Discovery of Hybrid Dual N-Acylhydrazone and Diaryl Urea Derivatives as Potent Antitumor Agents: Design, Synthesis and Cytotoxicity Evaluation
Abstract
:1. Introduction
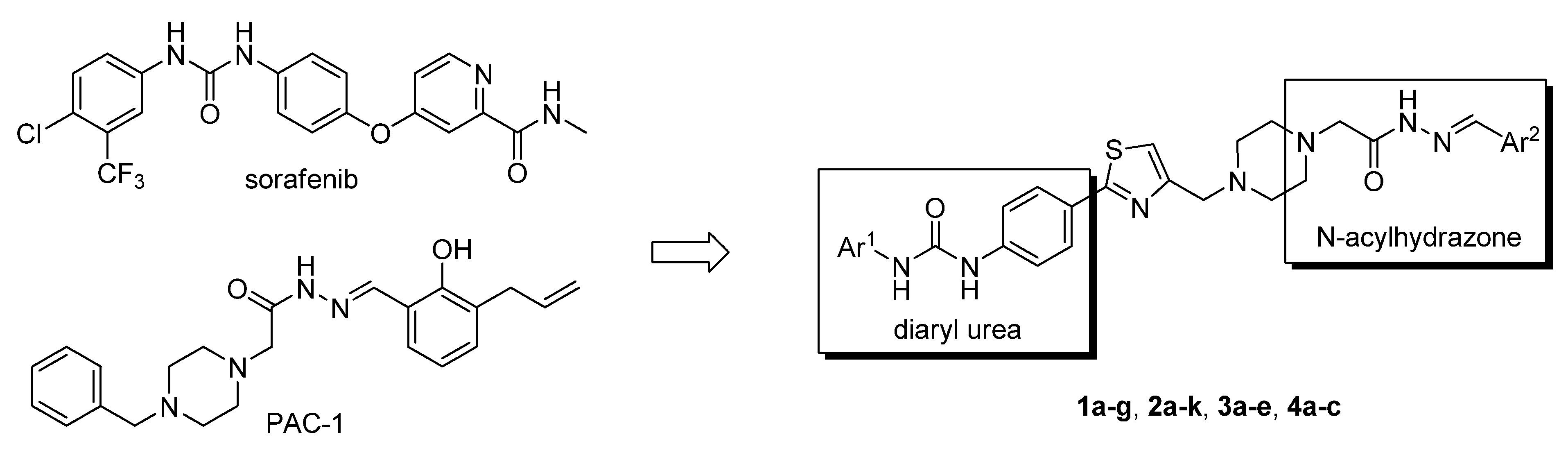
2. Results and Discussion
2.1. Chemistry
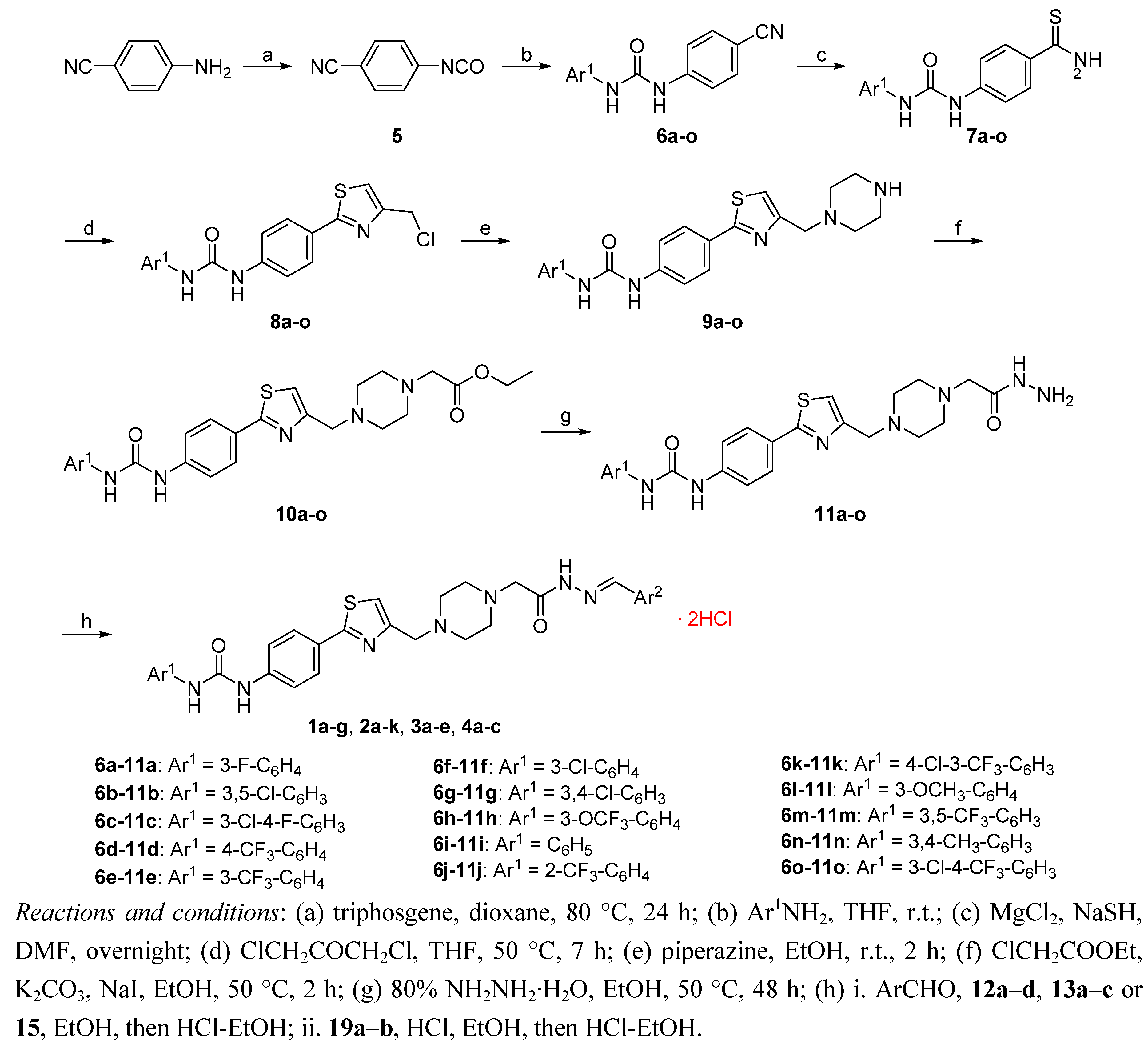
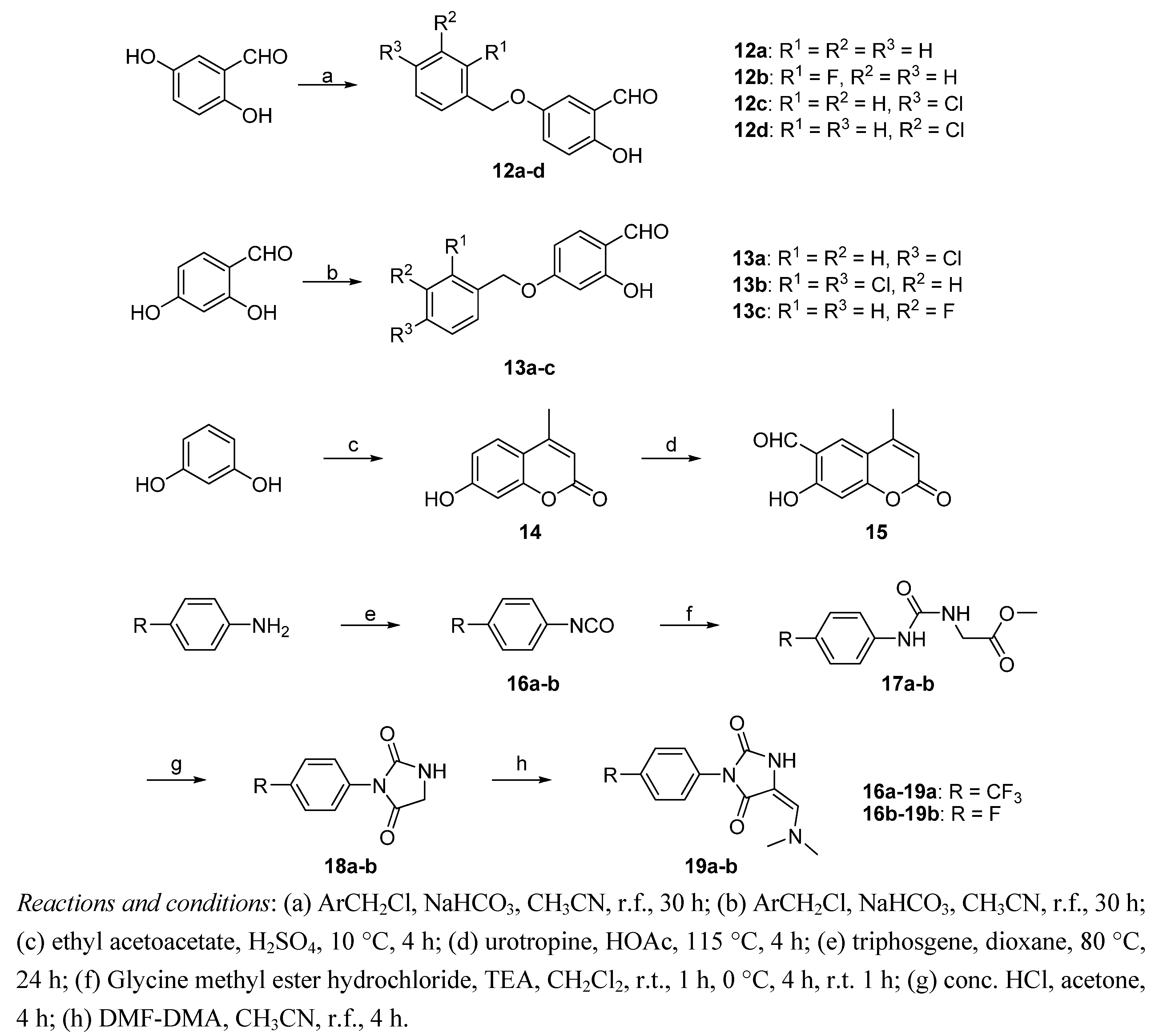
2.2. Biological Results and Discussion
| Compd. | Ar1 | Ar2 | IC50 (μmol/L) | ||
|---|---|---|---|---|---|
| HL-60 | A549 | MDA-MB-231 | |||
| 1a | 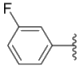 | 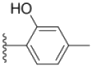 | ND | 0.64 ± 0.12 | 1.9 ± 0.16 |
| 1b |  |  | 0.56 ± 0.04 | 0.78 ± 0.02 | 0.48 ± 0.02 |
| 1c | 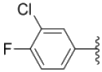 | 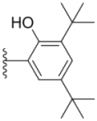 | 13.0 ± 0.37 | 0.48 ± 0.06 | 0.26 ± 0.01 |
| 1d | 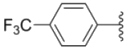 |  | 8.8 ± 0.31 | 5.1 ± 0.25 | 8.5 ± 0.44 |
| 1e |  | 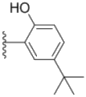 | 0.82 ± 0.08 | 1.6 ± 0.41 | 0.92 ± 0.24 |
| 1f |  |  | 0.63 ± 0.17 | 1.3 ± 0.16 | 0.82 ± 0.05 |
| 1g | 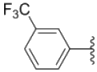 | 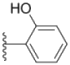 | 6.0 ± 0.09 | 0.50 ± 0.04 | 0.58 ± 0.03 |
| 2a | 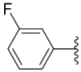 |  | 0.55 ± 0.09 | 1.6 ± 0.14 | 0.73 ± 0.06 |
| 2b | 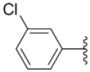 |  | 0.51 ± 0.01 | 1.2 ± 0.05 | 0.73 ± 0.02 |
| 2c |  | 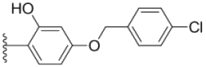 | 2.6 ± 0.11 | 0.59 ± 0.02 | 0.71 ± 0.01 |
| 2d |  |  | 3.8 ± 0.13 | 1.7 ± 0.12 | 0.53 ± 0.02 |
| 2e |  |  | 2.3 ± 0.11 | 0.49 ± 0.05 | 0.35 ± 0.02 |
| 2f | 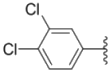 | 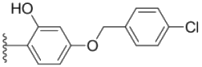 | 4.7 ± 0.19 | 2.8 ± 0.21 | 0.48 ± 0.05 |
| 2g |  | 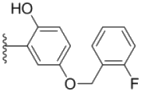 | 0.22 ± 0.01 | 0.34 ± 0.01 | 0.41 ± 0.3 |
| 2h | 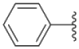 | 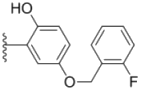 | 0.50 ± 0.004 | 1.8 ± 0.04 | 0.90 ± 0.006 |
| 2i |  | 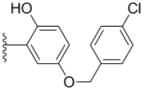 | 0.38 ± 0.01 | 0.54 ± 0.06 | 0.44 ± 0.04 |
| 2j | 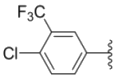 |  | 0.31 ± 0.14 | 0.96 ± 0.20 | 2.0 ± 0.12 |
| 2k | 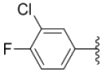 | 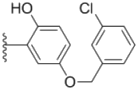 | 2.0 ± 0.11 | 2.3 ± 0.08 | 0.22 ± 0.04 |
| 3a | 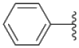 | 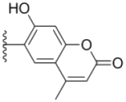 | 15.2 ± 0.22 | 17.0 ± 0.52 | 5.6 ± 0.36 |
| 3b |  | 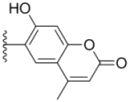 | 3.6 ± 0.12 | >50 | 3.8 ± 0.28 |
| 3c | 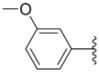 | 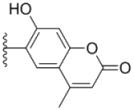 | 3.3 ± 0.25 | 6.4 ± 0.42 | 3.6 ± 0.28 |
| 3d |  |  | 4.0 ± 0.33 | 1.7 ± 0.15 | 1.8 ± 0.07 |
| 3e | 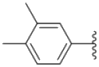 |  | 4.5 ± 0.13 | 19.0 ± 0.57 | 8.9 ± 0.41 |
| 4a |  | 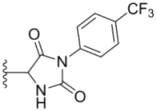 | 12.0 ± 0.32 | 37.2 ± 0.46 | 7.0 ± 0.18 |
| 4b |  |  | 25.5 ± 0.29 | 3.4 ± 0.10 | 13.3 ± 0.32 |
| 4c |  | 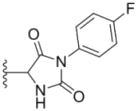 | >50 | 7.8 ± 0.20 | 13.1 ± 0.37 |
| Sorafenib | ND | 1.3 ± 0.06 | 2.7 ± 0.11 | ||
| PAC-1 | 4.5 ± 0.03 | 2.8 ± 0.10 | 2.0 ± 0.05 | ||
3. Experimental
3.1. Chemistry
3.2. 4-Isocyanatobenzonitrile (5)
3.3. General Procedure for Preparation of 1-(4-Cyanophenyl)-3-substituted Phenylureas 6a–o
3.4. General Procedure for Preparation of 4-(3-Substituted Phenylureido)benzothioamides 7a–o
3.5. General Procedure for Preparation of 1-(4-(4-Chloromethylthiazol-2-yl)phenyl)-3-substituted Phenylureas 8a–o
3.6. General Procedure for Preparation of 1-Substituted Phenyl-3-(4-(4-(piperazin-1-ylmethyl)thiazol-2-yl)phenyl)ureas 9a–o
3.7. General Procedure for Preparation of Ethyl 2-(4-((2-(4-(3-substituted phenylureido)phenyl)thiazol-4-yl)methyl)piperazin-1-yl)acetates 10a–o
3.8. General Procedure for Preparation of 1-Substituted phenyl-3-(4-(4-((4-(2-hydrazinyl-2-oxoethyl)piperazin-1-yl)methyl)thiazol-2-yl)phenyl)ureas 11a–o
3.9. General Procedure for Preparation of 5-Benzyloxy-2-hydroxybenzaldehydes 12a–d
3.10. General Procedure for Preparation of 4-Benzyloxy-2-hydroxybenzaldehydes 13a–c
3.11. 7-Hydroxy-4-methyl-2H-chromen-2-one (14)
3.12. 7-Hydroxy-4-methyl-2-oxo-2H-chromene-6-carbaldehyde (15)
3.13. General Procedure for Preparation of Substituted 4-Substituted Phenyl Isocyanates 16a–b
3.14. General Procedure for Preparation of Methyl 2-(3-(4-Substituted phenyl)ureido)acetates 17a–b
3.15. General Procedure for Preparation of 3-(4-Substituted phenyl)imidazolidine-2,4-diones 18a–b
3.16. General Procedure for Preparation of 5-((dimethylamino)methylene)-3-(4-substituted phenyl)imidazolidine-2,4-diones 19a–b
3.17. General Procedure for the Preparation of Target Compounds 1a–g, 2a–k, and 3a–e
3.18. General Procedure for Preparation of Target Compounds 4a–c
3.19. Evaluation of the Biological Activity
4. Conclusions
Acknowledgments
References
- Keating, G.M.; Santoro, A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Smalley, K.S.; Xiao, M.; Villanueva, J.; Nguyen, T.K.; Flaherty, K.T.; Letrero, R.; van Belle, P.; Elder, D.E.; Wang, Y.; Nathanson, K.L.; et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 2009, 28, 85–94. [Google Scholar] [CrossRef]
- Adnane, L.; Trail, P.A.; Taylor, I.; Wilhelm, S.M. Sorafenib (BAY 43-9006, Nexavar), a dual-actioninhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006, 407, 597–612. [Google Scholar] [CrossRef]
- Hyun, Y.W.; Jeong, H. Sorafenib in liver cancer. Expert Opin. Pharmacother. 2012, 13, 1059–1067. [Google Scholar] [CrossRef]
- Strumberg, D. Sorafenib for the treatment of renal cancer. Expert Opin. Pharmacother. 2012, 13, 407–419. [Google Scholar] [CrossRef]
- Peterson, Q.P.; Hsu, D.C.; Goode, D.R.; Novotny, C.J.; Totten, R.K.; Hergenrother, P.J. Procaspase-3 activation as an anti-cancer strategy: Structure-activity relationship of PAC-1, and its cellular co-localization with caspase-3. J. Med. Chem. 2009, 52, 5721–5731. [Google Scholar]
- Lucas, P.W.; Schmit, J.M.; Peterson, Q.P.; West, D.C.; Hsu, D.C.; Novotny, C.J.; Dirikolu, L.; Churchwell, M.I.; Doerge, D.R.; Garrett, L.D.; et al. Pharmacokinetics and Derivation of an anticancer dosing regimen for PAC-1, a preferential small molecule activator of procaspase-3, in healthy dogs. Invest. New Drugs 2011, 29, 901–911. [Google Scholar] [CrossRef]
- Peterson, Q.P.; Goode, D.R.; West, D.C.; Ramsey, K.N.; Lee, J.J.; Hergenrother, P.J. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol. 2009, 388, 144–158. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Zhai, X.; Wang, L.; Yang, J.; Tan, Z.; Gong, P. Design, synthesis and anticancer activities of diaryl urea derivatives bearing N-acylhydrazone moiety. Chem. Pharm. Bull. 2012, 60, 1046–1054. [Google Scholar] [CrossRef]
- Putt, K.S.; Chen, G.W.; Pearson, J.M.; Sandhorst, J.S.; Hoagland, M.S.; Kwon, J.T.; Hwang, S.K.; Jin, H.; Churchwell, M.I.; Cho, M.H.; et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat. Chem. Biol. 2006, 2, 543–550. [Google Scholar] [CrossRef]
- Kudo, M.; Hanashima, T.; Muranaka, A.; Sato, H.; Uchiyama, M.; Azumaya, I.; Hirano, T.; Kagechika, H.; Tanatani, A. Identification of Absolute Helical Structures of Aromatic Multilayered Oligo(m-Phenylurea)s in Solution. J. Org. Chem. 2009, 74, 8154–8163. [Google Scholar] [CrossRef]
- Duff, J.C.; Bills, E.J. Reactions between Hexamethylenetetramine and Phenolic Compounds. Part I. A New Method for the Preparation of 3- and 5-Aldehydosalicylic Acids. J. Chem. Soc. 1932, 1932, 1987–1988. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Fazio, N.; Scriba, G.K.; Poppitz, W.; Cannata, F.; Poupaert, J.H.; Wouters, J.; Lambert, J.D. Substituted 2-Thioxoimidazolidin-4-Ones and Imidazolidine-2,4-Diones as Fatty Acid Amide Hydrolase Inhibitors Templates. J. Med. Chem. 2006, 49, 417–425. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhai, X.; Huang, Q.; Jiang, N.; Wu, D.; Zhou, H.; Gong, P. Discovery of Hybrid Dual N-Acylhydrazone and Diaryl Urea Derivatives as Potent Antitumor Agents: Design, Synthesis and Cytotoxicity Evaluation. Molecules 2013, 18, 2904-2923. https://doi.org/10.3390/molecules18032904
Zhai X, Huang Q, Jiang N, Wu D, Zhou H, Gong P. Discovery of Hybrid Dual N-Acylhydrazone and Diaryl Urea Derivatives as Potent Antitumor Agents: Design, Synthesis and Cytotoxicity Evaluation. Molecules. 2013; 18(3):2904-2923. https://doi.org/10.3390/molecules18032904
Chicago/Turabian StyleZhai, Xin, Qiang Huang, Nan Jiang, Di Wu, Hongyu Zhou, and Ping Gong. 2013. "Discovery of Hybrid Dual N-Acylhydrazone and Diaryl Urea Derivatives as Potent Antitumor Agents: Design, Synthesis and Cytotoxicity Evaluation" Molecules 18, no. 3: 2904-2923. https://doi.org/10.3390/molecules18032904
APA StyleZhai, X., Huang, Q., Jiang, N., Wu, D., Zhou, H., & Gong, P. (2013). Discovery of Hybrid Dual N-Acylhydrazone and Diaryl Urea Derivatives as Potent Antitumor Agents: Design, Synthesis and Cytotoxicity Evaluation. Molecules, 18(3), 2904-2923. https://doi.org/10.3390/molecules18032904




