Abstract
In the present study, the chemical investigation of the leaves of Annona reticulata has resulted in the identification of nine compounds, including annonaretin A, (1), a new triterpenoid. The purified compounds were subjected to the examination of their effects on NO inhibition in LPS-activated mouse peritoneal macrophages and most of them exhibited significant NO inhibition, with IC50 values in the range of 48.6 ± 1.2 and 99.8 ± 0.4 μM.
1. Introduction
The Annona genus (Annonaceae) consists of about 119 species, most of which are shrubs and trees widely distributed in the tropical and subtropical regions, including the Southeast Asia countries such as Malaysia, Indonesia, Thailand, Cambodia, Laos, and Vietnam. In Indian folk medicine, various species of Annona have been used as vermifuges, anti-inflammatory agents, in wound healing, as antimalarial agents and in the treatment of diarrhoea and dysentery [1]. The bark of the plant Annona reticulata L. is a powerful astringent and given as tonic. The plant has been used as an anti-inflammatory agent in wound healing, anti-anxiety, anti-stress, anti-mutagenic, and spasmolytic agent. Leaf and stem extract shows inotropic, positive chronotropic and spasmolytic activities [2]. The plant is reported to contain acetogenins mainly cis- and trans-isomurisolenin [3], annoreticuin, bullatacin, squamosine, rolliniastatin [4], reticullacinone, rolliniastatin-2, molvizarin [5], 14-hydroxy-25-deoxy-rollinicin [6]. Reticulatacin and kaurane diterpenes were also identified from the bark of the plant [7]. Other terpenes such as spathenelol, muurolene, copaene and eudesmol were reported in the previous literature [8]. In our continued program aimed at the identification of anti-inflammatory drug leads from natural sources, the chemical composition of the leaves of A. reticulata was investigated to search for the bioactive constituents by assays of inhibitory effects on NO inhibition in LPS-activated mouse peritoneal macrophages. In the present study, we wished to report the characterization of nine compounds, including the structural determination of new compound 1, as well as their NO inhibitory effects.
2. Results and Discussion
2.1. Isolation and Characterization of Compounds
Air-dried and powdered leaves of A. reticulata L. were soaked with methanol at room temperature, and the combined extracts were concentrated to give a deep brown syrup. The crude extract was suspended into water and partitioned with n-hexane, ethyl acetate, and n-butanol, successively to afford n-hexane, ethyl acetate, n-butanol, and water solubles, respectively. Purification of the n-hexane soluble fraction by column chromatography yielded annonaretin A (1) (Figure 1), kaurenoic acid (2) [9], taraxerol (3) [10], β-sitosterol (4) [11], 16α-hydro-19-al-ent-kauran-17-oic acid (5) [9], 6β-hydroxystigmast-4-en-3-one (6) [12], and 17-acetoxy-16β-ent-kauran-19-oic acid 24 (7) [13]. Purification of the ethyl acetate soluble afforded 16α-hydro-ent-kauran-17,19-dioic acid (8) [9], and (2S)-di-O-methylquiritigenin (9) [14]. Compounds 2–9 (Figure 2) are known compounds, and their structures were identified by comparison of their physical and spectroscopic data with those reported in the literature. Compound 1 is new.
2.2. Structural Elucidation of New Compound 1
The purified colorless powder 1 was visualized by spraying with 1% (w/v) Ce(SO4)2 in 10% (v/v) aqueous H2SO4 followed by heating at 120 °C and displayed purplish black spots on TLC plate. It also displayed a positive response in the Lieberman–Burchard test. These results suggested that compound 1 possessed a basic triterpenoid skeleton [15]. The molecular formula of 1 was established as C33H56O3 by the pseudomolecular [M+Na]+ ion peak at m/z 523.4122 in HR-ESI-MS analysis and was further supported by its 13C-NMR spectrum which showed signals for all 33 carbons of the molecule, including one set of terminal methylenes (δC 111.9, 147.4), three oxygenated carbons (δC 62.8, 71.0, 83.2), and seven methyl carbons (δC 15.1, 18.1, 19.0, 19.3, 20.7, 21.3, 25.7). In the 1H-NMR spectrum of 1, there were five singlets at δ 0.82 (3H, s, CH3-18), 0.91 (3H, s, CH3-21), 0.97 (3H, s, CH3-22), 0.98 (3H, s, CH3-19), and 1.60 (3H, s, CH3-30); and two doublets at δ 0.80 (3H, d, J = 9.5 Hz, CH3-32) and 0.93 (3H, d, J = 6.0 Hz, CH3-33), respectively. In addition, two high field cyclopropyl proton doublets at δ 0.40 (1H, d, J = 4.5 Hz, H-20) and 0.62 (1H, d, J = 4.5 Hz, H-20) evidenced that this compound was a triterpenoid derivative with the cycloartanol skeleton. In the downfield region, four oxygenated protons at δ 3.01 (1H, d, J = 9.5 Hz, H-3), 3.54 (1H, dd, J = 11.0, 6.0 Hz, H-24), 3.64 (1H, br dd, J = 16.0, 9.0 Hz, H-2), and 3.70 (1H, br d, J = 11.0 Hz, H-24) were located at C-2, -3, and -24 determined by the 2J, 3J-HMBC correlations through H-2 to C-3 (δ 83.2), C-4 (δ 40.3); H-3 to C-1 (δ 25.7), C-2 (δ 71.0), C-4, and C-18 (δ 15.1); and H-24 to C-23 (δ 46.2), C-25 (δ 27.7), respectively. The position of terminal methylene group at δ 4.64 (1H, br s, H-29) and 4.77 (1H, br s, H-29) was also establsihed with the HMBC analysis of correlations between H-29 and C-27 (δ 55.4), C-30 (δ 19.0). In the HMBC spectral analysis (Figure 2), correlation peaks between H-32 and C-33, C-31, C-27; H-33 and C-32, C-31, C-27, supported that one isopropyl group was attached at C-27. The high field doublets at δ 0.40 and 0.62 displayed HMBC correlations with the carbons at δ 25.7 (C-1), 39.7 (C-11), and 47.1 (C-5) also confirmed the presence of cyclopropyl functionality as C-20 connected with C-9 and C-10. The full assignments of 1H- and 13C-NMR signals were substantiated by extensive 2D NMR experimental analysis. Therefore the chemical structure of 1 was established as shown in Figure 1 and the compound was given the trivial name annonaretin A.
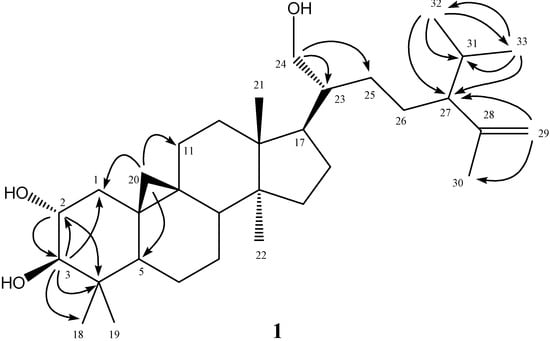
Figure 1.
Structure and significant HMBC (→) correlations of compound 1.
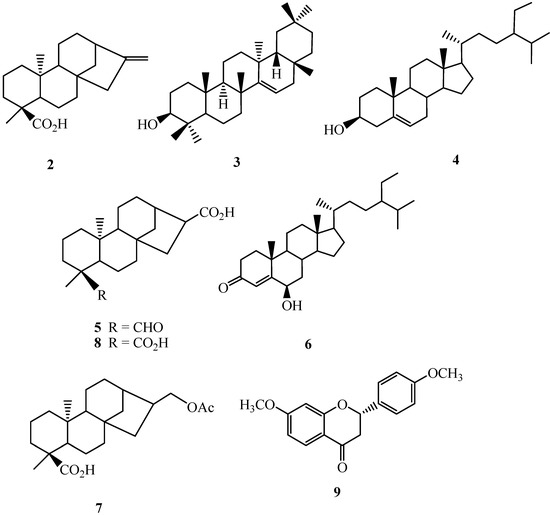
Figure 2.
Structures of purified known compounds 2–9.
2.3. The Inhibitory Effects of Isolated Compounds on NO Production
The isolated compounds 1–9 were subjected into the examination of their effects on LPS-induced iNOS-dependent NO production in RAW 264.7 cells. Cells cultured with 1–9 at different concentrations used in the presence of 100 ng/mL LPS for 24 h (Table 1). Some cell toxicity was observed in cells treated with compounds 1, 2, 4, and 9, whereas other compounds had no influence on cell viability. NO production was significantly decreased by the treatment with 2, 3, and 5–8 in a dose-dependent manner, with IC50 values in the range of 48.6 ± 1.2 and 99.8 ± 0.4 μM (Table 1). The inhibitory effects of 1, 4 and 9 were less obvious. 2, 3, and 5–8 did not exhibit significant cytotoxicity in the concentration range of 12.5–100 μM (Table 1), thus the NO inhibiting effects were probably not due to cytotoxicity. Among the tested compounds, 2, and 5–8 belonged to the ent-kaurane diterpenoids noted for the occurrence in the Annona species [16,17]. In the previous literature, kaurenoic acid (2) was reported to inhibit NO production, prostaglandin E2 release, cyclooxygenase-2, and inducible nitric oxide synthase expression in LPS-induced RAW264.7 macrophages [18]. It also exhibited anti-inflammatory [19,20], cytotoxic [21,22], antiplasmodial [21], antimicrobial [16], hypoglycaemic [23], vasorelaxant [24,25], and antispasmodic [25] bioactivities. 16α-Hydro-19-al-ent-kauran-17-oic acid (5) displayed the most significant inhibitory effect on NO production with the lowest IC50 value of 48.6 ± 1.2 μM. It was also reported from the stems of A. squamosa to exhibit anti-platelet aggregation activity [26]. In addition, taraxerol (3) could downregulate the expression of proinflammatory mediators in macrophages by interfering with the activation of TAK1 and Akt, thus preventing NF-κB activation [27]. In our experimental results, 2, 3, and 5 displayed NO inhibitory effects similar to these anti-inflammatory reports.

Table 1.
Inhibitory effects of purified samples from the leaves of A. reticulata on LPS-induced iNOS-dependent NO production in RAW 264.7 cells.
| Dose (μM) | Cell viability(% of control) | NO level | NO inhibition(% of control) | IC50(μM) | |
|---|---|---|---|---|---|
| Control | (−) | 100.0 ± 4.9 | −0.5 ± 0.1 | (−) | (−) |
| LPS | (+) | 98.7 ± 8.0 | 45.4 ± 2.7 ### | (−) | (−) |
| 1 | 12.5 25 50 100 | 88.0 ± 1.5 81.2 ± 1.8 73.0 ± 3.6 * 66.9 ± 5.5 ** | 36.6 ± 1.9 33.5 ± 0.9 (−) (−) | 19.3 ± 4.2 26.2 ± 2.0 (−) (−) | (−) |
| 2 | 12.5 25 50 100 | 98.0 ± 2.2 91.0 ± 3.6 82.9 ± 1.2 65.7 ± 8.6 ** | 34.3 ± 4.7 35.1 ± 1.2 22.7 ± 0.5 ** (−) | 24.5 ± 10.2 22.7 ± 2.6 50.0 ± 1.0 (−) | 50.0 ± 0.3 |
| 3 | 12.5 25 50 100 | 93.5 ± 1.8 92.6 ± 3.1 88.7 ± 1.8 81.4 ± 2.3 | 38.5 ± 2.3 39.5 ± 0.6 35.6 ± 0.9 * 22.6 ± 0.4 ** | 15.2 ± 5.0 13.1 ± 1.2 21.6 ± 2.0 50.1 ± 0.9 | 99.8 ± 0.4 |
| 4 | 12.5 25 50 100 | 82.8 ± 2.7 80.7 ± 3.8 65.3 ± 2.3 ** 55.8 ± 2.7 ** | 38.4 ± 0.7 38.6 ± 3.3 (−) (−) | 15.5 ± 1.5 15.1 ± 7.3 (−) (−) | (−) |
| 5 | 12.5 25 50 100 | 98.9 ± 5.2 96.5 ± 9.2 95.9 ± 8.6 80.2 ± 5.7 | 38.3 ± 1.3 33.1 ± 1.0 * 22.1 ± 1.5 ** 14.8 ± 0.7 *** | 15.5 ± 2.8 27.1 ± 2.3 51.4 ± 3.2 67.3 ± 1.5 | 48.6 ± 1.2 |
| 6 | 12.5 25 50 100 | 94.8 ± 3.1 83.7 ± 3.5 83.5 ± 3.0 81.3 ± 2.8 | 37.0 ± 1.8 33.1 ± 2.4 * 22.9 ± 1.1 ** 15.1 ± 0.3 *** | 18.5 ± 4.0 27.1 ± 5.3 49.5 ± 2.4 66.7 ± 0.6 | 51.5 ± 0.5 |
| 7 | 12.5 25 50 100 | 98.6 ± 3.1 93.6 ± 4.0 92.5 ± 2.8 83.7 ± 1.6 | 42.6 ± 0.7 40.6 ± 2.8 23.7 ± 0.2 ** 14.3 ± 0.3 *** | 6.2 ± 1.5 10.6 ± 6.1 47.7 ± 0.3 68.5 ± 0.7 | 55.5 ± 0.3 |
| 8 | 12.5 25 50 100 | 98.4 ± 3.2 94.0 ± 2.6 88.6 ± 2.6 82.4 ± 1.2 | 39.8 ± 1.2 35.4 ± 0.8 * 23.5 ± 0.9 ** 14.4 ± 0.3 *** | 12.3 ± 2.6 21.9 ± 1.8 48.2 ± 2.0 68.3 ± 0.7 | 54.5 ± 0.8 |
| 9 | 12.5 25 50 100 | 99.9 ± 4.8 90.0 ± 1.6 82.6 ± 2.7 76.2 ± 1.1 * | 42.0 ± 1.2 40.4 ± 0.7 25.9 ± 2.3 (−) | 7.5 ± 2.7 11.0 ± 1.6 42.9 ± 5.0 (−) | (−) |
The data were presented as mean ± S.D. ### Compared with sample of control group. * p < 0.05, ** p < 0.01, and *** p < 0.001 were compared with LPS-alone group. (−): Not determined.
3. Experimental
3.1. General Procedures
Melting point was determined using an Electrothermal IA-9200 melting point measuring apparatus without correction. The UV spectrum was recorded on an Agilent UV-VIS recording spectrophotometer. The IR spectra (KBr) were obtained, on a Hitachi 270-30 type spectrometer. Optical rotations were measured with a Jasco DIP-1000 KUY polarimeter. The electrospray ionization (ESI) mass spectra were determined using an Agilent 1200 LC-MSD Trap spectrometer, and the HR-ESI-MS was completed with the aid of a Bruker APEX II mass spectrometer. 1H- and 13C-NMR, COSY, NOESY, HMQC, and HMBC spectra were recorded on a Bruker Avance-500 NMR spectrometer, using tetramethylsilane (TMS) as the internal standard. Standard pulse sequences and parameters were used for the NMR experiments and all chemical shifts were reported in parts per million (ppm, δ). Column chromatography (CC) was performed on silica gel (Kieselgel 60, 70–230 mesh and 230–400 mesh, E. Merck, Darmstadt, Germany).
3.2. Plant Materials
The leaves of Annona reticulata L. (Annonaceae) were collected from Tiengiang, Vietnam, during October 2010 and the plant materials were identified and authenticated by Dr. Tran Huy Thai, Institute of Ecology and Biological Resources, Vietnamese Academy of Science and Technology. A voucher specimen (Viet-TSWu-20101015) was deposited at the Herbarium of the Vinh University.
3.3. Extraction and Isolation
The leaves of Annona reticulata L. (5.0 kg) were air-dried and powdered and soaked (24 h) with methanol (20 L × 3) at room temperature, and the combined extracts were concentrated under reduced pressure to give deep brown syrup (316.0 g). The crude extract was suspended into water (1 L) and partitioned with n-hexane (1 L × 5), ethyl acetate (1 L × 5), and n-butanol (1 L × 5), successively to afford n-hexane (31.0 g), ethyl acetate (82.0 g), n-butanol (47.0 g), and water solubles (52.0 g), respectively, after removal of the corresponding solvent.
The n-hexane soluble extracts were purified by silica gel column chromatography eluted with n-hexane and acetone gradients to afford 14 fractions. Fraction 1 was subjected to silica gel column chromatography eluted with n-hexane/acetone (25:1) to yield kaurenoic acid (2, 968 mg). Fraction 2 was isolated by silica gel column chromatography eluted with n-hexane/acetone (15:1) to afford taraxerol (3, 78 mg). Purification of fraction 3 by column chromatography with silica gel eluted by step gradients of n-hexane/acetone (15:1 and 9:1) afforded β-sitosterol (4, 302 mg). Fraction 4 was subjected to silica gel column chromatography eluted with n-hexane/acetone (15:1) to yield 16α-hydro-19-al-ent-kauran-17-oic acid (5, 26 mg). Fraction 5 was purified by silica gel column chromatography eluted with n-hexane/acetone (9:1) to yield 6β-hydroxystigmast-4-en-3-one (6, 31 mg). Isolation of fraction 6 by column chromatography with silica gel eluted by n-hexane/acetone (7:1) yielded 17-acetoxy-16β-ent-kauran-19-oic acid (7, 22 mg). Silica gel column chromatography of fraction 9 with step gradient elution of n-hexane/acetone (7:1 and 5:1) led to the purification of annonaretin A (1, 789 mg).
The ethyl acetate soluble extracts were applied to silica gel column chromatography with chloroform and methanol gradients (20:1 to 5:1) to afford eight fractions. Fraction 1 was subjected to silica gel column chromatography eluted with n-hexane/acetone (9:1) to yield 16α-hydro-ent-kauran-17,19-dioic acid (8, 38 mg). Silica gel column chromatography of fraction 6 with step gradient elution of chloroform and methanol (9:1 and 5:1) led to the isolation of (2S)-di-O-methylquiritigenin (9, 23 mg).
3.4. Spectral Data
Annonaretin A (1): colorless powder; m.p. 120−121 °C (CHCl3); 1H-NMR (CDCl3) δ 0.40 (1H, d, J = 4.5 Hz, H-20), 0.62 (1H, d, J = 4.5 Hz, H-20), 0.78 (1H, m, H-6), 0.80 (3H, d, J = 9.5 Hz, CH3-32), 0.82 (3H, s, CH3-18), 0.91 (3H, s, CH3-21), 0.93 (3H, d, J = 6.0 Hz, CH3-33), 0.97 (3H, s, CH3-22), 0.98 (3H, s, CH3-19), 1.04 (1H, m, H-7), 1.10–1.20 (3H, m, H-1, -25, -16), 1.31–1.43 (7H, m, H-1, -12, -26, -5, -17, -25), 1.52–1.69 (9H, m, H-31, -11, -8, -15, -27, -6, -30, -16), 1.60 (3H, s, CH3-30), 1.86–2.05 (3H, m, H-23, -26, -7), 3.01 (1H, d, J = 9.5 Hz, H-3), 3.40 (1H, br s, OH), 3.54 (1H, dd, J = 11.0, 6.0 Hz, H-24), 3.64 (1H, br dd, J = 16.0, 9.0 Hz, H-2), 3.70 (1H, br d, J = 11.0 Hz, H-24), 4.64 (1H, br s, H-29), 4.77 (1H, br s, H-29); 13C-NMR (CDCl3) δ 15.1 (C-18), 18.1 (C-19), 19.0 (C-30), 19.2 (C-10), 19.3 (C-21), 20.7 (C-33), 20.9 (C-6), 21.3 (C-32), 25.1 (C-9), 25.7 (C-22), 25.7 (C-1), 26.5 (C-7), 26.8 (C-16), 27.3 (C-26), 27.7 (C-25), 29.7 (C-20), 30.2 (C-31), 31.8 (C-15), 35.3 (C-12), 39.7 (C-11), 40.3 (C-4), 43.1 (C-17), 45.0 (C-13), 46.2 (C-23), 47.1 (C-5), 47.7 (C-8), 48.7 (C-14), 55.4 (C-27), 62.8 (C-24), 71.0 (C-2), 83.2 (C-3), 111.9 (C-29), 147.4 (C-28); ESI-MS m/z (rel. int.) 523 ([M+Na]+, 100); HR-ESI-MS m/z 523.4122 [M+Na]+ (calcd for C33H56O3Na, 523.4127).
3.5. Determination of Inhibitory Effects on NO Production
3.5.1. Cell Culture
A murine macrophage cell line RAW264.7 (BCRC No. 60001) was purchased from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in plastic dishes containing Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma, USA) in a CO2 incubator (5% CO2 in air) at 37°C and subcultured every 3 days at a dilution of 1:5 using 0.05% trypsin–0.02% EDTA in Ca2+-, Mg2+- free phosphate-buffered saline (DPBS).
3.5.2. Cell Viability
Cells (2 × 105) were cultured in 96-well plate containing DMEM supplemented with 10% FBS for 1 day to become nearly confluent. Then cells were cultured with samples in the presence of 100 ng/mL LPS for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μL of 0.5 mg/mL MTT for 2 h at 37 °C testing for cell viability. The medium was then discarded and 100 μL dimethyl sulfoxide (DMSO) was added. After 30-min incubation, absorbance at 570 nm was read using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3.5.3. Measurement of Nitric oxide/Nitrite
NO production was indirectly assessed by measuring the nitrite levels in the cultured media and serum determined by a colorimetric method based on the Griess reaction [28]. The cells were incubated with a test sample in the presence of LPS (100 ng/mL) at 37 °C for 24 h. Then, cells were dispensed into 96-well plates, and 100 μL of each supernatant was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthyl ethylenediamine dihydrochloride, and 5% phosphoric acid) and incubated at room temperature for 10 min, the absorbance was measured at 540 nm with a Micro-Reader (Molecular Devices). By using sodium nitrite to generate a standard curve, the concentration of nitrite was measured form absorbance at 540 nm.
3.5.4. Statistical Analysis
Experimental results were presented as the mean ± standard deviation (SD) of three parallel measurements. Statistical comparisons were made by Student’s t-test. IC50 values were estimated using a non-linear regression algorithm (SigmaPlot 8.0; SPSS Inc. Chicago, IL, USA). Statistical significance is expressed as * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Conclusion
In summary, nine compounds were characterized from the leaves of A. reticulata L. and their inhibitory activity on NO production was examined. The results provide a potential explanation for the use of the leaves of A. reticulata as a herbal medicine in the treatment of inflammatory diseases, and they may be potentially useful in developing new anti-inflammatory agents.
Acknowledgments
The authors are thankful to the National Science Council, Taiwan, for financial support of the present research. This study was supported in part by the NAFOSTED (104.01-2010.27) (Vietnam).
References
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors: Deharadun, India, 1987; pp. 68–69. [Google Scholar]
- Anonymous. The Useful Plants of India; Council of Scientific and Industrial Research: New Delhi, India, 1994; p. 43. [Google Scholar]
- Chang, F.R.; Chen, J.L.; Chiu, H.F.; Wu, M.J.; Wu, Y.C. Acetogenins from seeds of Annona reticulata. Phytochemistry 1998, 47, 1057–1061. [Google Scholar]
- Maeda, U.; Hara, N.; Fujimoto, Y.; Shrivastava, A.; Gupta, Y.K.; Sahai, M. N-fatty acyl tryptamines from Annona reticulata. Phytochemistry 1993, 34, 1633–1635. [Google Scholar] [CrossRef]
- Hisham, A.; Sunitha, C.; Sreekala, U.; Pieters, L.; De Bruyne, T.; Van den Heuvel, H.; Claeys, M. Reticulacinone, an acetogenin from Annona reticulata. Phytochemistry 1994, 35, 1325–1329. [Google Scholar] [CrossRef]
- Etse, J.T.; Waterman, P.G. Chemistry in the Annonaceae, XXII. 14-Hydroxy-25-desoxyrollinicin from the stem bark of Annona reticulata. J. Nat. Prod. 1986, 49, 684–686. [Google Scholar] [CrossRef]
- Saad, J.M.; Hui, Y.H.; Rupprecht, J.K.; Anderson, J.E.; Kozlowski, J.F.; Zhao, G.X.; Wood, K.V.; McLaughlin, J.L. Reticulatacin: A new bioactive acetogenin from Annona reticulata (Annonaceae). Tetrahedron 1991, 47, 2751–2756. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Saidutty, A. Analysis of the essential oils of the leaves and roots of Annona reticulata from South-India. Ernaehrung 1998, 22, 9–10. [Google Scholar]
- Hsieh, T.J.; Wu, Y.C.; Chen, S.C.; Huang, C.S.; Chen, C.Y. Chemical constituents from Annona glabra. J. Chin. Chem. Soc. 2004, 51, 869–876. [Google Scholar]
- Kanlayavattanakul, M.; Ruangrungsi, N.; Watanabe, T.; Kawahata, M.; Therrien, B.; Yamaguchi, K.; Ishikawa, T. ent-Halimane diterpenes and a guaiane sesquiterpene from Cladogynos orientalis. J. Nat. Prod. 2005, 68, 7–10. [Google Scholar] [CrossRef]
- Nes, W.D.; Norton, R.A.; Benson, M. Carbon-13-NMR studies on sitosterol biosynthesized from [13C]mevalonates. Phytochemistry 1992, 31, 805–811. [Google Scholar]
- Kuo, Y.H.; Chu, P.H. Studies on the constituents from the bark of Bauhinia purpurea. J. Chin. Chem. Soc. 2002, 49, 269–274. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Wu, Y.C. The constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1997, 44, 313–320. [Google Scholar]
- Yoshida, T.; Feng, W.S.; Okuda, T. Two polyphenol glycosides and tannins from Rosa cymosa. Phytochemistry 1993, 32, 1033–1036. [Google Scholar] [CrossRef]
- Srivastava, A.; Shukla, Y.N. Aryl esters and a coumarin from Aygyreia speciosa. Indian J. Chem. Sect. B 1998, 37B, 192–194. [Google Scholar]
- Okoye, T.C.; Akah, P.A.; Okoli, C.O.; Ezike, A.C.; Omeje, E.O.; Odoh, U.E. Antimicrobial effects of a lipophilic fraction and kaurenoic acid isolated from the root bark extracts of Annona senegalensis. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Guillopé, R.; Escobar-Khondiker, M.; Guérineau, V.; Laprévote, O.; Höglinger, G.U.; Champy, P. Kaurenoic acid from pulp of Annona cherimolia in regard to Annonaceae-induced Parkinsonism. Phytother. Res. 2011, 25, 1861–1864. [Google Scholar] [CrossRef]
- Choi, R.J.; Shin, E.M.; Jung, H.A.; Choi, J.S.; Kim, Y.S. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine 2011, 18, 677–682. [Google Scholar] [CrossRef]
- Mizokami, S.S.; Arakawa, N.S.; Ambrosio, S.R.; Zarpelon, A.C.; Casagrande, R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Kaurenoic acid from Sphagneticola trilobata inhibits inflammatory pain: Effect on cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. J. Nat. Prod. 2012, 75, 896–904. [Google Scholar] [CrossRef]
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of Aralia continentalis. Arch. Pharm. Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef]
- Batista, R.; García, P.A.; Castro, M.A.; Miguel Del Corral, J.M.; Speziali, N.L.; de P Varotti, F.; de Paula, R.C.; García-Fernández, L.F.; Francesch, A.; San Feliciano, A.; et al. Synthesis, cytotoxicity and antiplasmodial activity of novel ent-kaurane derivatives. Eur. J. Med. Chem. 2013, 62C, 168–176. [Google Scholar]
- De Andrade, B.B.; Moreira, M.R.; Ambrosio, S.R.; Furtado, N.A.; Cunha, W.R.; Heleno, V.C.; Silva, A.N.; Simão, M.R.; Da Rocha, E.M.; Martins, C.H.; et al. Evaluation of ent-kaurenoic acid derivatives for their anticariogenic activity. Nat. Prod. Commun. 2011, 6, 777–780. [Google Scholar]
- Raga, D.D.; Alimboyoguen, A.B.; Del Fierro, R.S.; Ragasa, C.Y. Hypoglycaemic effects of tea extracts and ent-kaurenoic acid from Smallanthus sonchifolius. Nat. Prod. Res. 2010, 24, 1771–1782. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; da Costa, F.B.; Coutinho, S.T.; de Oliveira, D.C.; de Oliveira, A.M. Analysis of the mechanisms underlying the vasorelaxant action of kaurenoic acid in the isolated rat aorta. Eur. J. Pharmacol. 2004, 492, 233–241. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Tirapelli, C.R.; Coutinho, S.T.; de Oliveira, D.C.; de Oliveira, A.M.; da Costa, F.B. Role of the carboxylic group in the antispasmodic and vasorelaxant action displayed by kaurenoic acid. J. Pharm. Pharmacol. 2004, 56, 1407–1413. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chang, F.R.; Wu, C.C.; Wang, W.Y.; Wu, Y.C. New ent-kaurane diterpenoids with anti-platelet aggregation activity from Annona squamosa. J. Nat. Prod. 2002, 65, 1462–1467. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. Int.Immunopharmacol. 2013, 15, 316–324. [Google Scholar] [CrossRef]
- Chang, C.T.; Huang, S.S.; Lin, S.S.; Amagaya, S.; Ho, H.Y.; Hou, W.C.; Shie, P.H.; Wu, J.B.; Huang, G.J. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya japonica ex vivo and in vivo. Food Chem. 2011, 127, 1131–1137. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–9 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).