Steroidal Triterpenes of Cholesterol Synthesis
Abstract
:1. Introduction
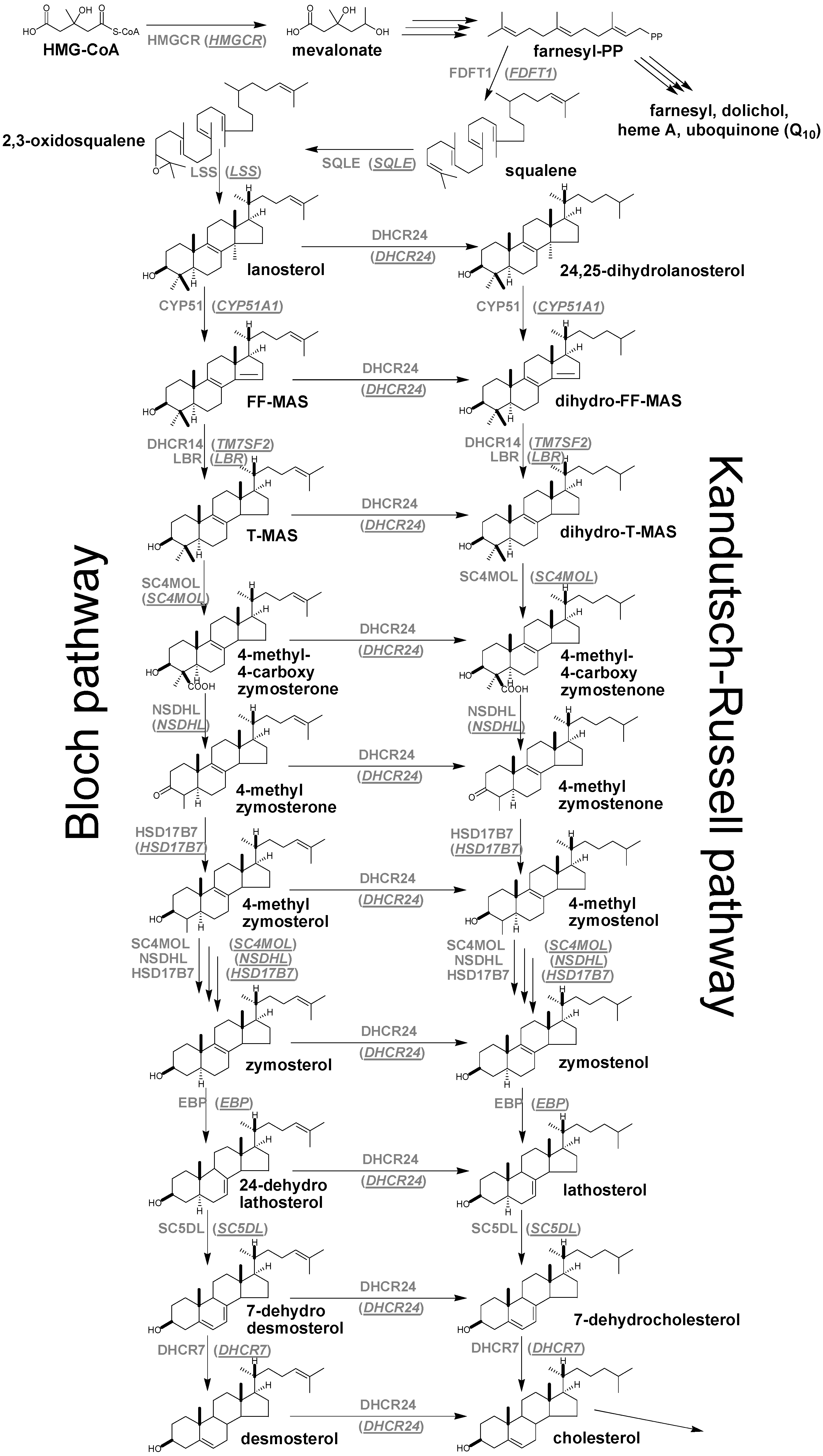
| Enzyme/Gene symbol | Enzyme/Gene name | Enzyme Commission number |
|---|---|---|
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase | EC:1.1.1.34 |
| FDFT1 | farnesyl-diphosphate farnesyl transferase 1 | EC:2.5.1.21 |
| SQLE | squalene epoxidase/monooxygenase | EC:1.14.13.132 |
| LSS | lanosterol synthase | EC:5.4.99.7 |
| CYP51A1 | lanosterol-14α-demethylase | EC:1.14.13.70 |
| DHCR14 (TM7SF2) | sterol-Δ14-reductase | EC:1.3.1.70 |
| LBR | lamin B receptor | 3930 a |
| SC4MOL | sterol-C4-methyl oxidase | EC:1.14.13.72 |
| NSDHL | 3β-hydroxy-Δ5-steroid-dehydrogenase | EC:1.1.1.170 |
| HSD17B7 | 3β-keto-reductase | EC:1.1.1.270 |
| EBP | sterol-Δ8-7-isomerase | EC:5.3.3.5 |
| SC5DL | sterol-C5-desaturase/lathosterol oxidase | EC:1.14.21.6 |
| DHCR7 | sterol-Δ7-reductase | EC:1.3.1.21 |
| DHCR24 | sterol-Δ24-reductase | EC:1.3.1.72 |
2. Nomenclature and Numbering
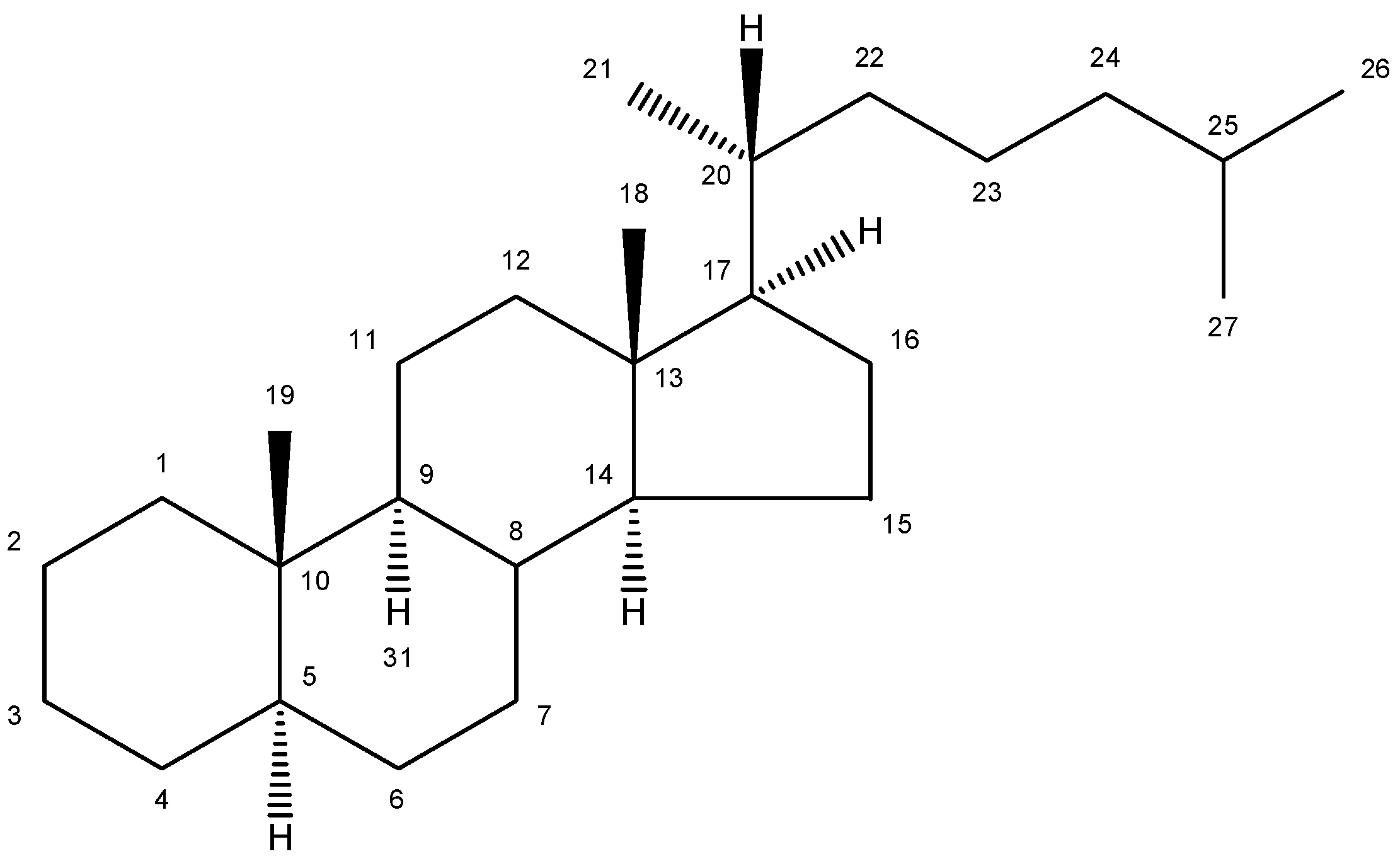
| Trivial name | Chemical name | Structure | Formula | Mw |
|---|---|---|---|---|
| Lanosterol | 4,4,14α-trimethyl-5α-cholesta-8(9),24-dien-3β-ol | 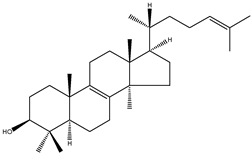 | C30H50O | 426.72 |
| 24,25-dihydrolanosterol | 4,4,14α-trimethyl-5α-cholest-8(9)en-3β-ol |  | C30H52O | 428.73 |
| FF-MAS | 4,4-dimethyl-5α-cholesta-8(9),14,24-trien-3β-ol | 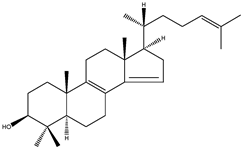 | C29H46O | 410.67 |
| Dihydro-FF-MAS | 4,4-dimethyl-5α-cholesta-8(9),14-dien-3β-ol | 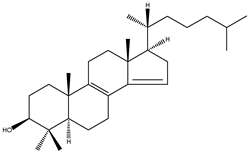 | C29H48O | 412.69 |
| T-MAS | 4,4-dimethy-5α-cholesta-8(9),24-dien-3β-ol | 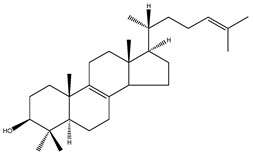 | C29H48O | 412.69 |
| Dihydro-T-MAS | 4,4-dimethy-5α-cholest-8(9)-en-3β-ol |  | C29H50O | 414.69 |
| Zymosterol | 5α-cholesta-8(9),24-dien-3β-ol | 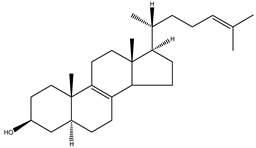 | C27H44O | 384.64 |
| Zymostenol | 5α-cholest-8(9)-en-3β-ol | 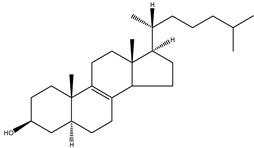 | C27H46O | 386.65 |
| 24-dehydrolathosterol | 5α-cholesta-7,24-dien-3β-ol | 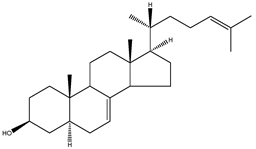 | C27H44O | 384.64 |
| Lathosterol | 5α-cholest-7-en-3β-ol | 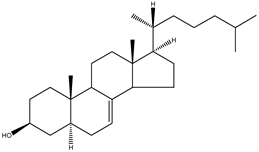 | C27H46O | 386.66 |
| 7-dehydrodesmosterol | 5α-cholesta-5,7,24-trien-3β-ol | 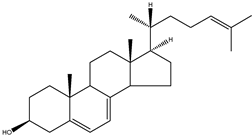 | C27H46O | 386.66 |
| 7-dehydrocholesterol | 5α-cholesta-5,7-dien-3β-ol | 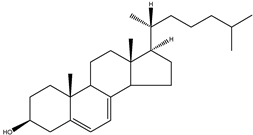 | C27H44O | 384.64 |
| Desmosterol | 5α-cholesta-5,24-dien-3β-ol | 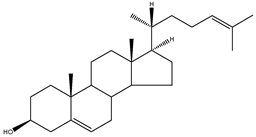 | C27H44O | 384.64 |
| Cholesterol | 5α-cholest-5-en-3β-ol | 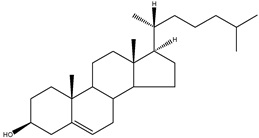 | C27H46O | 386.66 |
3. Commercial and Laboratory Standards Together with Some Chemical Syntheses
| Sterol | Providers | ||
|---|---|---|---|
| Unlabelled | Deuterium labelled | Tritium labelled | |
| Lanosterol | APL a, SA b, S c, ARC f | APL | ARC |
| 24,25-dihydrolanosterol | APL, S | APL | ARC |
| FF-MAS | APL | ||
| T-MAS | APL | APL | |
| Zymosterol | APL | APL, MI d | |
| Zymostenol | APL | ||
| Lathosterol | APL, SA, S | APL, MI | |
| 7-dehydrodesmosterol | MI | MI | |
| 7-dehydrocholesterol | APL, SA, S, MI | APL, CIL e | |
| Desmosterol | APL, SA, S, MI | APL, MI | ARC |
4. Enzymes of Post-Squalene Cholesterol Synthesis Pathway and Their Biological Significance
| Enzyme | Substrate sterol | Vmax [nmol/min per mg of protein] | Km [µM] | kcat [Vmax/Km] | Relative kcat (fold) | Reference |
|---|---|---|---|---|---|---|
| CYP51A1 | Lanosterol | 262.3 | 10.5 | 24.1 | [25] | |
| 24,25-dihydrolanosterol | 377.4 | 20.0 | 18.87 | |||
| DHCR14 | 4,4-dimethy-5α-cholesta-7,24-dien-3β-ol | 1.13 | 29.4 | [26] | ||
| EBP | Zymosterol | 0.325 | 25 | [27] | ||
| SC5DL | Lathosterol | 0.017 | 15.4a | [28] | ||
| DHCR7 | 7-dehydrocholesterol | 0.85 | 30 | [22] | ||
| DHCR24 | Lanosterol | 0.361 | 109 | 0.0033 | 1.0 | [7] |
| Zymosterol | 3.550 | 176 | 0.0201 | 6.1 | ||
| 7-dehydrodesmosterol | 2.176 | 37 | 0.0586 | 17.8 | ||
| Desmosterol | 2.945 | 163 | 0.0180 | 5.5 |
5. Biological Role and Toxicity
| Organism | Sterol | Blood plasma [µg/mL] | Liver [µg/g] | Brain [µg/g] | Testis [µg/g] |
|---|---|---|---|---|---|
| Mus musculus a | Lanosterol | 0.08–0.86 | 0.92–7.12 | 3.2–4.7 | 4.3–17.8 |
| 24,25-dihydrolanosterol | 0.08–1.17 | 0.10–0.13 | |||
| FF-MAS | 1.10–2.15 | ||||
| T-MAS | 0.61–5.65 | 4.70–8.50 | 31–110 | ||
| Zymosterol | |||||
| Lathosterol | 25.3–30.8 | 7.6–11.6 | |||
| 7-dehydrocholesterol | 0.22–2.39 | 0.94–9.31 | 3.31–7.70 | 2.6–4.8 | |
| Desmosterol | 0.43–2.27 | 0.78–4.14 | 29.4–97.8 | 39–91 | |
| Cholesterol | 405–1,997 | 1,965–6,700 | 12,500–15,500 | 1,337–2,829 | |
| Rattus | Lanosterol | 0.75–1.38 b | ~4 c | ||
| norvegicus | 24,25-dihydrolanosterol | ||||
| FF-MAS | |||||
| T-MAS | ~2 c | ~49 c | |||
| Zymosterol | |||||
| Lathosterol | 15.5–10.4 b | ||||
| 7-dehydrocholesterol | 2.63–3.31 b | ||||
| Desmosterol | 4.55–5.39 b | ~2 c | |||
| Cholesterol | 589–856 b | 2,041–2,618 b | ~1544 c |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Analysis of neurosterols by GC-MS and LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2778–2805. [Google Scholar] [CrossRef]
- Porter, F.D. Malformation syndromes due to inborn errors of cholesterol synthesis. J. Clin. Invest. 2002, 110, 715–724. [Google Scholar]
- Horvat, S.; McWhir, J.; Rozman, D. Defects in cholesterol synthesis genes in mouse and in humans: lessons for drug development and safer treatments. Drug Metab. Rev. 2011, 43, 69–90. [Google Scholar] [CrossRef]
- Kandutsch, A.A.; Russell, A.E. Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol. J. Biol. Chem. 1960, 235, 2256–2261. [Google Scholar]
- Marijanovic, Z.; Laubner, D.; Moller, G.; Gege, C.; Husen, B.; Adamski, J.; Breitling, R. Closing the gap: identification of human 3-ketosteroid reductase, the last unknown enzyme of mammalian cholesterol biosynthesis. Mol. Endocrinol. 2003, 17, 1715–1725. [Google Scholar] [CrossRef]
- Bae, S.H.; Paik, Y.K. Cholesterol biosynthesis from lanosterol: development of a novel assay method and characterization of rat liver microsomal lanosterol delta 24-reductase. Biochem. J. 1997, 326, 609–616. [Google Scholar]
- Liu, J.; Nes, W.D. Steroidal triterpenes: design of substrate-based inhibitors of ergosterol and sitosterol synthesis. Molecules 2009, 14, 4690–4706. [Google Scholar] [CrossRef]
- Goad, L.J.; Akishisa, T. Analysis of Sterols; Blackie Academic & Professional: London, UK, 1997; Chapter 1; p. 20. [Google Scholar]
- Bloch, K.; Gautschi, F.; Johnston, J.D. Isolation of lanosterol from isocholesterol. J. Biol. Chem. 1957, 224, 185–190. [Google Scholar]
- Kandutsch, A.A.; Russell, A.E. Preputial gland tumor sterols. I. The occurrence of 24,25-dihydrolanosterol and a comparison with liver and the normal gland. J. Biol. Chem. 1959, 234, 2037–2042. [Google Scholar]
- Byskov, A.G.; Andersen, C.Y.; Nordholm, L.; Thogersen, H.; Xia, G.; Wassmann, O.; Andersen, J.V.; Guddal, E.; Roed, T. Chemical structure of sterols that activate oocyte meiosis. Nature 1995, 374, 559–562. [Google Scholar] [CrossRef]
- Ruan, B.; Watanabe, S.; Eppig, J.J.; Kwoh, C.; Dzidic, N.; Pang, J.; Wilson, W.K.; Schroepfer, G.J., Jr. Sterols affecting meiosis: novel chemical syntheses and the biological activity and spectral properties of the synthetic sterols. J. Lipid Res. 1998, 39, 2005–2020. [Google Scholar]
- Murray, A.; Gronvald, F.C.; Nielsen, J.K.; Faarup, P. Synthesis of FF-MAS from lithocholic acid. Bioorg. Med. Chem. Lett. 2000, 10, 1067–1068. [Google Scholar] [CrossRef]
- Ruan, B.; Wilson, W.K.; Schroepfer, G.J., Jr. An alternative synthesis of 4,4-dimethyl-5 alpha-cholesta-8,14,24-trien-3 beta-ol, an intermediate in sterol biosynthesis and a reported activator of meiosis and of nuclear orphan receptor LXR alph. Bioorg. Med. Chem. Lett. 1998, 8, 233–236. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Parish, E.J.; Tsuda, M.; Kandutsch, A.A. Inhibitors of sterol synthesis. Chemical syntheses, properties and effects of 4,4-dimethyl-15-oxygenated sterols on sterol synthesis and on 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured mammalian cells. Chem. Phys. Lipids 1998, 47, 187–207. [Google Scholar]
- Thoma, R.; Schulz-Gasch, T.; D’Arcy, B.; Benz, J.; Aebi, J.; Dehmlow, H.; Hennig, M.; Stihle, M.; Ruf, A. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 2004, 432, 118–122. [Google Scholar] [CrossRef]
- Wendt, K.U.; Schulz, G.E.; Corey, E.J.; Liu, D.R. Enzyme Mechanisms for Polycyclic Triterpene Formation. Angew. Chem. Int. Ed. Engl. 2000, 39, 2812–2833. [Google Scholar] [CrossRef]
- Debeljak, N.; Fink, M.; Rozman, D. Many facets of mammalian lanosterol 14alpha-demethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch. Biochem. Biophys. 2003, 409, 159–171. [Google Scholar] [CrossRef]
- Bennati, A.M.; Schiavoni, G.; Franken, S.; Piobbico, D.; Della Fazia, M.A.; Caruso, D.; de Fabiani, E.; Benedetti, L.; Cusella De Angelis, M.G.; Gieselmann, V.; et al. Disruption of the gene encoding 3beta-hydroxysterol Delta-reductase (Tm7sf2) in mice does not impair cholesterol biosynthesis. FEBS J. 2008, 275, 5034–5047. [Google Scholar] [CrossRef]
- Wassif, C.A.; Brownson, K.E.; Sterner, A.L.; Forlino, A.; Zerfas, P.M.; Wilson, W.K.; Starost, M.F.; Porter, F.D. HEM dysplasia and ichthyosis are likely laminopathies and not due to 3beta-hydroxysterol Delta14-reductase deficiency. Hum. Mol. Genet. 2007, 16, 1176–1187. [Google Scholar] [CrossRef]
- Moebius, F.F.; Fitzky, B.U.; Lee, J.N.; Paik, Y.K.; Glossmann, H. Molecular cloning and expression of the human delta7-sterol reductase. Proc. Natl. Acad. Sci. USA 1998, 95, 1899–1902. [Google Scholar]
- Bae, S.H.; Lee, J.N.; Fitzky, B.U.; Seong, J.; Paik, Y.K. Cholesterol biosynthesis from lanosterol. Molecular cloning, tissue distribution, expression, chromosomal localization, and regulation of rat 7-dehydrocholesterol reductase, a Smith-Lemli-Opitz syndrome-related protein. J. Biol. Chem. 1999, 274, 14624–14631. [Google Scholar] [CrossRef]
- Rozman, D.; Monostory, K. Perspectives of the non-statin hypolipidemic agents. Pharmacol. Ther. 2010, 127, 19–40. [Google Scholar]
- Nitahara, Y.; Aoyama, Y.; Horiuchi, T.; Noshiro, M.; Yoshida, Y. Purification and characterization of rat sterol 14-demethylase P450 (CYP51) expressed in Escherichia coli. J. Biochem. 1999, 126, 927–933. [Google Scholar] [CrossRef]
- Paik, Y.K.; Trzaskos, J.M.; Shafiee, A.; Gaylor, J.L. Microsomal enzymes of cholesterol biosynthesis from lanosterol. Characterization, solubilization, and partial purification of NADPH-dependent delta 8,14-steroid 14-reductase. J. Biol. Chem. 1984, 259, 13413–13423. [Google Scholar]
- Moebius, F.F.; Reiter, R.J.; Bermoser, K.; Glossmann, H.; Cho, S.Y.; Paik, Y.K. Pharmacological analysis of sterol delta8-delta7 isomerase proteins with [3H]ifenprodil. Mol. Pharmacol. 1998, 54, 591–598. [Google Scholar]
- Takakuwa, Y.; Nishino, H.; Ishibe, Y.; Ishibashi, T. Properties and kinetics of membrane-bound enzymes when both the enzyme and substrate are components of the same microsomal membrane. Studies on lathosterol 5-desaturase. J. Biol. Chem. 1994, 269, 27889–27893. [Google Scholar]
- Kelley, R.I.; Hennekam, R.C. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 2000, 37, 321–335. [Google Scholar] [CrossRef]
- Waterham, H.R.; Koster, J.; Romeijn, G.J.; Hennekam, R.C.; Vreken, P.; Andersson, H.C.; FitzPatrick, D.R.; Kelley, R.I.; Wanders, R.J. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001, 69, 685–694. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Corso, G.; Rossi, M.; Ferrari, P.; Balli, F.; Rivasi, F.; Annunziata, I.; Ballabio, A.; Russo, A.D.; Andria, G.; et al. Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of 3beta-hydroxysteroid-delta5-desaturase. Am. J. Hum. Genet. 2002, 71, 952–958. [Google Scholar] [CrossRef]
- Krakowiak, P.A.; Wassif, C.A.; Kratz, L.; Cozma, D.; Kovarova, M.; Harris, G.; Grinberg, A.; Yang, Y.; Hunter, A.G.; Tsokos, M.; et al. Lathosterolosis: An inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum. Mol. Genet. 2003, 12, 1631–1641. [Google Scholar] [CrossRef]
- Waterham, H.R.; Koster, J.; Mooyer, P.; Noort Gv, G.; Kelley, R.I.; Wilcox, W.R.; Wanders, R.J.; Hennekam, R.C.; Oosterwijk, J.C. Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene. Am. J. Hum. Genet. 2003, 72, 1013–1017. [Google Scholar] [CrossRef]
- Konig, A.; Happle, R.; Bornholdt, D.; Engel, H.; Grzeschik, K.H. Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrom. Am. J. Med. Genet. 2000, 90, 339–346. [Google Scholar] [CrossRef]
- Braverman, N.; Lin, P.; Moebius, F.F.; Obie, C.; Moser, A.; Glossmann, H.; Wilcox, W.R.; Rimoin, D.L.; Smith, M.; Kratz, L.; et al. Mutations in the gene encoding 3 beta-hydroxysteroid-delta 8, delta 7-isomerase cause X-linked dominant Conradi-Hunermann syndrome. Nat. Genet. 1999, 22, 291–294. [Google Scholar] [CrossRef]
- Bloch, K.E. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 1983, 14, 47–92. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed; John Wiley & Sons: Hoboken, NJ, USA, 2011; Chapter 25; pp. 951–954. [Google Scholar]
- Tulenko, T.N.; Boeze-Battaglia, K.; Mason, R.P.; Tint, G.S.; Steiner, R.D.; Connor, W.E.; Labelle, E.F. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 2006, 47, 134–143. [Google Scholar]
- Song, B.L.; Javitt, N.B.; DeBose-Boyd, R.A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 2005, 1, 179–189. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef]
- Fitzky, B.U.; Moebius, F.F.; Asaoka, H.; Waage-Baudet, H.; Xu, L.; Xu, G.; Maeda, N.; Kluckman, K.; Hiller, S.; Yu, H.; et al. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J. Clin. Invest. 2001, 108, 905–915. [Google Scholar]
- Byskov, A.G.; Andersen, C.Y.; Leonardsen, L.; Baltsen, M. Meiosis activating sterols (MAS) and fertility in mammals and man. J. Exp. Zool. 1999, 285, 237–242. [Google Scholar] [CrossRef]
- Vaknin, K.M.; Lazar, S.; Popliker, M.; Tsafriri, A. Role of meiosis-activating sterols in rat oocyte maturation: effects of specific inhibitors and changes in the expression of lanosterol 14alpha-demethylase during the preovulatory period. Biol. Reprod. 2001, 64, 299–309. [Google Scholar] [CrossRef]
- Grondahl, C. Oocyte maturation. Basic and clinical aspects of in vitro maturation (IVM) with special emphasis of the role of FF-MAS. Dan. Med. Bull. 2008, 55, 1–16. [Google Scholar]
- Keber, R.; Rozman, D.; Horvat, S. Sterols in spermatogenesis and sperm maturation. J. Lipid Res. 2013, 54, 20–33. [Google Scholar] [CrossRef]
- Connor, W.E.; Lin, D.S.; Neuringer, M. Biochemical markers for puberty in the monkey testis: desmosterol and docosahexaenoic acid. J. Clin. Endocrinol. Metab. 1997, 82, 1911–1916. [Google Scholar] [CrossRef]
- Acimovic, J.; Kosir, R.; Kastelec, D.; Perse, M.; Majdic, G.; Rozman, D.; Kosmelj, K.; Golicnik, M. Circadian rhythm of cholesterol synthesis in mouse liver: A statistical analysis of the post-squalene metabolites in wild-type and Crem-knock-out mice. Biochem. Biophys. Res. Commun. 2011, 408, 635–641. [Google Scholar] [CrossRef]
- Ali, Z.; Heverin, M.; Olin, M.; Acimovic, J.; Lovgren-Sandblom, A.; Shafaati, M.; Bavner, A.; Meiner, V.; Leitersdorf, E.; Bjorkhem, I. On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and cyp27a1−/− mice. J. Lipid Res. 2013, 54, 1033–1043. [Google Scholar] [CrossRef]
- Bjorkhem-Bergman, L.; Acimovic, J.; Torndal, U.B.; Parini, P.; Eriksson, L.C. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res. 2010, 30, 1105–1112. [Google Scholar]
- Tacer, K.F.; Haugen, T.B.; Baltsen, M.; Debeljak, N.; Rozman, D. Tissue-specific transcriptional regulation of the cholesterol biosynthetic pathway leads to accumulation of testis meiosis-activating sterol (T-MAS). J. Lipid Res. 2002, 43, 82–89. [Google Scholar]
- Xu, F.; Rychnovsky, S.D.; Belani, J.D.; Hobbs, H.H.; Cohen, J.C.; Rawson, R.B. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14551–14556. [Google Scholar]
- Rodriguez-Acebes, S.; de la Cueva, P.; Fernandez-Hernando, C.; Ferruelo, A.J.; Lasuncion, M.A.; Rawson, R.B.; Martinez-Botas, J.; Gomez-Coronado, D. Desmosterol can replace cholesterol in sustaining cell proliferation and regulating the SREBP pathway in a sterol-Delta24-reductase-deficient cell line. Biochem. J. 2009, 420, 305–315. [Google Scholar] [CrossRef]
- Flaxman, N. Mer-29 (Triparanol) and Cataract. Med. Trial Tech. Q. 1963, 10, 11–32. [Google Scholar]
- Kirby, T.J. Cataracts produced by triparanol. (MER-29). Trans. Am. Ophthalmol. Soc. 1967, 65, 494–543. [Google Scholar]
- Perdriel, G. Cataract and Triparanol. Bull. Soc. Ophtalmol. Fr. 1964, 64, 699–701. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ačimovič, J.; Rozman, D. Steroidal Triterpenes of Cholesterol Synthesis. Molecules 2013, 18, 4002-4017. https://doi.org/10.3390/molecules18044002
Ačimovič J, Rozman D. Steroidal Triterpenes of Cholesterol Synthesis. Molecules. 2013; 18(4):4002-4017. https://doi.org/10.3390/molecules18044002
Chicago/Turabian StyleAčimovič, Jure, and Damjana Rozman. 2013. "Steroidal Triterpenes of Cholesterol Synthesis" Molecules 18, no. 4: 4002-4017. https://doi.org/10.3390/molecules18044002
APA StyleAčimovič, J., & Rozman, D. (2013). Steroidal Triterpenes of Cholesterol Synthesis. Molecules, 18(4), 4002-4017. https://doi.org/10.3390/molecules18044002




