Abstract
Two new sesquiterpenoids, (−)-(1S*,2S*,3R*)-3-ethoxycupar-5-ene-1,2-diol (1) and (−)-(1S*,4S*,9S*)-1,9-epoxybisabola-2,10-diene-4-ol (2), along with six known compounds 3−8, were isolated from the EtOH extract of the herb of Leonurus japonicus. Their structures were elucidated by physical and spectroscopic analysis. In the in vitro assays, compounds 7 and 8 showed obvious antibacterial activity against several bacteria strains, while compound 3 significantly inhibited abnormal increase of platelet aggregation induced by ADP.
1. Introduction
Species of the genus Leonurus (Labiatae) are widely distributed in Eurasia, from Western Europe to China [1]. A number of bioactive secondary metabolites, including alkaloids [2,3], phenylethanoid glycosides [1], iridoid glucosides [4], cyclic peptides [5], diterpenoids [6,7,8,9] and triterpenoids [10], have been reported from several plants of this genus. Leonurus japonicus (synonyms Leonurus heterophyllus) is commonly used in Chinese herbal medicine for regulating menstrual disturbance, invigorating blood circulation, diuretics, and dispel edema [11,12]. In our previous study, chemical composition and antibacterial activity of essential oils from different parts of L. japonicus have been investigated [13]. The result showed that the oil of the herb (“Yimucao” in Chinese) had antibacterial activity against various Gram-positive bacteria and mainly consisted of sesquiterpenes and diterpenes, while the oil of the fruit (“Chongweizi” in Chinese) mainly made up of bornyl acetate and aliphatic hydrocarbons was inactive in the antibacterial assay. In searching for bioactive natural products from L. japonicus, we carried out a continuing investigation of the ethanolic extract of “Yimucao”. Two new sesquiterpenoids 1−2 and six known compounds were isolated from the EtOAc soluble portion of the ethanolic extract. This paper describes the isolation, structure elucidation, and bioassays of these isolates.
2. Results and Discussion
The EtOH extract of the herb of L. japonicus was suspended in water and successively partitioned with EtOAc and n-BuOH. Separation of the EtOAc fraction by column chromatography provided compounds 1−8 (Figure 1). The known compounds 3−8 were identified by comparing the spectroscopic data with those reported in the corresponding literature as (2S,5S)-2-hydroxy-2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-en-8-one (3) [14], 3-oxo-α-ionone (4) [15], (+)-dehydrovomifoliol (5) [16], (+)-3-hydroxy-β-ionone (6) [17], arteannuin B (7) [18], chamigrenal (8) [19].
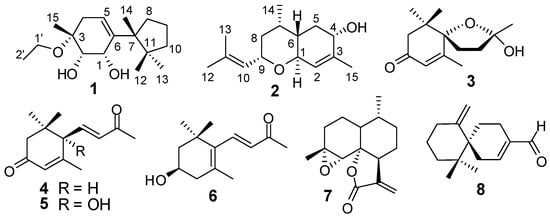
Figure 1.
Structures of compounds 1–8.
Compound 1 showed IR absorptions for hydroxyl (3,418 cm−1) and olefinic (3,039 and 1,463 cm−1) functionalities. The molecular formula C17H30O3 of 1, with three hydrogen deficiencies, was indicated by HR-ESI-MS and NMR data. The 1H-NMR spectrum of 1 displayed resonances attributable to four tertiary methyl groups [δH 0.82 (H3-12), 1.00 (H3-13), 1.06 (H3-14), and 1.25 (H3-15)], an ethoxyl group [δH 1.07 (t, J = 6.8 Hz, H3-2′), 3.49 (m, H-1′a), and 3.41 (m, H-1′b)], two oxymethines [δH 3.65 (m, H-2), 4.33 (m, H-1)], and an olefinic methine group [δH 5.62 (dd, J = 4.0 and 3.2 Hz, H-5)]. In addition, it showed resonances assignable to two exchangeable hydroxyl protons [δH 3.25 (d, J = 4.8 Hz, OH-1), 3.88 (d, J = 4.0 Hz, OH-2)] and partially overlapped resonances ascribable to several aliphatic methylenes between δH 1.40 and 2.40 (Table 1). The 13C-NMR and DEPT spectra of 1 revealed 17 carbon resonances (Table 1) corresponding to the above protonated units and four quaternary carbons (δC 45.9, 50.8, 76.1, and 143.1). These data suggested that 1 was a cuparene analogue with substitution of two hydroxyl groups and an ethoxyl group [20]. This conjecture was further confirmed by 2D NMR data analysis. The gHSQC spectrum of 1 furnished assignments of the proton-bearing carbon and corresponding proton resonances in the NMR spectra (Table 1). In the 1H-1H gCOSY spectrum of 1, homonuclear coupling correlations of H-1/H-2, H2-4/H-5, and H2-8/H2-9/H2-10 revealed the presence of structural units containing the vicinal coupling protons (Figure 2). In the HMBC spectrum, correlations of H-1/C-2, C-3, C-5, and C-6; OH-1/C-1, C-2, and C-6; H-2/C-3, C-4, C-6, and C-15; OH-2/C-1, C-2, and C-3; H-5/C-1, C-3, C-4, C-6, and C-7; H3-12 and H3-13/C-7, C-10, and C-11; H3-14/C-6, C-7, C-8, and C-11; H3-15/C-2, C-3, and C-4; H2-1′/C-3 (Figure 2), in combination with the shifts of these proton and carbon resonances, demonstrated a gross structure of 3-ethoxycupar-5-ene-1,2-diol for 1.

Table 1.
NMR data (Ā) for compounds 1 and 2 in acetone-d6 a.
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 4.33 m | 70.1 | 4.25 br d (9.6) | 69.1 |
| 2 | 3.65 m | 74.9 | 5.38 br s | 127.1 |
| 3 | – | 76.1 | – | 139.3 |
| 4 | 2.30 dd (17.2, 4.0), 2.19 dd (17.2, 3.2) | 35.8 | 3.93 br d (4.8) | 66.8 |
| 5 | 5.62 dd (4.0, 3.2) | 123.1 | 2.16 dd (15.0, 3.6), 1.70 ddd (15.0, 4.8, 3.6) | 32.7 |
| 6 | – | 143.1 | 1.38 m | 39.5 |
| 7 | – | 50.8 | 2.24 m | 28.5 |
| 8 | 2.36 dd (8.4, 4.4), 1.71 dd (8.4, 3.2) | 36.9 | 1.42 ddd (12.6, 3.6, 3.0), 0.92 dd (12.6, 1.2) | 41.3 |
| 9 | 1.62 m | 19.7 | 4.22 m | 73.9 |
| 10 | 1.65 (overlapped), 1.44 dd (12.4, 4.0) | 40.4 | 5.11 d (7.8) | 128.4 |
| 11 | – | 45.9 | – | 133.8 |
| 12 | 0.82 s | 26.7 | 1.65 s | 18.4 |
| 13 | 1.00 s | 23.9 | 1.67 s | 25.7 |
| 14 | 1.06 s | 25.1 | 0.95 d (6.6) | 20.3 |
| 15 | 1.25 s | 19.7 | 1.79 s | 20.7 |
| 1′ | 3.49 m, 3.41 m | 56.8 | ||
| 2′ | 1.07 t (6.8) | 16.5 | ||
| OH-1 | 3.25 d (4.8) | – | ||
| OH-2 | 3.88 d (4.4) | – | ||
a 1H-NMR data were measured at 400 MHz for 1 and at 600 MHz for 2, respectively. Proton coupling constants (J) in Hz are given in parentheses. 13C NMR data were measured at 150 MHz for 1 and 2. The assignments were based on 1H-1H COSY, HSQC, and HMBC experiments.
The configuration of 1 was elucidated by the NOESY data analysis [20]. In the NOESY spectrum of 1, correlations of H-1 with H-2, H3-12, and H3-14; and H-2 with H-1, H3-14, and H3-15 (Figure 2) indicated that the orientations of H-1, H-2, and H3-15 were consistent with those of H3-12 and H3-14, but opposite that of H3-13. Thus, compound 1 was determined as (−)-(1S*,2S*,3R*)-3-ethoxycupar-5-ene-1,2-diol.
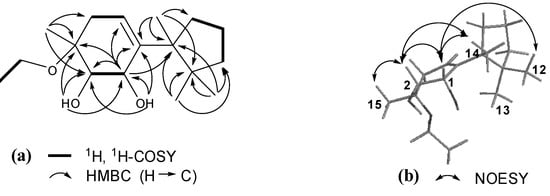
Figure 2.
(a) Key 1H, 1H-COSY and HMBC correlations of 1; (b) Key NOESY correlations of 1.
Compound 2, obtained as a colorless oil, had the molecular formula C15H24O2 with four degrees of unsaturation as indicated by HR-ESI-MS m/z 259.1668 [M+Na]+ (calcd for C15H24O2Na, 259.1674). The 1H-NMR spectrum of 2 (Table 1) showed signals ascribed to a secondary methyl [δH 0.95 (d, J = 6.6 Hz, H3-14)] and three olefinic tertiary methyl [δH 1.79 (H3-15), 1.67 (H3-13), and 1.65 (H3-12)] groups, three oxymethines [δH 4.25 (brd, J = 9.6 Hz, H-1), 4.22 (m, H-9), and 3.93 (brd, J = 4.8 Hz, H-4)], and two trisubstituted double bonds [δH 5.38 (brs, H-2) and 5.11 (d, J = 7.8 Hz, H-10)]. In addition, the proton signals attributed to aliphatic methylenes and methines between δH 0.90 and 2.30, together with the 13C-NMR and DEPT data, indicated the presence of two aliphatic methylenes and two methines in 2. These spectroscopic features suggested that 2 was a sesquiterpene and similar to (+)-bisabola-2,10-diene[1,9]oxide [21]. Comparison of their NMR data showed replacement of one methylene unit in (+)-bisabola-2,10-diene[1,9]oxide by an oxymethine (δH 3.93 and δC 66.8) in 2 (Table 1). Meanwhile, the olefinic proton signal for H-2 was changed from a doublet (J1,2 = 6.5 Hz) in (+)-bisabola-2,10-diene[1,9]oxide into a broad singlet in 2 (J1,2 ≈ 0 Hz). All the above spectroscopic data analysis indicated that 2 was an analogue of (+)-bisabola-2,10-diene[1,9]oxide with an additional hydroxy at C-4 and different configuration at C-1, which was proved by the 2D NMR experiments that amended the assignments of the NMR data (Figure 3). In the NOE difference spectrum of 2, irradiation of H-7 enhanced H-4, H-6, and H-9, while H-1 was enhanced upon irradiation of H3-14 (Figure 3). These enhancements revealed that the protons H-4/H-6/H-7/H-9 had to be on the same side of the ring system, H-1/OH-4/isobutenyl-9/H3-14 were located on the opposite side. Therefore, compound 2 was determined as (−)-(1S*,4S*,9S*)-1,9-epoxybisabola-2,10-diene-4-ol.

Figure 3.
(a) Key 1H, 1H-COSY and HMBC correlations of 2; (b) Key NOE correlations of 2.
The antibacterial activity of the isolates was assayed by the micro-dilution method [22]. Compound 7 showed the obvious activity against Escherichia coli and Enterobacter aerogenes with the MIC values of 25 μg/mL and 50 μg/mL, respectively, while compound 8 had the antibacterial activity against three Gram-positive strains, including Macrococcus caseolyticus, Staphylococcus auricularis,and Staphylococcus aureus (MIC 25, 50, 200 μg/mL, respectively). In addition, the inhibitory activity of the compounds against platelet aggregation induced by ADP was conducted by Born’s method [23]. The maximum aggregation ratio of the blank control was 61.4 ± 9.44%, while compound 3 evidently inhibited abnormal increase of platelet aggregation at a concentration of 10 μM, with the maximum aggregation ratio of 42.0 ± 15.63% (p < 0.01).
3. Experimental
3.1. General
NMR spectra were recorded on a Bruker-AV-400 or SYS-600 spectrometers. HRESIMS were measured with Waters Synapt G2 HDMS. IR were recorded on a Vector 22 FT-IR spectrometer. UV spectra were obtained on a Shimadzu UV-260 spectrophotometer. Optical rotations were measured with a Perkin-Elmer 341 plus. Platelet aggregation was recorded on Labor APACT-2 aggregation meter. Column chromatography was performed with silica gel (200–300 mesh, Yantai Institute of Chemical Technology, Yantai, China), MCI gel CHP 20P (75–150 μm, Mitsubishi Chemical, Co., Tokyo, Japan), and Sephadex LH-20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden). HPLC separation was performed on an instrument consisting of a Cometro 6000LDS pump and a Cometro 6000PVW UV/VIS detector with an Ultimate (250 ƀ 10 mm) preparative column packed with C18 (5 μm). TLC was carried out with glass precoated silica gel GF254 plates (Qingdao Marine Chemical Inc., Qingdao, China).
3.2. Plant Material
The herb of L. japonicus (“Yimucao”) was collected in May of 2012 from the field in Wenjiang District, Chengdu City, Sichuan Province, China. Plant identity was verified by Prof. Min Li (Chengdu University of TCM, Sichuan, China). A voucher specimen (SYMC-0522) was deposited at the School of Pharmacy, Chengdu University of TCM, China.
3.3. Extraction and Isolation
The air-dried herb of L. japonicus (20 kg) was extracted three times with 95% EtOH (3 × 160 L) at room temperature for 72 h. The ethanolic extract was evaporated under reduced pressure to yield a dark brown residue (1.2 kg). The residue was suspended in H2O and then successively partitioned into EtOAc (400 g) and n-BuOH (160 g) fractions. The EtOAc extract (400 g) was subjected to silica gel CC using a gradient elution of petroleum ether–acetone (100:1–0:1) to afford nineteen fractions (F1-F19). F4 was further separated by silica gel CC over petroleum ether–EtOAc (35:1) yield four subfractions (F4-1–F4-4). The successive separation of F4-3 with Sephadex LH-20 (petroleum ether-CHCl3-MeOH, 5:5:1) and with PTLC (petroleum ether-EtOAc 10:1) yielded 4 (14 mg) and 8 (120 mg). Eluting with a step gradient of 50%-100% MeOH in H2O, F7 was separated by flash chromatography over MCI gel, to give ten subfractions (F7-1-F7-10). F7-2 was purified via Sephadex LH-20 (petroleum ether-CHCl3-MeOH, 5:5:1) to give F7-2-1–F7-2-4. F7-2-2 was fractioned via PTLC (petroleum ether-acetone 8:1) followed by reversed-phase semipreparative HPLC (75% MeOH in H2O) purification to afford 3 (15 mg), 5 (4 mg), and 6 (3 mg). Separation of F7-2-3 by PTLC (petroleum ether-acetone 6:1) and reversed-phase semipreparative HPLC (60% MeOH in H2O) successively yielded 2 (2 mg) and 7 (20 mg). F7-3 was separated by silica gel CC over petroleum ether–acetone (20:1–1:1) to get F7-3-1–F7-3-6. F7-3-1 was further purified by reversed-phase semipreparative HPLC, using MeOH–H2O (85: 15) to afford 1 (11 mg).
(−)-(1S*,2S*,3R*)-3-ethoxycupar-5-ene-1,2-diol (1): Colorless oil, 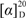 = −5.0 (c = 0.10, MeOH); IR (KBr) νmax: 3,418, 3,039, 2,963, 2,928, 2,874, 1,462, 1,368, 1,237, 1,096, 1,057 cm−1; ESI-MS m/z 305.2 [M+Na]+; HRESI-MS: m/z 305.2090 [M+Na]+ (calcd for C17H30O3Na, 305.2093); 1H- and 13C-NMR data see Table 1.
= −5.0 (c = 0.10, MeOH); IR (KBr) νmax: 3,418, 3,039, 2,963, 2,928, 2,874, 1,462, 1,368, 1,237, 1,096, 1,057 cm−1; ESI-MS m/z 305.2 [M+Na]+; HRESI-MS: m/z 305.2090 [M+Na]+ (calcd for C17H30O3Na, 305.2093); 1H- and 13C-NMR data see Table 1.
(−)-(1S*,4S*,9S*)-1,9-epoxybisabola-2,10-diene-4-ol (2): Colorless oil, 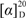 = −2.2 (c = 0.15, MeOH); IR (KBr) νmax: 3,478, 3,019, 2,925, 2,858, 1,461, 1,375, 1,230, 1,028 cm−1; ESI-MS m/z 259.2 [M+Na]+; HRESI-MS m/z 259.1668 [M+Na]+ (calcd for C15H24O2Na, 259.1674); 1H- and 13C-NMR data see Table 1.
= −2.2 (c = 0.15, MeOH); IR (KBr) νmax: 3,478, 3,019, 2,925, 2,858, 1,461, 1,375, 1,230, 1,028 cm−1; ESI-MS m/z 259.2 [M+Na]+; HRESI-MS m/z 259.1668 [M+Na]+ (calcd for C15H24O2Na, 259.1674); 1H- and 13C-NMR data see Table 1.
3.4. Antibacterial Activity Experiments
All bacteria were obtained from clinical samples and stored in the Department of Pharmacology of Chengdu University of TCM. The in vitro antibacterial activity was determined by the standard agar dilution method, according to NCCLS (National Committee for Clinical Laboratory Standard) [22]. 5 μL of cultures of test strains at the concentration of 1 × 106 CFU/mL were inoculated on Mueller Hinton agar containing different concentrations of the test compounds. The MIC values were determined after incubation at 35 °C for 24 h.
3.5. Platelet Aggregation Assay
SD rats were lightly anesthetized with ether. Blood was immediately taken from the femoral artery and anticoagulated with 3.8% trisodium citrate (9:1, v/v). Platelet rich plasma (PRP) was obtained by centrifugation of the whole blood at 800 g for 10 min. The precipitate of PRP was further centrifuged at 3000 g for 10 min to obtain platelet poor plasma (PPP). PRP was adjusted with PPP to about 2 × l08 ~ 4 × 108 platelets/L. Then, the platelet aggregation induced by ADP (final concentration: 0.05 mg/mL) was recorded on a dual sample aggregation meter according to Born’s method [23]. The antiplatelet efficacy was evaluated by comparing maximum aggregation response of the tested compound groups with that of control group.
4. Conclusions
Based on our previous study on the essential oil of L. japonicus obtained by hydrodistillation [13], we carried on a continuing examination of the EtOAc soluble portion of the ethanolic extract of the herb of this plant. Two new sesquiterpenoids, (−)-(1S*,2S*,3R*)-3-ethoxycupar-5-ene-1,2-diol (1) and (−)-(1S*,4S*,9S*)-1,9-epoxybisabola-2,10-diene-4-ol (2) were isolated, together with six known sesquiterpenoids. Among them, compounds 7 and 8 showed antibacterial activity against several bacteria strains, including E. coli, E. aerogenes, M. caseolyticus, S. auricularis, and S. aureus, with the MIC values in the range of 25 to 200 μg/mL. In addition, at a concentration of 10 μM, compound 3 displayed the inhibitory activity against platelet aggregation induced by ADP. According to the literature survey, cuparane- and chamigrane-type sesquiterpenoids were isolated from the genus Leonurus for the first time.
Acknowledgments
Financial support from the National Key Technology R&D Program for the “Eleventh Five-Year” Plan of China (grant No. 2009BAI84B00), and the Sichuan Science and Technology Support Program (grant No. 2011SZ0056) is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.X.; Chen, Z.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Hepatoprotective glycosides from Leonurus japonicus Houtt. Carbohydr. Res. 2012, 348, 42–46. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, L.L.; Chen, P.F.; Zhu, Y.Z. Leonurine improves ischemia-induced myocardial injury through antioxidative activity. Phytomedicine 2010, 17, 753–759. [Google Scholar] [CrossRef]
- Qi, J.; Hong, Z.Y.; Xin, H.; Zhu, Y.Z. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull. 2010, 33, 1958–1964. [Google Scholar] [CrossRef]
- Tasdemir, D.; Scapozza, L.; Zerbe, O.; Linden, A.; Çalis, I.; Sticher, O. Iridoid glycosides of Leonurus persicus. J. Nat. Prod. 1999, 62, 811–816. [Google Scholar] [CrossRef]
- Morita, H.; Iizuka, T.; Gonda, A.; Itokawa, H.; Takeya, K. Cycloleonuripeptides E and F, cyclic nonapeptides from Leonurus heterophyllus. J. Nat. Prod. 2006, 69, 839–841. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Jin, H.G.; Woo, E.R.; Kang, S.S.; Baek, S.W.; Kim, J.; Kim, Y.S. Anti-inflammatory mechanism of 15,16-epoxy-3α-hydroxylabda-8,13(16),14-trien-7-one via inhibition of LPS-induced multicellular signaling pathways. J. Nat. Prod. 2012, 75, 67–71. [Google Scholar] [CrossRef]
- Boalino, D.M.; McLean, S.; Reynolds, W.F.; Tinto, W.F. Labdane diterpenes of Leonurus sibiricus. J. Nat. Prod. 2004, 67, 714–717. [Google Scholar] [CrossRef]
- Moon, H.T.; Jin, Q.; Shin, J.E.; Choi, E.J.; Han, H.K.; Kim, Y.S.; Woo, E.R. Bis-spirolabdane-type diterpenoids from Leonurus sibiricus. J. Nat. Prod. 2010, 73, 123–126. [Google Scholar]
- Gong, H.Q.; Wang, R.; Shi, Y.P. New labdane-type diterpenoids from Leonurus heterophyllus. Helv. Chim. Acta 2012, 95, 618–625. [Google Scholar] [CrossRef]
- Liu, Y.; Kubo, M.; Fukuyama, Y. Spirocyclic nortriterpenoids with NGF-potentiating activity from the fruits of Leonurus heterophyllus. J. Nat. Prod. 2012, 75, 1353–1358. [Google Scholar] [CrossRef]
- Commission of Chinese Pharmacopoeia, Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 272–273.
- Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1995; pp. 1609−1610,1954–1956.
- Xiong, L.; Peng, C.; Zhou, Q.M.; Wan, F.; Xie, X.F.; Guo, L.; Li, X.H.; He, C.J.; Dai, O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules 2013, 18, 963–973. [Google Scholar] [CrossRef]
- Knapp, H.; Weigand, C.; Gloser, J.; Winterhalter, P. 2-Hydroxy-2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-en-8-one: precursor of 8,9-dehydrotheaspirone in white-fleshed nectarines. J. Agric. Food Chem. 1997, 45, 1309–1313. [Google Scholar] [CrossRef]
- Ma, M.; Bell, S.G.; Yang, W.; Hao, Y.; Rees, N.H.; Bartlam, M.; Zhou, W.; Wong, L.; Rao, Z. Structural analysis of CYP101C1 from Novosphingobium aromaticivorans DSM12444. ChemBioChem. 2011, 12, 88–89. [Google Scholar]
- Kai, H.; Baba, M.; Okuyama, T. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007, 55, 133–136. [Google Scholar] [CrossRef]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Vishwakarma, R.A.; Jain, D.C.; Roy, R. High field NMR spectroscopic studies of arteannuin B and a reappraisal of the structure of arteannuin C. Phytochemistry 1991, 30, 3469–3471. [Google Scholar]
- Baek, N.I.; Han, J.T.; Ahn, E.M.; Park, J.K.; Cho, S.W.; Jeon, S.G.; Jang, J.S.; Kim, C.K.; Choi, S.Y. Isolation of anticonvulsant compounds from the fruits of Schizandra chinensis Baili. J. Korean Soc. Agric. Chem. Biotechnol. 2000, 43, 72–75. [Google Scholar]
- Nagashima, F.; Suzuki, M.; Takaoka, S.; Asakawa, Y. Sesqui- and diterpenoids from the Japanese liverwort Jungermannia infusca. J. Nat. Prod. 2001, 64, 1309–1317. [Google Scholar] [CrossRef]
- Warmers, U.; Rieck, A.; König, W.A.; Muhle, H. Bisabola-2,10-diene[1,9]oxide, a constituent of the liverwort Calypogeia suecica. Phytochemistry 1999, 51, 679–682. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS), Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Sixth Edition: Approved Standard M7-A6; NCCLS: Wayne, PA, USA, 2003.
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).