Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-Ethylidene-1,3-thiazol-4-ones
Abstract
:1. Introduction
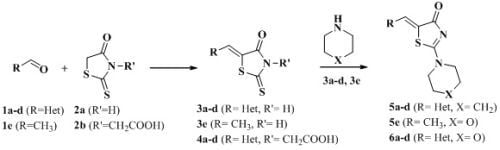
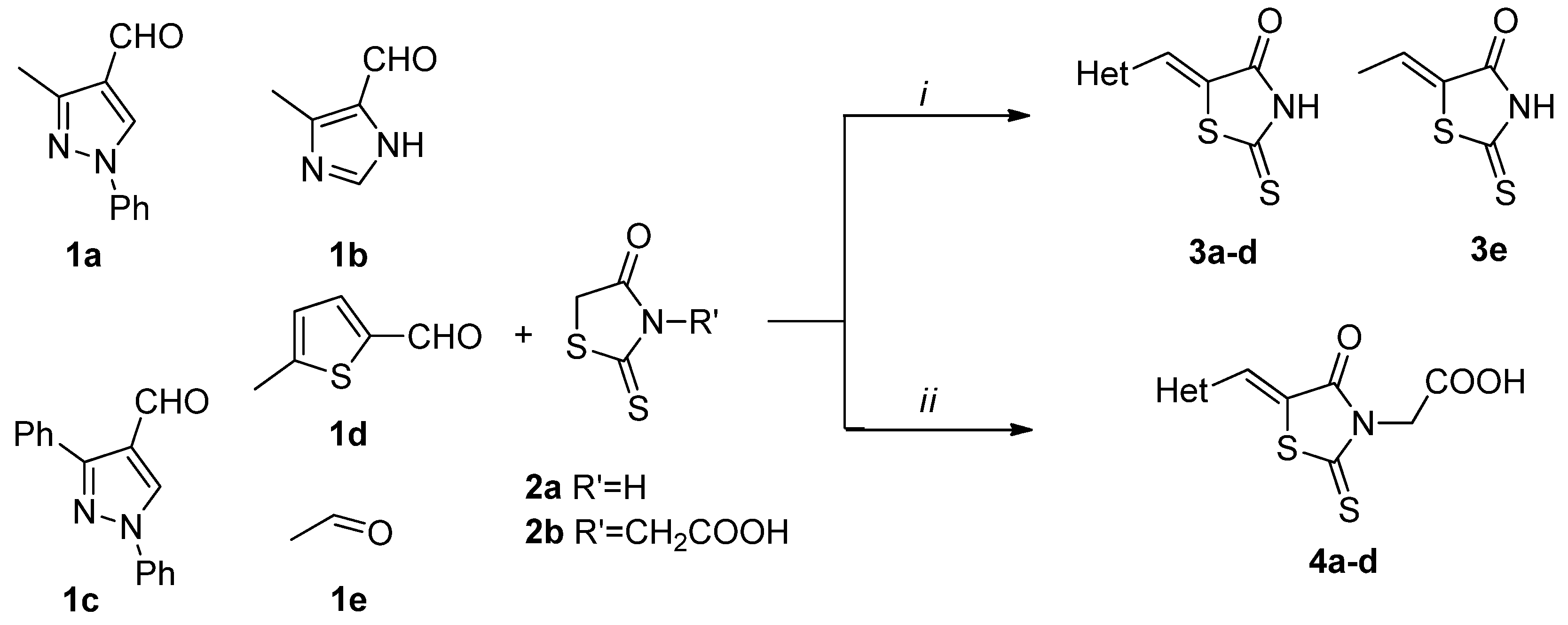
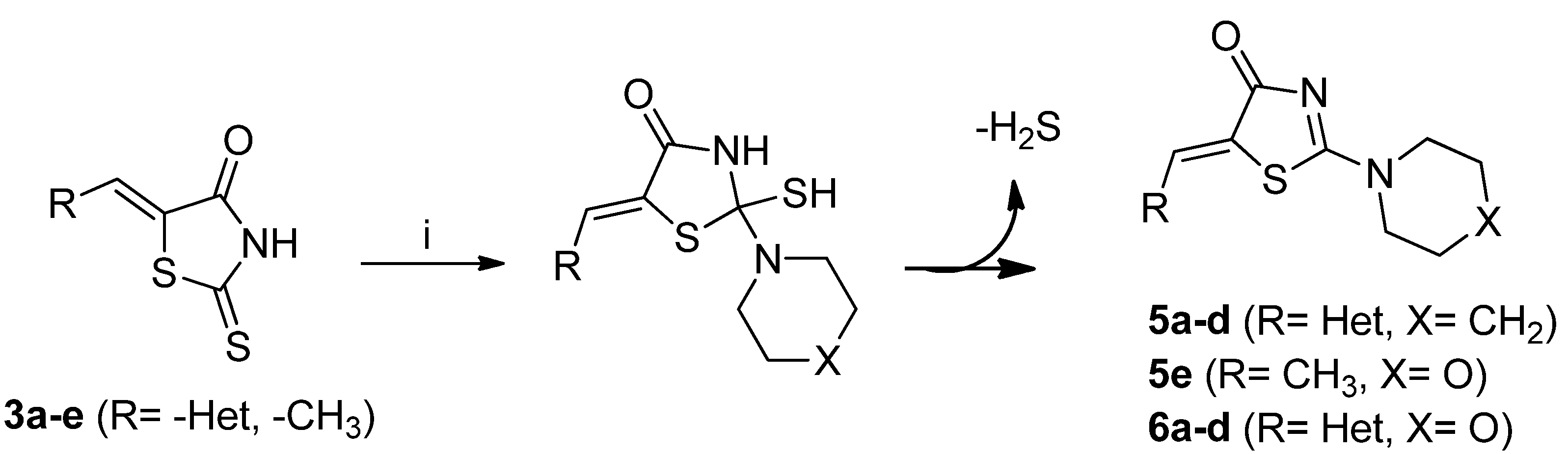
2. Results and Discussion
2.1. Chemistry
| Compound | m.p. (°C) | Yield (%) |
|---|---|---|
| 3a | 294–295 | 86 |
| 3b | 307–309 | 91 |
| 3c | 315–317 | 86 |
| 3d | 230–231 | 85 |
| 3e | 145–147 | 64 |
| 4a | 279–281 | 81 |
| 4b | 254–256 | 53 |
| 4c | 263–265 | 92 |
| 4d | 232–234 | 63 |
| Compound | -X- | m.p. (°C) | Yield (%) |
|---|---|---|---|
| 5a | -CH2- | 141–143 | 85 |
| 5b | -CH2- | 261–262 | 70 |
| 5c | -CH2- | 262–264 | 95 |
| 5d | -CH2- | 194–196 | 93 |
| 5e | -O- | 193–195 | 45 |
| 6a | -O- | 264–265 | 71 |
| 6b | -O- | 270–272 | 62 |
| 6c | -O- | 266–268 | 86 |
| 6d | -O- | 206–208 | 85 |
2.2. In Vitro Antifungal Activity
| Compound | Structure | Antifungal Activity MIC/MFC (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Ct | Sc | Cn | Afu | Afl | Ani | Mg | Tr | Tm | ||
| 3a |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 3b | 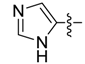 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 3c | 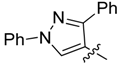 | >250 | >250 | >250 | 125/125 | 250/250 | 250/250 | 250/250 | 125/125 | 125/125 | 125/125 |
| 3d | 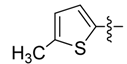 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | <250 | <250 | <250 |
| 3e | 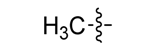 | 7.8/31.2 | 7.8/31.2 | 3.9/15.6 | 15.6/62.5 | 31.2/250 | 31.2/250 | 62.5/250 | 7.8/7.8 | 7.8/15.6 | 15.6/15.6 |
| 4a | 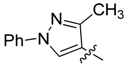 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | 125/125 | 62.5/62.5 | 62.5/62.5 |
| 4b | 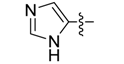 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 4c |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 4d |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 5a | 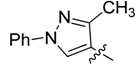 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 5b |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 5c | 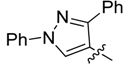 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 5d | 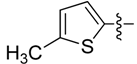 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 5e |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 6a | 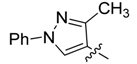 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 6b | 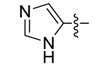 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 6c |  | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 6d | 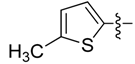 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| amphotericin B | - | 0.78 | 0.50 | 0.25 | 0.50 | 0.50 | 0.50 | 0.12 | 0.07 | 0.07 | |
| ketoconazole | - | 1.56 | 3.12 | 0.39 | 0.78 | 0.78 | 1.56 | 0.04 | 0.01 | 0.02 | |
| terbinafine | - | 0.50 | 0.50 | 0.25 | 0.12 | 0.50 | 0.25 | 0.05 | 0.02 | 0.02 | |
2.3. In Vitro Antitumor Activity
| Panel/Cell Line | Compound 3c | |
|---|---|---|
| GI50 b (μM) | LC50 c (μM) | |
| Leukemia | ||
| CCRF-CEM | 2.50 | >100 |
| HL-60(TB) | 4.83 | >100 |
| K562 | 7.54 | >100 |
| MOLT-4 | 14.8 | >100 |
| RPMI-8226 | 2.52 | >100 |
| SR | 7.29 | >100 |
| Non Small Cell Lung | ||
| A549/ATCC | 5.88 | >100 |
| EKVX | 3.03 | >100 |
| HOP-62 | 22.7 | >100 |
| HOP-92 | 0.62 | >100 |
| NCI-H226 | 2.03 | >100 |
| NCI-H23 | 2.68 | >100 |
| NCI-H322M | 7.63 | >100 |
| NCI-H460 | 5.50 | 54.4 |
| NCI-H522 | 2.96 | >100 |
| Colon Cancer | ||
| COLO 205 | 21.2 | >100 |
| HCC-2998 | 6.05 | >100 |
| HCT-116 | 5.62 | 70.2 |
| HCT-15 | 4.71 | 96.2 |
| HT29 | 12.5 | >100 |
| KM12 | 6.24 | 63.5 |
| SW-620 | 19.6 | >100 |
| Prostate Cancer | ||
| PC-3 | 5.66 | >100 |
| DU-145 | 12.6 | >100 |
| CNS Cancer | ||
| SF-268 | 17.2 | >100 |
| SF-295 | 3.33 | 82.6 |
| SF-539 | 5.53 | 61.0 |
| SNB-19 | 6.14 | >100 |
| SNB-75 | 17.8 | >100 |
| U251 | 5.54 | 64.8 |
| Melanoma | ||
| LOX IMVI | 10.0 | >100 |
| MALME-3M | 3.84 | 6.11 |
| M14 0.405 | 6.75 | >100 |
| MDA-MB-435 | 4.91 | >100 |
| SK-MEL-2 | 4.18 | 70.7 |
| SK-MEL-28 | 9.22 | >100 |
| SK-MEL-5 | 3.19 | 58.4 |
| UACC-257 | 13.2 | >100 |
| UACC-62 | 3.36 | 5.77 |
| Renal Cancer | ||
| 786-0 | 3.92 | >100 |
| A498 | 2.99 | 94.4 |
| ACHN | 7.40 | 52.1 |
| CAKI-1 | 7.15 | >100 |
| RXF 393 | 22.4 | >100 |
| SN12C | 9.93 | >100 |
| TK-10 | 8.00 | >100 |
| UO-31 | 4.39 | >100 |
| Breast Cancer | ||
| MCF7 | 8.31 | >100 |
| MDA-MB231/ATCC | 10.1 | >100 |
| HS 578T | 6.03 | >100 |
| BT-549 | 5.20 | 86.5 |
| T-47D | 4.59 | >100 |
| MDA-MB-468 | 6.83 | >100 |
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of (Z)-5-Hetarylmethylidene-2-thioxothiazolidin-4-ones 3a–d
3.2.2. General Procedure for the Synthesis of 2-(5Z)-(Hetarylmethylidene)-4-oxo-2-thioxothiazolidin-3-yl) Acetic Acids 4a–d
3.2.3. General Procedure for the Synthesis of 5-Hetarylmethylidene-2-(piperidin-1-yl)thiazol-4-ones, 5-hetarylmethylidene-2-morpholinothiazol-4-ones and (Z)-5-Ethylidene-2-morpholinothiazol-4(5H)-ones 5a–d, 5e and 6a–d
3.3. Antifungal Activity
3.4. Antifungal Susceptibility Testing
3.5. Antitumor Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ramirez, M.A.; Borja, N.L. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 2008, 28, 646–655. [Google Scholar] [CrossRef]
- Ravi, S.; Chiruvella, K.K.; Rajesh, K.; Prabhu, V.; Raghavan, S.C. 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. Eur. J. Med. Chem. 2010, 45, 2748–2752. [Google Scholar] [CrossRef]
- Heerding, D.A.; Christmann, L.T.; Clark, T.J.; Holmes, D.J.; Rittenhouse, S.F.; Takata, D.T.; Venslavsky, J.W. New Benzylidenethiazolidinediones as Antibacterial Agents. Bioorg. Med. Chem. Lett. 2003, 13, 3771–3773. [Google Scholar] [CrossRef]
- Dolezel, J.; Hirsova, P.; Opletalova, V.; Dohnal, J.; Marcela, V.; Kunes, J.; Jampilek, J. Rhodanineacetic acid derivatives as potential drugs: Preparation, hydrophobic properties and antifungal activity of (5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic acids. Molecules 2009, 14, 4197–4212. [Google Scholar] [CrossRef]
- Orchard, M.G.; Neuss, J.C.; Galley, C.M.S.; Carr, A.; Porter, D.W.; Smith, P.; Scopes, D.I.C.; Haydon, D.; Vousden, K.; Stubberfield, C.R.; et al. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1). Bioorg. Med. Chem. Lett. 2004, 14, 3975–3978. [Google Scholar] [CrossRef]
- Sortino, M.; Delgado, P.; Juárez, S.; Quiroga, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Rodero, L.; Garibotto, F.M.; Enriz, R.D.; et al. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg. Med. Chem. 2007, 15, 484–494. [Google Scholar]
- Insuasty, B.; Gutiérrez, A.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Svetaz, L.; Raimondi, M.; Zacchino, S. Fungicide activity of 5-(4-chlorobenzylidene)-(Z)-2-dimethylamino-1,3-thiazol-4-one against cryptococcus neoformans. Arch. Pharm. 2010, 343, 48–53. [Google Scholar]
- Insuasty, B.; Tigreros, A.; Martínez, H.; Quiroga, J.; Abonia, R.; Gutierrez, A.; Nogueras, M.; Cobo, J. An efficient two-step synthesis of novel thiazolo[2,3-b]pyrazolo[3,4-f][1,3,5]triazepines. J. Heterocycl. Chem. 2009, 46, 756–761. [Google Scholar] [CrossRef]
- Xu, L.-L.; Zheng, C.-J.; Sun, L.-P.; Miao, J.; Piao, H.-R. Synthesis of novel 1,3-diaryl pyrazole derivatives bearing rhodanine-3-fatty acid moieties as potential antibacterial agents. Eur. J. Med. Chem. 2012, 48, 174–178. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zheng, C.-J.; Sun, L.-P.; Piao, H.-R. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med. Chem. 2010, 45, 5739–5743. [Google Scholar] [CrossRef]
- Delgado, P.; Quiroga, J.; Cobo, J.; Low, J.N.; Glidewell, C. Supramolecular structures of four (Z)-5-arylmethylene-2-thioxothiazolidin-4-ones: Hydrogen-bonded dimers, chains of rings and sheets. Acta Crystallogr. C 2005, 61, o477–o482. [Google Scholar] [CrossRef]
- Delgado, P.; Quiroga, J.; de la Torre, J.M.; Cobo, J.; Low, J.N.; Glidewell, C. Three substituted (Z)-5-benzylidene-2-thioxothiazolidin-4-ones: Hydrogen-bonded dimers that can be effectively isolated or linked into chains either by aromatic [pi]-[pi] stacking interactions or by dipolar carbonyl-carbonyl interactions. Acta Crystallogr. C 2006, 62, o382–o385. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, approved standard-3rd ed.; CLSI Document M27-A3; CLSI: Wayne, PA, USA, 2008; Volume 28, Nº 14, pp. 1–25. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, approved standard-2nd ed.; CLSI Document M38-A2; CLSI: Wayne, PA, USA, 2008; Volume 28, Nº 16, pp. 1–3. [Google Scholar]
- Hubbard, W.C.; Alley, M.C.; Gray, G.N.; Green, K.C.; McLemore, T.L.; Boyd, M.R. Evidence for prostanoid biosynthesis as a biochemical feature of certain subclasses of non-small cell carcinomas of the lung as determined in established cell lines derived from human lung tumors. Cancer Res. 1989, 49, 826–832. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Insuasty, A.; Ramírez, J.; Raimondi, M.; Echeverry, C.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Rodríguez, M.V.; Zacchino, S.A.; et al. Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-Ethylidene-1,3-thiazol-4-ones. Molecules 2013, 18, 5482-5497. https://doi.org/10.3390/molecules18055482
Insuasty A, Ramírez J, Raimondi M, Echeverry C, Quiroga J, Abonia R, Nogueras M, Cobo J, Rodríguez MV, Zacchino SA, et al. Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-Ethylidene-1,3-thiazol-4-ones. Molecules. 2013; 18(5):5482-5497. https://doi.org/10.3390/molecules18055482
Chicago/Turabian StyleInsuasty, Alberto, Juan Ramírez, Marcela Raimondi, Carlos Echeverry, Jairo Quiroga, Rodrigo Abonia, Manuel Nogueras, Justo Cobo, María Victoria Rodríguez, Susana A. Zacchino, and et al. 2013. "Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-Ethylidene-1,3-thiazol-4-ones" Molecules 18, no. 5: 5482-5497. https://doi.org/10.3390/molecules18055482
APA StyleInsuasty, A., Ramírez, J., Raimondi, M., Echeverry, C., Quiroga, J., Abonia, R., Nogueras, M., Cobo, J., Rodríguez, M. V., Zacchino, S. A., & Insuasty, B. (2013). Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-Ethylidene-1,3-thiazol-4-ones. Molecules, 18(5), 5482-5497. https://doi.org/10.3390/molecules18055482