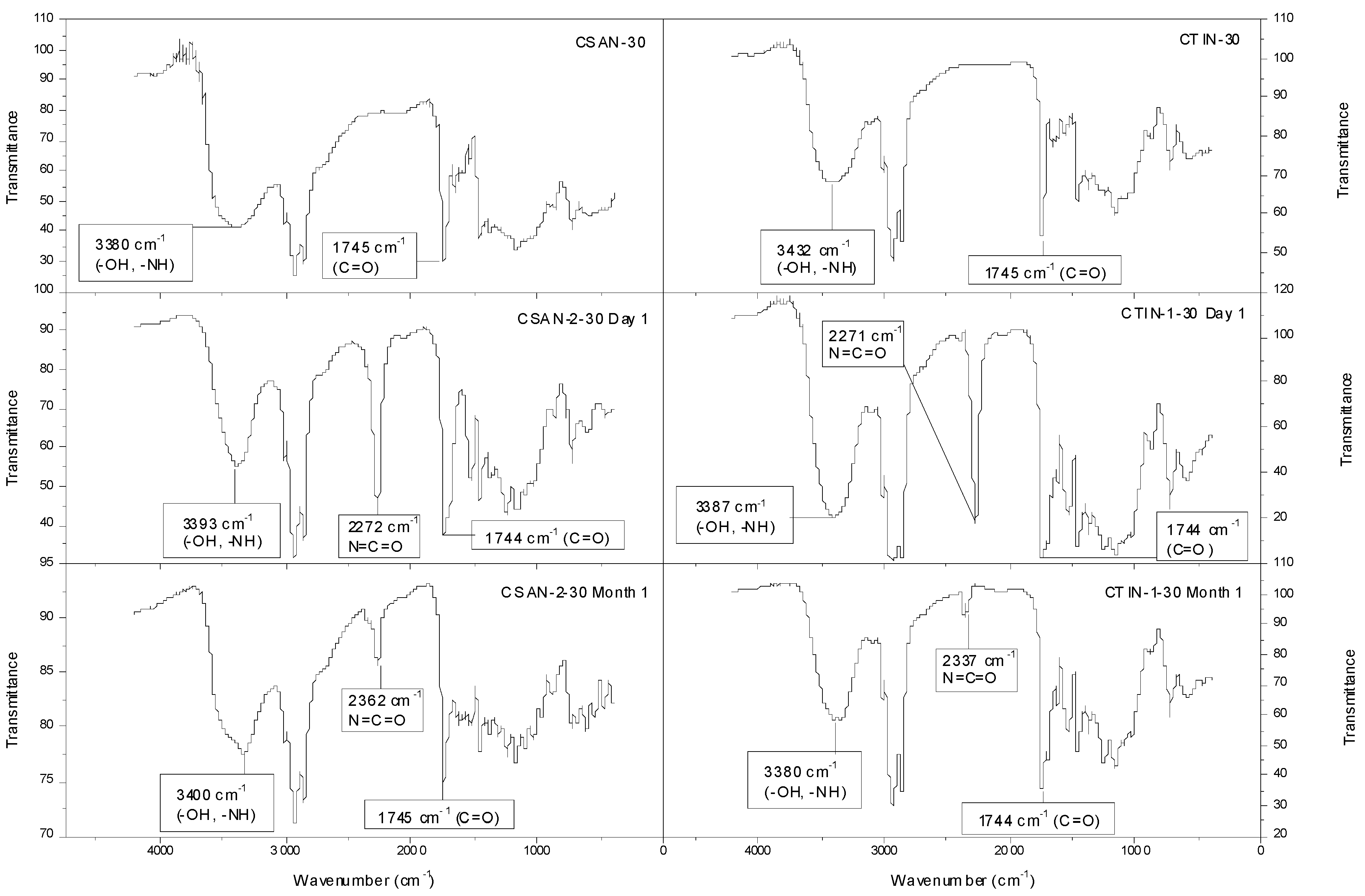
2.4. Rheological Characterization of Functionalized Chitin and Chitosan Gel-Like Dispersions in Castor Oil
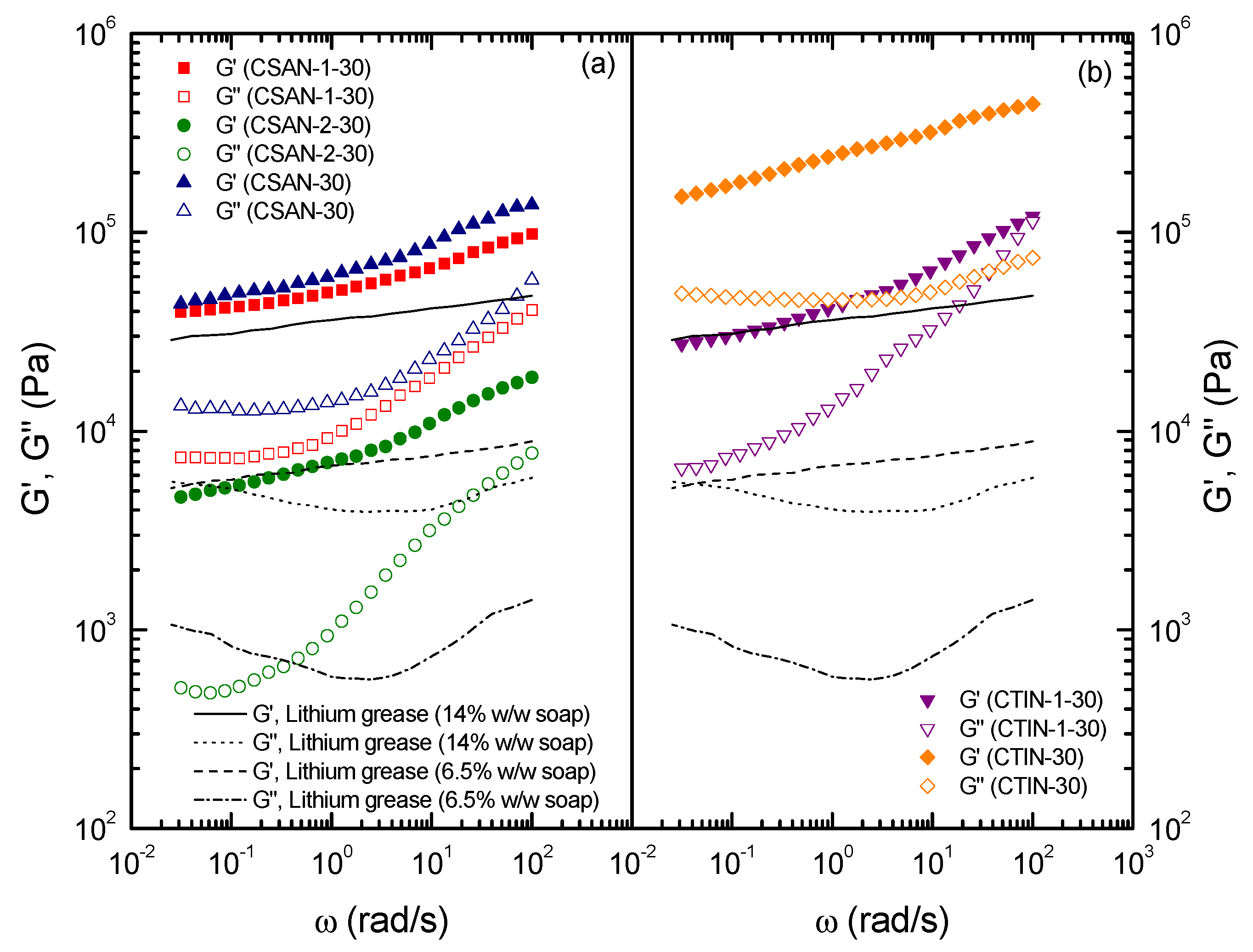
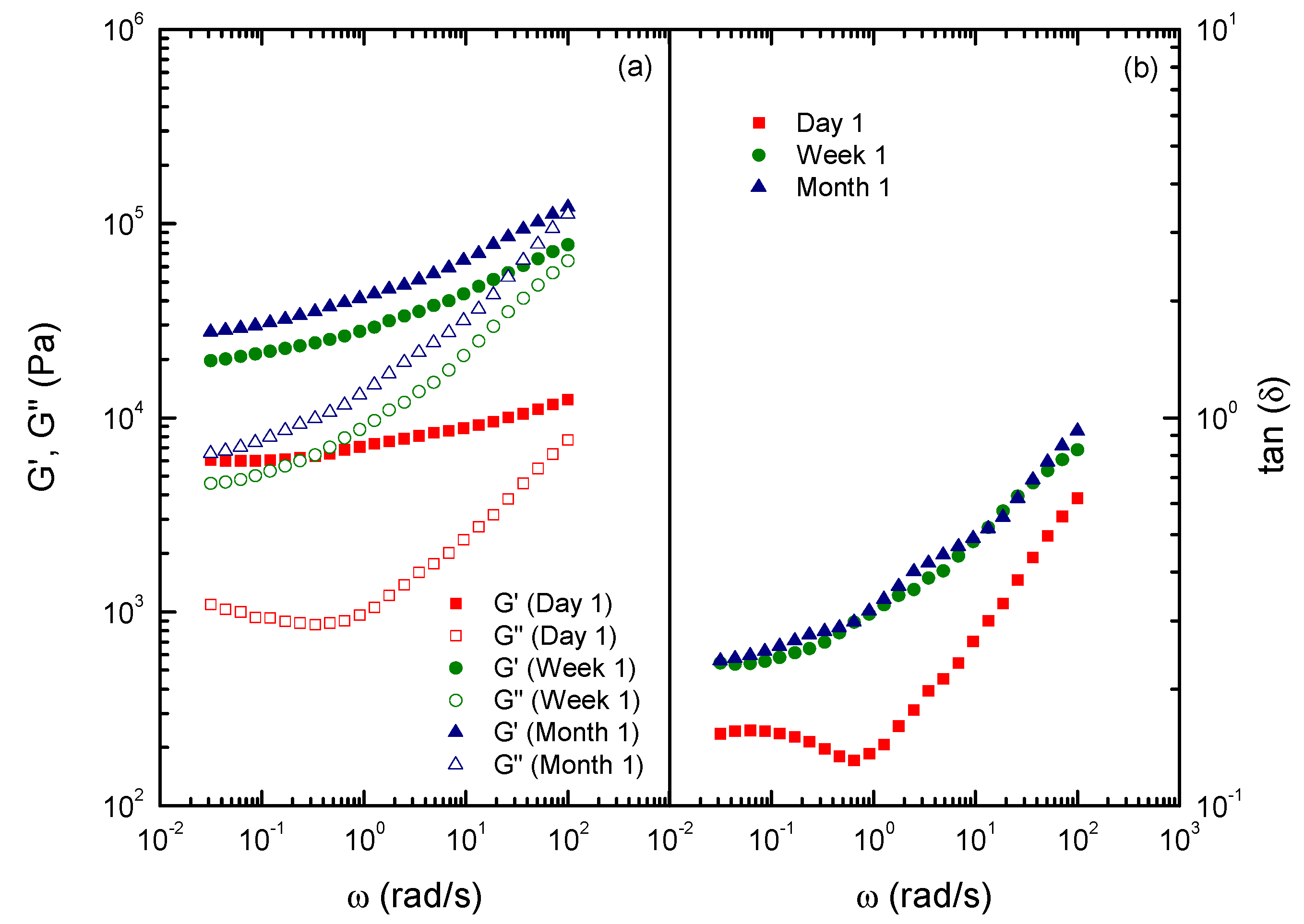
3. Experimental
3.1. Materials
3.2. General Methods
3.3. Functionalization Reaction of Chitosan and Chitin with HMDI
3.3.1. CSAN-1 Synthesis
3.3.2. CSAN-2 Synthesis
3.3.3. CTIN-1 Synthesis
3.4. Preparation of Oleogels
| Thickener agent | Code applied |
|---|---|
| CSAN-1 | CSAN-1-30 |
| CSAN-2 | CSAN-2-30 |
| Chitosan | CSAN-30 |
| CTIN-1 | CTIN-1-30 |
| Chitin | CTIN-30 |
3.5. Thermogravimetric Analysis (TGA)
3.6. Nuclear Magnetic Resonance of Protons (1H-NMR)
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8. Rheological Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lea, C.W. European development of lubricant derived from renewable resources. Ind. Lubric. Tribol. 2002, 54, 268–274. [Google Scholar] [CrossRef]
- Wilson, B. Lubricants and functional fluids from renewable sources. Ind. Lubric. Tribol. 1998, 50, 6–15. [Google Scholar] [CrossRef]
- Kumar, R.; Muzzarelli, M.N.V.; Muzzarelli, R.A.A.; Sashiwa, C.H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Skjak-Braek, G.; Anthonsen, T.; Sanford, P.A. Chitin and Chitosan-sources, Chemistry, Biochemistry, Physical Properties and Applications; Elsevier: London, UK, 1989; pp. 51–70. [Google Scholar]
- Muzzarelli, R.A.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bersch, P.C.; Nies, B.; Liebendorfer, A. Evaluation of the biological properties of different wound dressing materials. J. Mater. Sci. Mater. Med. 1995, 6, 231–240. [Google Scholar]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Shi, T.C.; Jing, Y.M.; Sui, D.X. Affinity chromatography of trypsin using chitosan as ligand support. J. Chromatogr. 1996, 742, 107–112. [Google Scholar] [CrossRef]
- Krajewska, B.; Leszko, M.; Zoborska, W. Urease immobilized on chitosan membrane–preparation and properties. J. Chem. Technol. Biot. 1990, 48, 337–350. [Google Scholar] [CrossRef]
- Shahabeddin, L.; Damour, O.; Bethod, F. Reconstructed skin from cocultured human keratinocytes and fibroblasts on a chitosane cross–linked collagen–gag matrix. J. Mater. Sci. -Mater. Med. 1991, 2, 222–226. [Google Scholar] [CrossRef]
- Muzzarell, R.; Biagini, G.; Paugnaloni, A. Reconstruction of parodontal tissue with chitosan. Biomaterials 1989, 10, 598–603. [Google Scholar] [CrossRef]
- Costain, D.J.; Kennedy, R.; Ciona, C.; McAlister, V.C.; Lee, T.D.G. Prevention of post surgical adhesions with N,O-carboxymethyl chitosan: Examination of the most efficacious preparation and the effect of N,O-carboxymethyl chitosan on post surgical healing. Surgery 1997, 121, 314–319. [Google Scholar] [CrossRef]
- Hirano, S.; Ohe, Y.; Metsuda, N.; Metsuda. 2-Deoxy-2-nicotinamido derivatives of some mono-saccha rides, oligo-saccharides, and polysaccharides. Carbohydr. Res. 1978, 60, 404–406. [Google Scholar] [CrossRef]
- Hirano, S.; Tobetto, K.; Hasagawn, M.; Matsuda, N.J. Permeability properties of gels and membranes derived from chitosan. J. Biomed. Mater. Res. 1980, 14, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Ishii, K.; Nadai, T. The use of chitin and chitosan as drug carriers. Chem. Pharm. Bull. 1981, 29, 3067–3069. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug. Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Kojima, T.; Munakata, T.; Akao, H.; Mori, T.; Nishiyama, Y. Preparation of non-natural branched chitin and chitosan. Chem. Lett. 1998, 27, 317–318. [Google Scholar] [CrossRef]
- Sashiwa, H.; Shigemasa, Y. Chemical modification of chitin and chitosan 2: preparation and water soluble property of N-acylated or N-alkylated partially deacetylated chitins. Carbohydr. Polym. 1999, 39, 127–138. [Google Scholar] [CrossRef]
- Heras, A.; Rodriguez, N.M.; Ramos, V.M. N-methylene phosphonic chitosan: a novel soluble derivative. Carbohydr. Polym. 2001, 44, 1–8. [Google Scholar] [CrossRef]
- Grcic, J.F.; Voinovich, D.; Moneghini, M.; Lacan, M.B.; Magarotto, L.; Jalsenjak, I. Chitosan microspheres with hydrocortisone and hydrocortisone-hydroxypropyl-b-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 2000, 9, 373–379. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharmaceut. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of chitosan hydrochloride-mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Chiu, S.; Chung, T.; Giridhar, R.; Wu, W. Immobilization of β-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Res. Int. 2004, 37, 217–223. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan derivatives bearing cyclodextrin cavities as novel adsorbent matrices. Carbohydr. Polym. 2006, 63, 153–166. [Google Scholar] [CrossRef]
- Schüßlera, S.; Blaubacha, N.; Stolle, A.; Cravottob, G.; Ondruschkaa, B. Application of a cross-linked Pd–chitosan catalyst in liquid-phase-hydrogenation using molecular hydrogen. Appl. Catal. A-Gen 2012, 445–446, 231–238. [Google Scholar] [CrossRef]
- Martinaa, K.; Leonhardtb, S.E.S.; Ondruschkab, B.; Curinic, M.; Binelloa, A.; Cravottoa, G. In situ cross-linked chitosan Cu(I) or Pd(II) complexes as a versatile, Eco-friendly recyclable solid catalyst. J. Mol. Catal. A-Chem. 2011, 334, 60–64. [Google Scholar] [CrossRef]
- Zha, F.; Li, S.; Chang, Y. Preparation and adsorption property of chitosan beads bearing β-cyclodextrin cross-linked by 1,6-hexamethylene diisocyanate. Carbohydr. Polym. 2008, 72, 456–461. [Google Scholar] [CrossRef]
- Arrascue, M.L.; Garcia, H.M.; Horna, O.; Guibal, E. Gold sorption on chitosan derivatives. Hydrometallurgy 2003, 71, 191–200. [Google Scholar] [CrossRef]
- Ziaa, K.M.; Bhattia, I.A.; Barikanib, M.; Zuberc, M.; Sheikh, M.A. XRD studies of chitin-based polyurethane elastomers. Int. J. Biol. Macromol. 2008, 43, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Stringari, G.B.; Franco, J.M.; Valencia, C.; Gallegos, C. Use of chitin, Chitosan and acylated derivatives as thickener agents of vegetable oils for bio-lubricant applications. Carbohydr. Polym. 2011, 85, 705–714. [Google Scholar] [CrossRef]
- Gallego, R.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Chemical modification of methyl cellulose with HMDI to modulate the thickening properties in castor oil. Cellulose 2013, 20, 495–507. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm chrysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Nuñez, N.; Martin-Alfonso, J.E.; Eugenio, M.E.; Valencia, C.; Diaz, M.J.; Franco, J.M. Preparation and characterization of gel-like dispersions based on cellulosic pulps and castor oil for lubricant applications. Ind. Eng. Chem. Res. 2011, 50, 5618–5627. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Núñez, N.; Valencia, C.; Franco, J.M.; Diaz, M.J. Formulation of new biodegradable lubricating greases using ethylated cellulose pulp as thickener agent. J. Ind. Eng. Chem. 2011, 17, 818–823. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C.; Sánchez, M.C.; Franco, J.M.; Gallegos, C. Rheological modification of lubricating greases with recycled polymers from different plastics waste. Ind. Eng. Chem. Res. 2009, 48, 4136–4144. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C.; Sánchez, M.C.; Franco, J.M.; Gallegos, C. Evaluation of different polyolefins as rheology modifier additives in lubricating grease formulations. Mater. Chem. Phys. 2011, 128, 530–538. [Google Scholar] [CrossRef]
- Gupta, R.K. Polymer and Composite Rheology, 2nd ed.; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Nuñez, N.; Martín-Alfonso, J.E.; Valencia, C.; Sánchez, M.C.; Franco, J.M. Rheology of new green lubricating grease formulations containing cellulose pulp and its methylated derivative as thickener agents. Ind. Crop. Prod. 2012, 37, 500–507. [Google Scholar] [CrossRef]
- Sanchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Development of new green lubricating grease formulations based on cellulosic derivatives and castor oil. Green Chem. 2009, 11, 686–693. [Google Scholar] [CrossRef]
- Sánchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Thermal and mechanical characterization of cellulosic derivatives-based oleogels potentially applicable as bio-lubricating greases: Influence of ethyl cellulose molecular weight. Carbohydr. Polym. 2011, 83, 151–158. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C.; Sanchez, M.C.; Franco, J.M.; Gallegos, C. Recycled and virgin ldpe as rheology modifiers of lithium lubricating greases. a comparative study. Polym. Eng. Sci. 2008, 48, 1112–1119. [Google Scholar] [CrossRef]
- Franco, J.M.; Delgado, M.A.; Valencia, C.; Sánchez, M.C.; Gallegos, C. Mixing rheometry for studying the manufactured of lubricating greases. Chem. Eng. Sci. 2005, 60, 2409–2418. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term “gel”. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Moreno, G.; Valencia, C.; Franco, J.M.; Gallegos, C.; Diogo, A.; Bordado, J.C.M. Influence of molecular weight and free NCO content on the rheological properties of lithium lubricating greases modified with NCO-terminated prepolymers. Eur. Polym. J. 2008, 44, 2262–2274. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallego, R.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Isocyanate-Functionalized Chitin and Chitosan as Gelling Agents of Castor Oil. Molecules 2013, 18, 6532-6549. https://doi.org/10.3390/molecules18066532
Gallego R, Arteaga JF, Valencia C, Franco JM. Isocyanate-Functionalized Chitin and Chitosan as Gelling Agents of Castor Oil. Molecules. 2013; 18(6):6532-6549. https://doi.org/10.3390/molecules18066532
Chicago/Turabian StyleGallego, Rocío, Jesús F. Arteaga, Concepción Valencia, and José M. Franco. 2013. "Isocyanate-Functionalized Chitin and Chitosan as Gelling Agents of Castor Oil" Molecules 18, no. 6: 6532-6549. https://doi.org/10.3390/molecules18066532
APA StyleGallego, R., Arteaga, J. F., Valencia, C., & Franco, J. M. (2013). Isocyanate-Functionalized Chitin and Chitosan as Gelling Agents of Castor Oil. Molecules, 18(6), 6532-6549. https://doi.org/10.3390/molecules18066532






