Functionalization of Multi-Walled Carbon Nanotubes with Thermo-Responsive Azide-Terminated Poly(N-isopropylacrylamide) via Click Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of MWNTs with Alkyne Groups (MWNTs-alk)
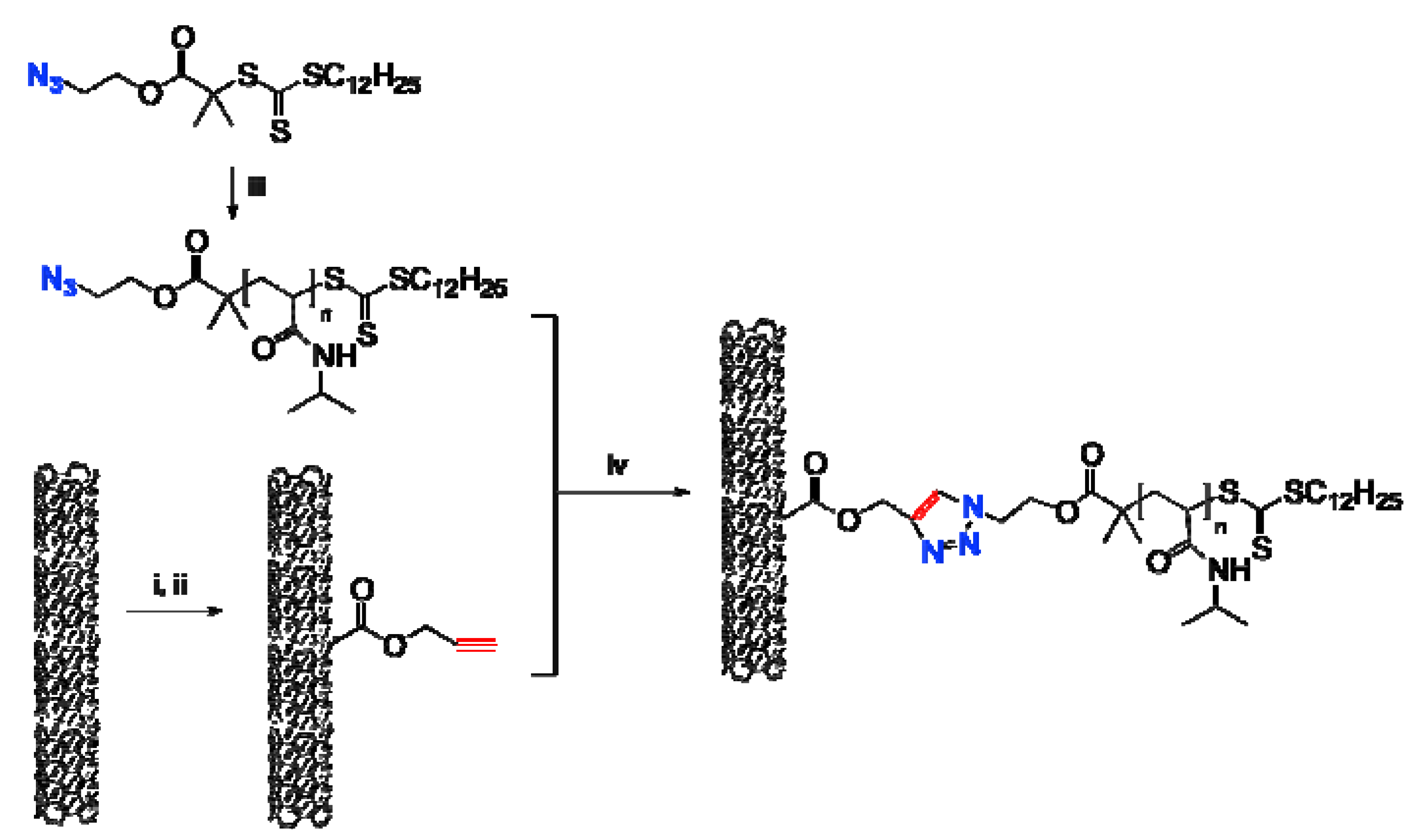
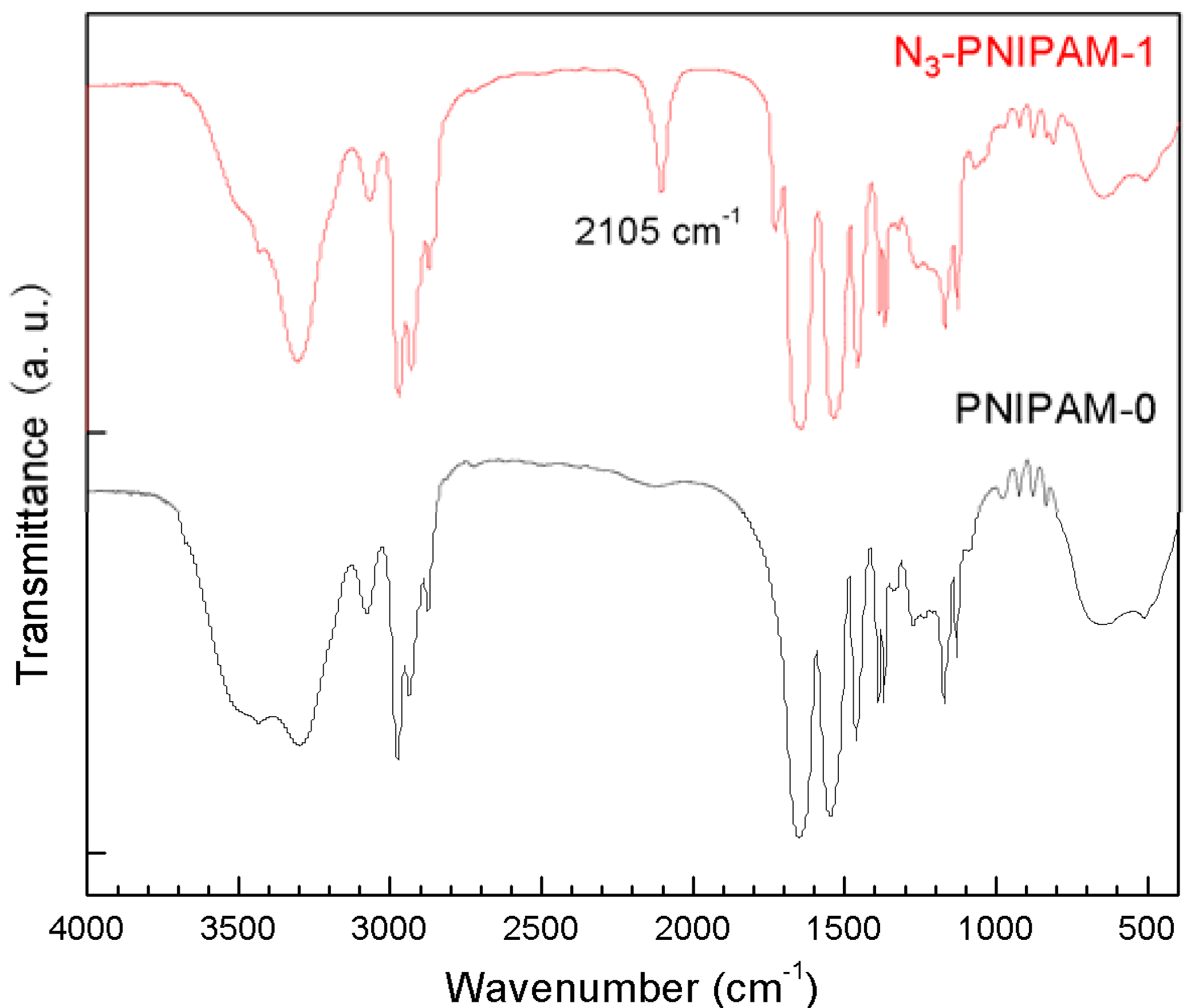
2.2. Synthesis and Characterization of N3-PNIPAM
| Entry | Sample | [M]:[CTA]:[I] | Mtheo (g·mol−1) | Mn (g·mol−1) | PDI |
|---|---|---|---|---|---|
| 1 | N3-PNIPAM-1 | 100:5:1 | 2200 | 2,180 | 1.19 |
| 2 | N3-PNIPAM-2 | 250:5:1 | 5600 | 4,790 | 1.26 |
| 3 | N3-PNIPAM-3 | 500:5:1 | 11,000 | 9,440 | 1.22 |
| 4 | PNIPAM-0 | 200:1 * | / | 8,220 | 2.83 |
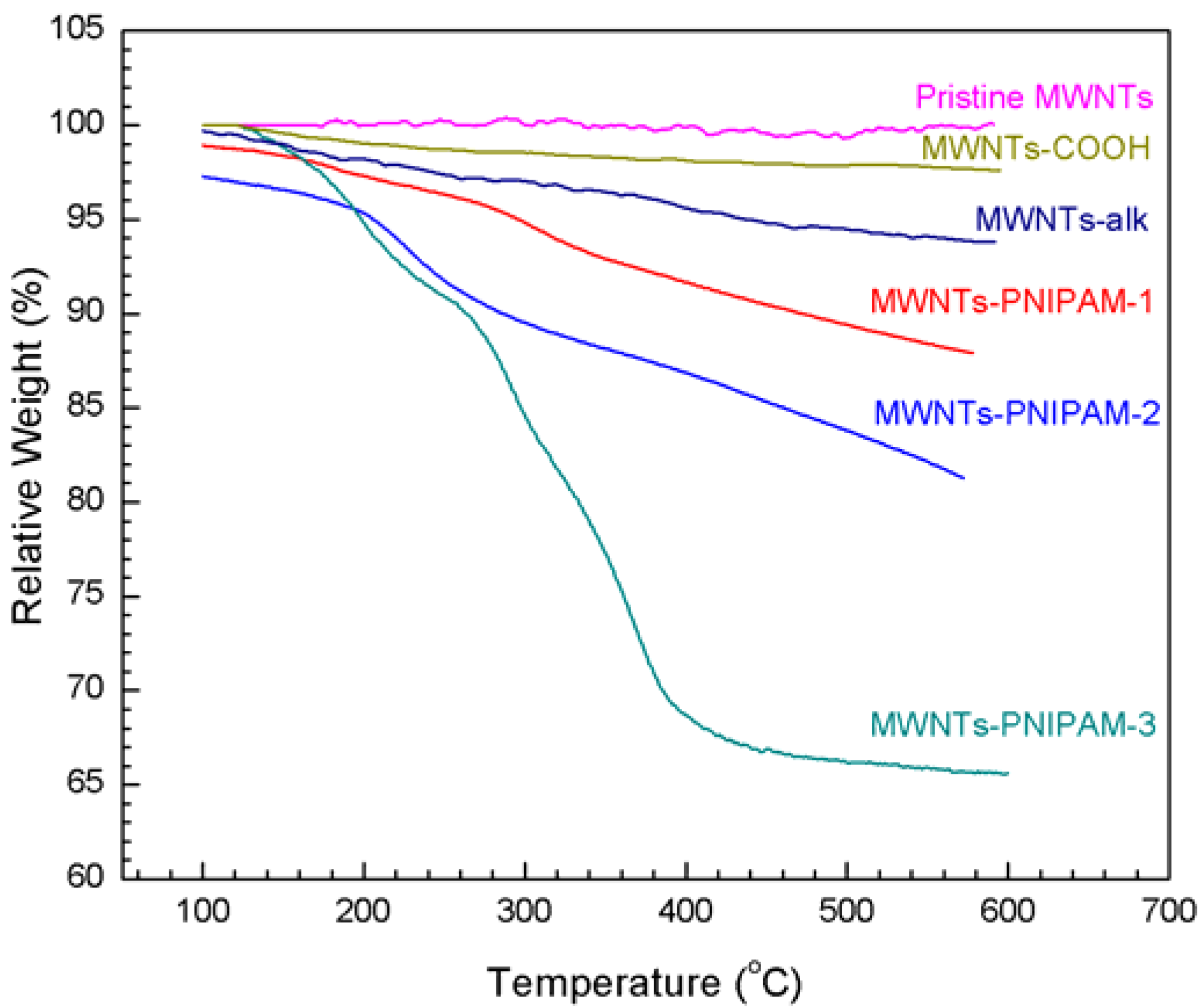
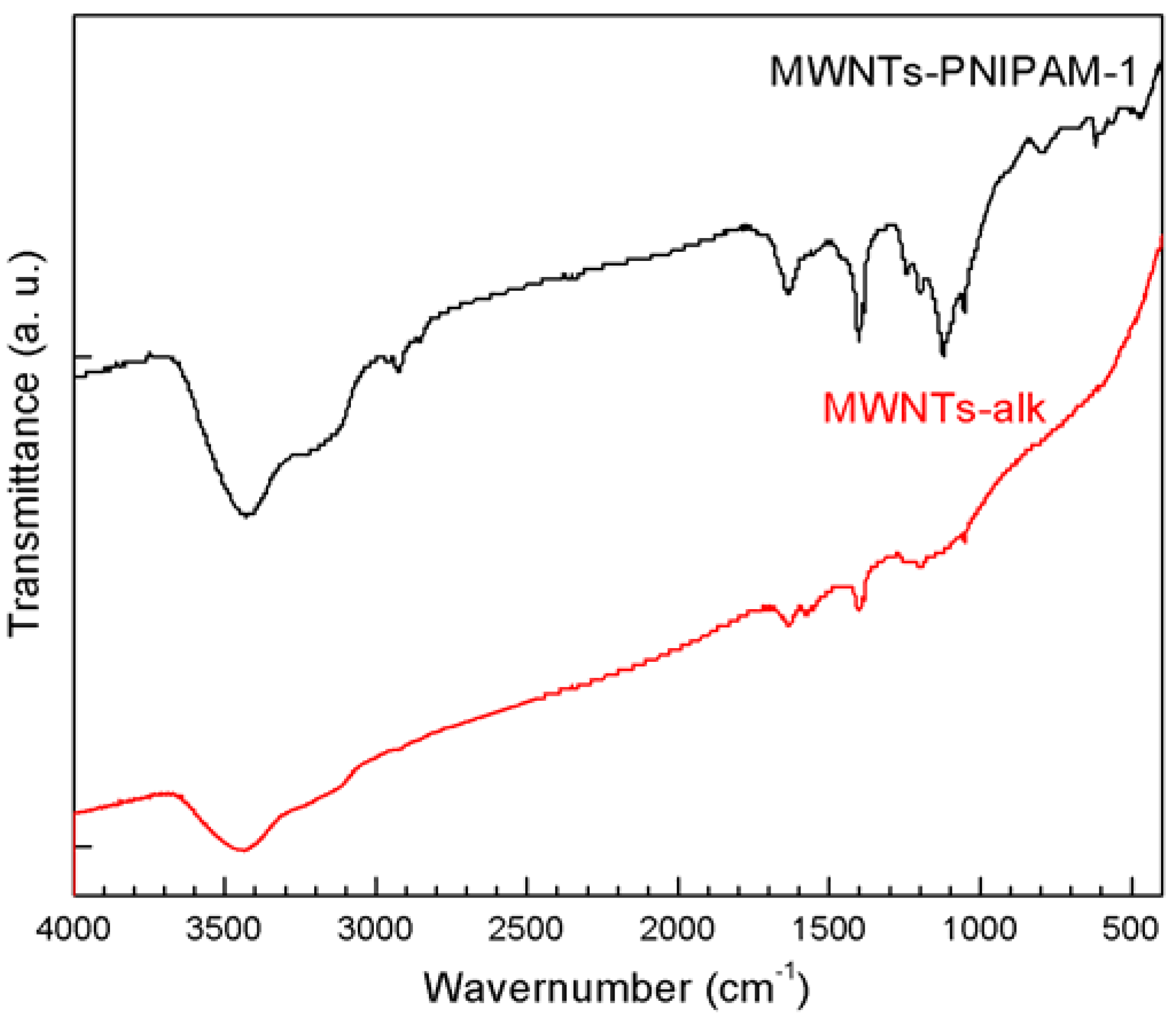
2.3. Coupling of Polymers onto MWNTs by Click Reaction
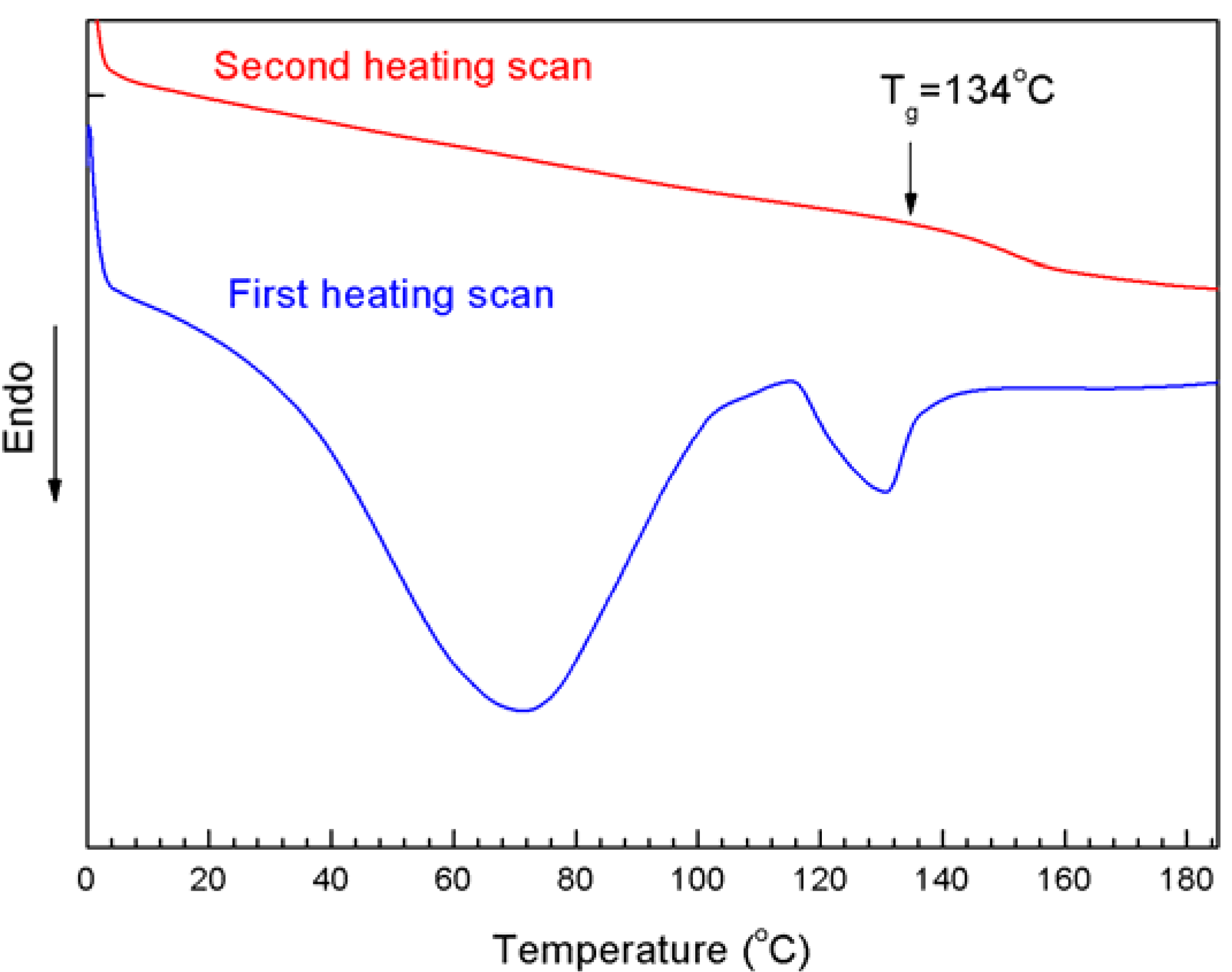
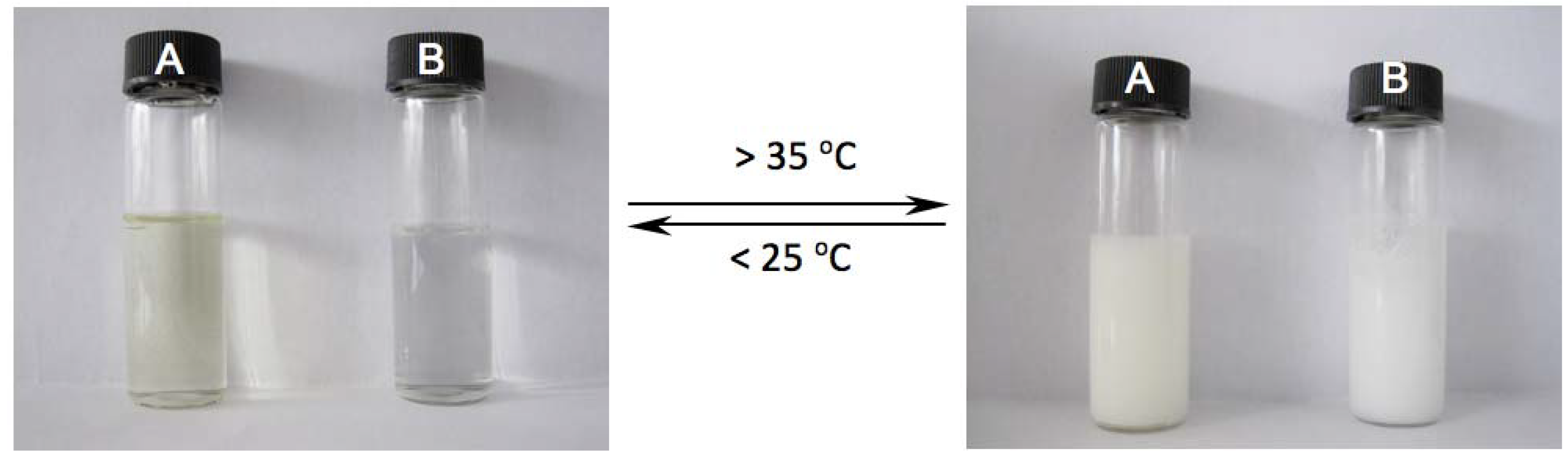
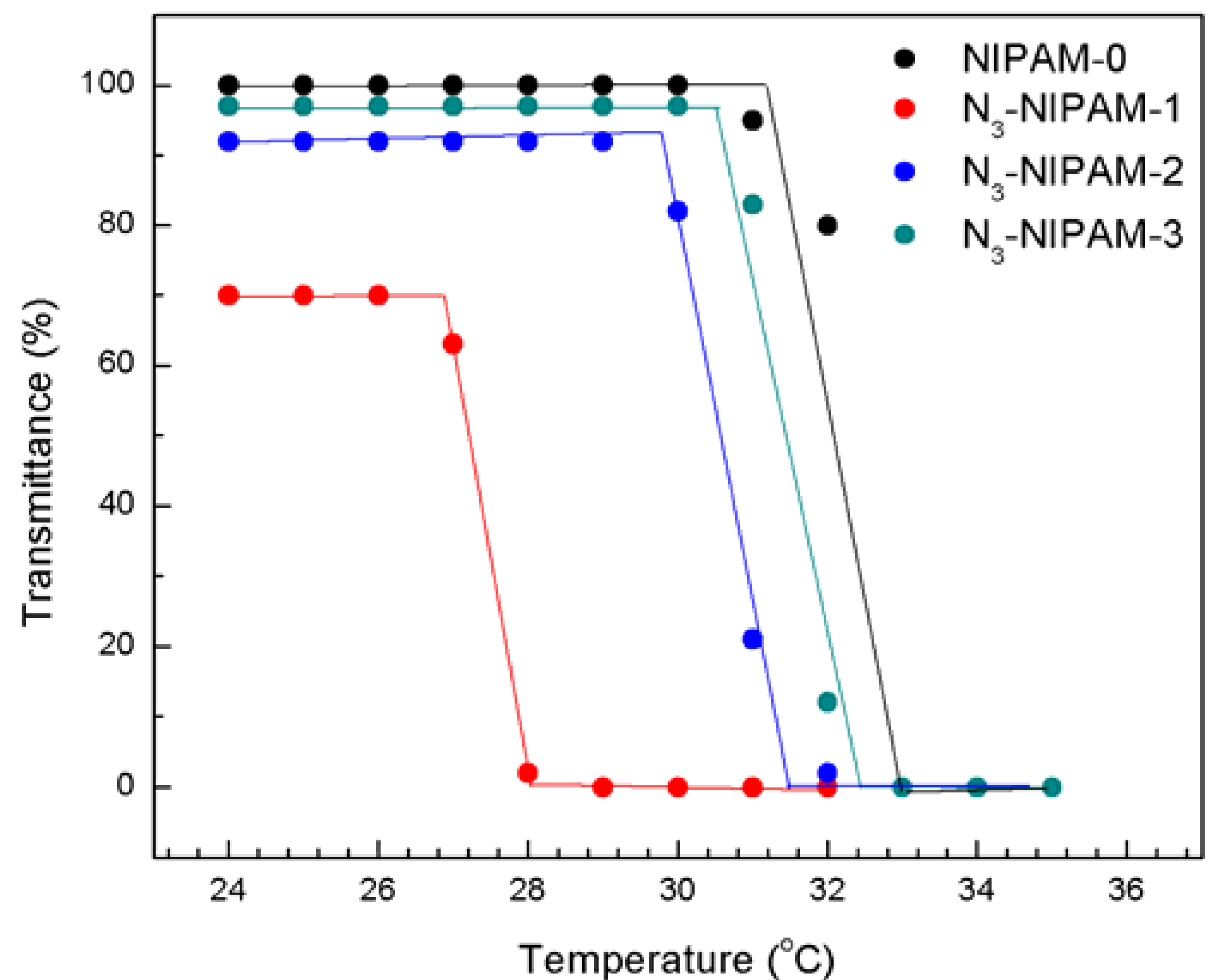
2.4. Dispersion Behavior of MWNTs-PNIPAM in Water
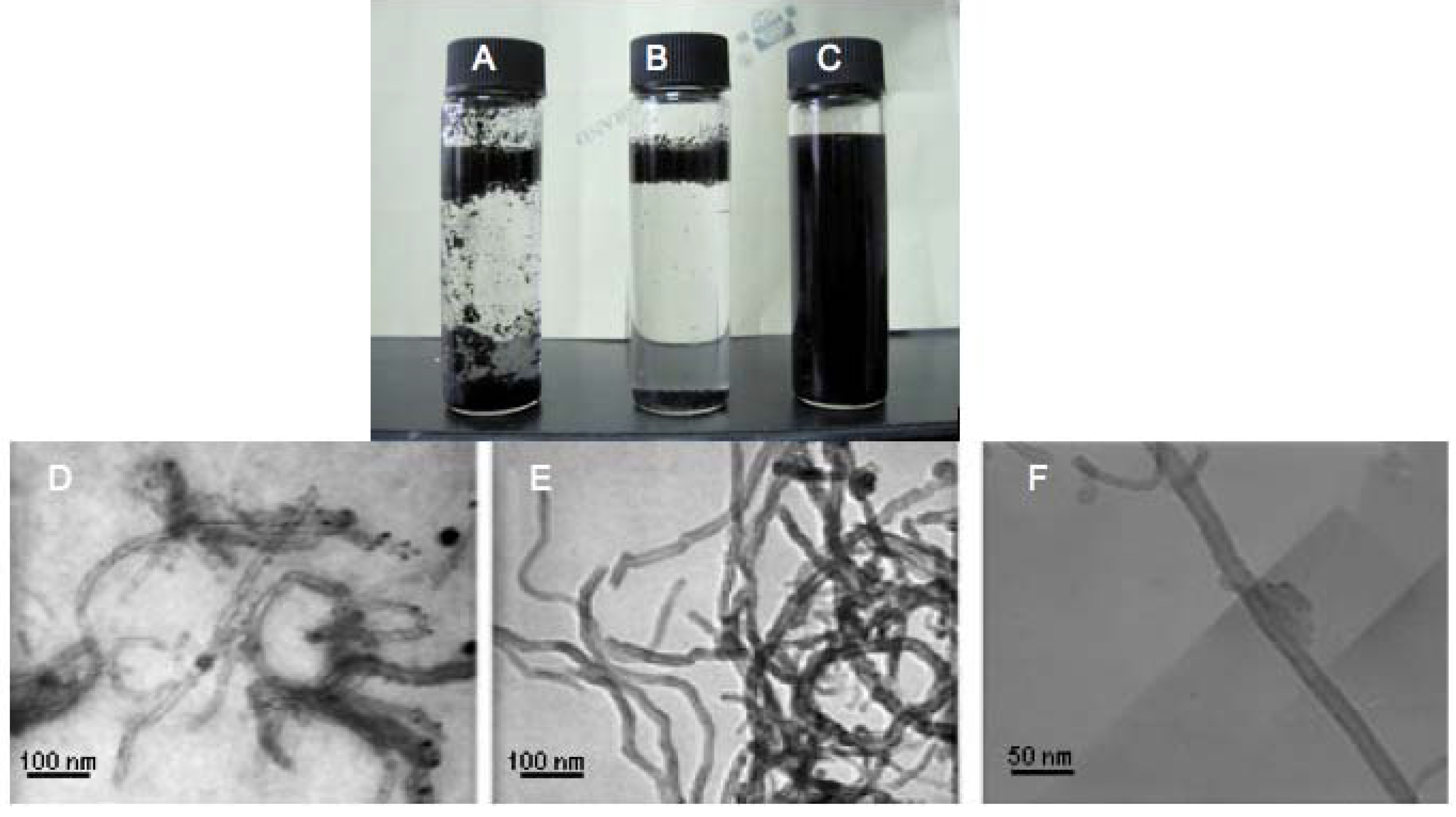
3. Experimental
3.1. Materials
3.2. Synthesis of Alkyne-Modified Multi-Walled Carbon Nanotubes (MWNTs-alk)
3.3. Synthesis of N3-PNIPAM
3.4. Coupling of N3-PNIPAM and MWNTs-alk via Click Reaction
3.5. Characterizations
4. Conclusions
Selected Abbreviations and Acronyms
| CNTs | Carbon nanotubes |
| MWNTs | Multi-walled carbon nanotubes |
| SWNTs | Single-walled carbon nanotubes |
| PNIPAM | poly(N-isopropylacrylamide) |
| N3-CTA | Azide-capped chain transfer agent |
| N3-PNIPAM | Azide-terminated poly(N-isopropylacrylamide) |
| MWNTs-alk | MWNTs with alkyne groups |
| MWNTs-PNIPAM | MWNTs functionalized by N3-PNIPAM |
Acknowledgments
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Vigolo, B.; Penicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef]
- Postma, H.W.C.; Teepen, T.; Yao, Z.; Grifoni, M.; Dekker, C. Carbon nanotube single-electron transistors at room temperature. Science 2001, 293, 76–79. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—the route towards applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Nativ-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of individual carbon nanotubes in aqueous solutions. Nano. Lett. 2002, 2, 25–28. [Google Scholar] [CrossRef]
- Wang, C.C.; Guo, Z.X.; Fu, S.K.; Wu, W.; Zhu, D.B. Polymers containing fullerene or carbon nanotube structures. Prog. Polym. Sci. 2004, 29, 1079–1141. [Google Scholar] [CrossRef]
- Liu, P. Modifications of carbon nanotubes with polymers. Eur. Polym. J. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Sun, Y.P.; Fu, K.F.; Lin, Y.; Huang, W.J. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Hill, D.E.; Lin, Y.; Rao, A.M.; Allard, L.F.; Sun, Y.P. Functionalization of carbon nanotubes with polystyrene. Macromolecules 2002, 35, 9466–9471. [Google Scholar] [CrossRef]
- Kong, H.; Gao, C.; Yan, D.Y. Functionalization of multiwalled carbon nanotubes by atom transfer radical polymerization and defunctionalization of the products. Macromolecules 2004, 37, 4022–4030. [Google Scholar] [CrossRef]
- Cui, J.; Wang, W.P.; You, Y.Z.; Liu, C.H.; Wang, P.H. Functionalization of multiwalled carbon nanotubes by reversible addition fragmentation chain-transfer polymerization. Polymer 2004, 45, 8717–8721. [Google Scholar] [CrossRef]
- Kong, H.; Gao, C.; Yan, D.Y. Controlled functionalization of multiwalled carbon nanotubes by in situ atom transfer radical polymerization. J. Am. Chem. Soc. 2004, 126, 412–413. [Google Scholar] [CrossRef]
- Xu, G.Y.; Wu, W.T.; Wang, Y.S.; Pang, W.M.; Zhu, Q.R.; Wang, P.H.; You, Y.Z. Constructing polymer brushes on multiwalled carbon nanotubes by in situ reversible addition fragmentation chain transfer polymerization. Polymer 2006, 47, 5909–5918. [Google Scholar] [CrossRef]
- Wang, G.J.; Huang, S.Z.; Wang, Y.; Liu, L.; Qiu, J.; Li, Y. Synthesis of water-soluble single-walled carbon nanotubes by RAFT polymerization. Polymer 2007, 48, 728–733. [Google Scholar] [CrossRef]
- Hong, C.Y.; You, Y.Z.; Pan, C.Y. Synthesis of water-soluble multi-walled carbon nanotubes with grafted grafted temperature-responsive shells by surface RAFT polymerization. Chem. Mater. 2005, 17, 2247–2254. [Google Scholar] [CrossRef]
- Hiromi, K.; Kazutaka, T.; Yasutaka, A. Functionalization of single-walled carbon nanotube by the covalent modification with polymer chains. J. Colloid Interface Sci. 2007, 306, 28–33. [Google Scholar] [CrossRef]
- Bromberg, L.E.; Ron, E.S. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Delivery Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef]
- Chen, G.H.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Koshti, N.; Franchina, J.G.; Frels, J.D. Sequestration of trace metals using water-soluble and fluorous phase-soluble polymers. Angew. Chem. Int. Ed. 2000, 39, 1039–1042. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Caraway, J.W. Thermoresponsive polymer-bound substrates. J. Am. Chem. Soc. 1996, 118, 6092–6093. [Google Scholar] [CrossRef]
- Sun, T.L.; Wang, G.J.; Feng, L.; Liu, B.Q.; Ma, Y.M.; Jiang, L.; Zhu, D.B. Reversible switching between superhydrophilicity and superhydrophobicity. Angew. Chem. Int. Ed. 2004, 43, 357–360. [Google Scholar] [CrossRef]
- You, Y.Z.; Hong, C.Y.; Pan, C.Y. Preparation of smart polymer/carbon nanotube conjugates via stimuli-responsive linkages. Adv. Funct. Mater. 2007, 17, 2470–2477. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lutz, J.F. 1,3-Dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Johnson, J.A.; Finn, M.G.; Koberstein, J.T.; Turro, N.J. Construction of linear polymers, dendrimers, networks, and other polymeric architectures by copper-catalyzed azide-alkyne cycloaddition “click” chemistry. Macromol. Rapid Commun. 2008, 29, 1052–1072. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. “Click” chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Duft, A.M.; Adronov, A. Functionalization of single-walled carbon nanotubes with well-defined polystyrene by “click” coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar]
- Liu, J.; Nie, Z.; Gao, Y.; Adronov, A.; Li, H. “Click” coupling between alkyne-decorated multiwalled carbon nanotubes and reactive PDMA-PNIPAM micelles. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 7187–7199. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mahapatra, S.S.; Cho, J.W.; Lee, J.Y. Functionalization of multiwalled carbon nanotubes with poly(styrene-b-(ethylene-co-butylene)-b-styrene) by click coupling. J. Phys. Chem. C 2010, 114, 11395–11400. [Google Scholar] [CrossRef]
- Rana, S.; Cho, J.W. Functionalization of carbon nanotubes via Cu(I)-catalyzed Huisgen [3+2] cycloaddition “click” chemistry. Nanoscale 2010, 2, 2550–2556. [Google Scholar] [CrossRef]
- Clave, G.; Campidelli, S. Efficient covalent functionalisation of carbon nanotubes: The use of “click chemistry”. Chem. Sci. 2011, 2, 1887–1896. [Google Scholar] [CrossRef]
- Ju, X.J.; Chu, L.Y.; Mi, P.; Song, H.; Lee, Y.M. Synthesis and characterization of a novel thermo-sensitive copolymer of N-isopropylacrylamide and dibenzo-18-crown-6-diacrylamide. Macromol. Rapid Commun. 2006, 27, 2072–2077. [Google Scholar] [CrossRef]
- Kucklingl, D.P.; Adler, H.J.; Ling, L.; Habicher, W.D.; Arndt, K.F. Temperature sensitive polymers based on 2-dimethylmaleinimido)-N-ethyl-acrylamide: Copolymers with N-isopropylacrylamide. Polym. Bull. 2000, 44, 269–276. [Google Scholar] [CrossRef]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef]
- Lai, J.T.; Filla, D.; Shea, R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar]
- Li, C.; Hu, J.; Yin, J.; Liu, S. Click coupling fullerene onto thermoresponsive water-soluble diblock copolymer and homopolymer chains at defined positions. Macromolecules 2009, 42, 5007–5016. [Google Scholar] [CrossRef]
- Sample Availability: MWNTs are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, X.; Shuai, Y.; Guo, Z.; Feng, Y. Functionalization of Multi-Walled Carbon Nanotubes with Thermo-Responsive Azide-Terminated Poly(N-isopropylacrylamide) via Click Reactions. Molecules 2013, 18, 4599-4612. https://doi.org/10.3390/molecules18044599
Su X, Shuai Y, Guo Z, Feng Y. Functionalization of Multi-Walled Carbon Nanotubes with Thermo-Responsive Azide-Terminated Poly(N-isopropylacrylamide) via Click Reactions. Molecules. 2013; 18(4):4599-4612. https://doi.org/10.3390/molecules18044599
Chicago/Turabian StyleSu, Xin, Ya Shuai, Zanru Guo, and Yujun Feng. 2013. "Functionalization of Multi-Walled Carbon Nanotubes with Thermo-Responsive Azide-Terminated Poly(N-isopropylacrylamide) via Click Reactions" Molecules 18, no. 4: 4599-4612. https://doi.org/10.3390/molecules18044599
APA StyleSu, X., Shuai, Y., Guo, Z., & Feng, Y. (2013). Functionalization of Multi-Walled Carbon Nanotubes with Thermo-Responsive Azide-Terminated Poly(N-isopropylacrylamide) via Click Reactions. Molecules, 18(4), 4599-4612. https://doi.org/10.3390/molecules18044599




