Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition
Abstract
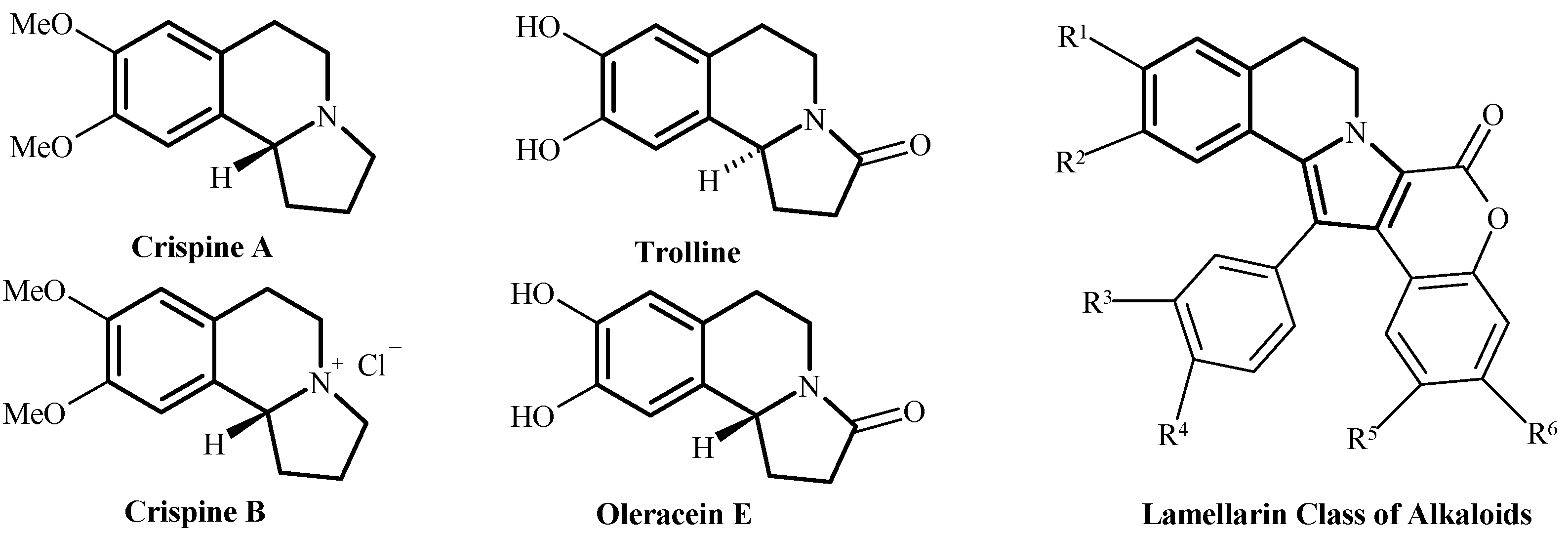
:1. Introduction
2. Results and Discussion
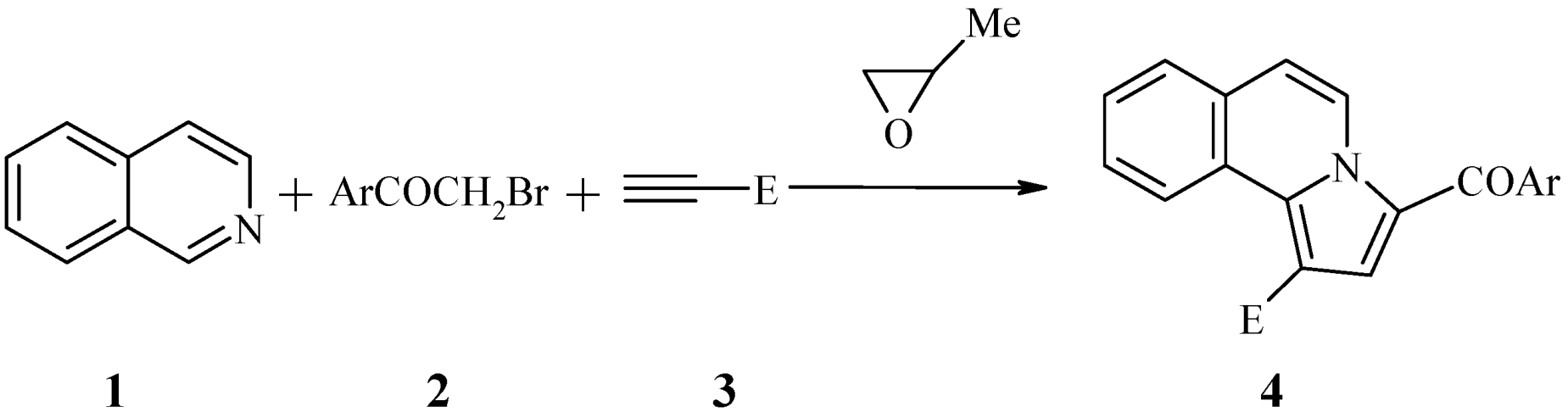
| No. | R | E | Ar | M.p. (°C) | Yield (%) |
|---|---|---|---|---|---|
| 4a | H | COMe | 4-MeOC6H4 | 171–173 | 71 |
| 4b | H | COMe | 3-NO2C6H4 | 218–220 | 70 |
| 4c | H | COMe | 3,4-(MeO)2C6H3 | 198–200 | 65 |
| 4d | H | CO2Me | 2-ClC6H4 | 222–225 | 60 |
| 4e | H | CO2Me | 2,4-Cl2C6H3 | 205–208 | 69 |
| 4f | H | CO2Me | 3-NO2C6H4 | 209–212 | 70 |
| 4g | H | CO2Me | 4-NO2C6H4 | 208–211 | 64 |
| 4h | H | CO2Et | 1-naphthyl | 162–164 | 72 |
| 4i | H | CO2Et | 2-napthyl | 150–152 | 67 |
| 4j | H | CO2Et | 2-NO2C6H4 | 186–187 | 69 |
| 4k | H | CO2Et | 3-NO2C6H4 | 201–203 | 78 |
| 4l | H | CO2Et | 4-NO2C6H4 | 209–211 | 63 |
| 4m | H | CO2Et | 4-FC6H4 | 140–142 | 65 |
| 4n | H | CO2Et | 2,4-Cl2C6H3 | 180–186 | 52 |
| 4o | H | CO2Et | 4-BrC6H4 | 190–192 | 66 |
| 4p | H | CO2Et | 2-HOC6H4 | 152–154 | 64 |
| 4q | H | CO2Et | 4-MeOC6H4 | 151–153 | 61 |
| 4r | H | CO2Et | 3,4-(MeO)2C6H3 | 173–176 | 69 |
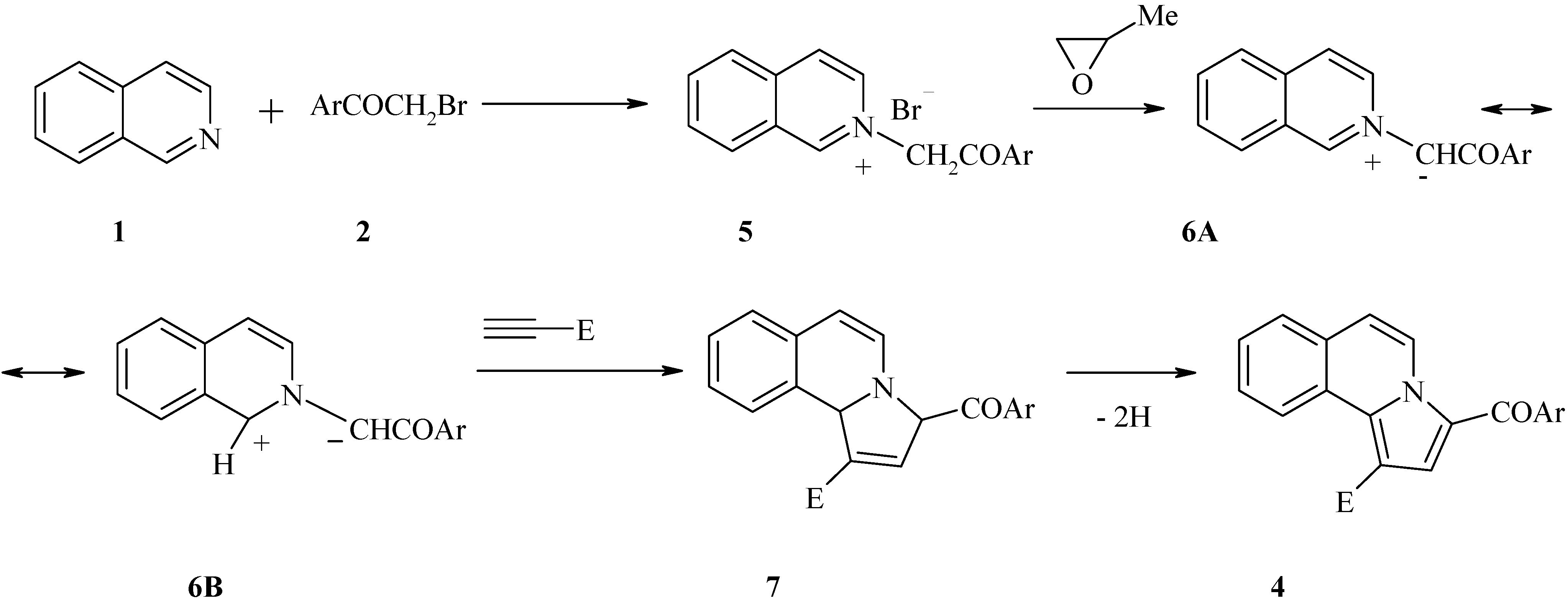
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of pyrrolo[2,1-a]isoquinolines 4
4. Conclusions
Conflict of Interest
References
- Zhang, Q.; Tu, G.; Zhao, Y.; Cheng, T. Novel bioactive isoquinoline alkaloids from Carduus crispus. Tetrahedron 2002, 58, 6795–6798. [Google Scholar] [CrossRef]
- Yioti, E.G.; Mati, I.K.; Arvanitidis, A.G.; Massen, Z.S.; Alexandraki, E.S.; Gallos, J.K. Synthesis of (±)-Crispine A by a nitrosoalkene hetero-Diels-Alder addition to ethyl vinyl ether. Synthesis 2011, 142–146. [Google Scholar]
- Agarwal, S.; Kataeva, O.; Schmidt, U.; Knölker, H.-J. Transition metals in organic synthesis, Part 107. Silver(I)-promoted oxidative cyclization to pyrrolo[2,1-a]isoquinolines and application to the synthesis of (±)-crispine A. RSC Adv. 2013, 3, 1089–1096. [Google Scholar]
- Meyer, N.; Opatz, T. One-pot synthesis of (±)-crispine A and its C-ring-substituted analogs. Eur. J. Org. Chem. 2006, 17, 3997–4002. [Google Scholar] [CrossRef]
- Opatz, T. The chemistry of deprotonated a-aminonitriles. Synthesis 2009, 1941–1959. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother. Res. 2009, 23, 1032–1035. [Google Scholar] [CrossRef]
- Wang, R.F.; Yang, X.W.; Ma, C.M.; Cai, S.Q.; Li, J.N. Shoyama, Y. A bioactive alkaloid from the flowers of Trollius chinensis. Heterocycles 2004, 63, 1443–1448. [Google Scholar] [CrossRef]
- Pla, D.; Albericio, F.; Alvarez, M. Progress on lamellarins. Med. Chem. Commun. 2011, 2, 689–697. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Total synthesis of Lamellarins D, H, and R and Ningalin B. Org. Lett. 2011, 13, 312–315. [Google Scholar]
- Shen, L.; Yang, X.; Yang, B.; He, Q.; Hu, Y. Novel hybrids from lamellarin D and combretastatin A4 as cytotoxic agents. Eur. J. Med. Chem. 2010, 45, 11–18. [Google Scholar] [CrossRef]
- Yadav, J.S.; Gayathri, K.U.; Reddy, B.V.S.; Prasad, A.R. Modular total synthesis of lamellarin G trimethyl ether. Synlett 2009, 43–46. [Google Scholar]
- Baunbæk, D.; Trinkler, N.; Ferandin, Y.; Lozach, O.; Ploypradith, P.; Rucirawat, S.; Ishibashi, F.; Iwao, M.; Meijer, L. Anticancer alkaloid lamellarins inhibit protein kinases. Mar. Drugs 2008, 6, 514–527. [Google Scholar] [CrossRef]
- Neagoie, C.; Vedrenne, E.; Buron, F.; Mérour, J.-Y.; Rosca, S.; Bourg, S.; Lozach, O.; Meijer, L.; Baldeyrou, B.; Lansiaux, A.; et al. Synthesis of chromeno[3,4-b]indoles as Lamellarin D analogues: A novel DYRK1A inhibitor class. Eur. J. Med. Chem. 2012, 49, 379–396. [Google Scholar] [CrossRef]
- Mikhailovskii, G.; Shklyaev, V.S. Pyrrolo[2,1-a]isoquinolines. Chem. Heterocycl. Compd. 1997, 33, 243–265. [Google Scholar] [CrossRef]
- Kulikova, Yu. A.; Surikova, O.V.; Mikhailovskii, A.G.; Vakhrin, M.I. Synthesis of 2-(3-coumarinyl)pyrrolo[2,1-a]isoquinoline derivatives by the Chichibabin reaction. Chem. Heterocycl. Compd. 2011, 47, 290–293. [Google Scholar]
- Naskar, S.; Banerjee, M.; Hazra, A.; Mondal, S.; Maity, A.; Paira, R.; Sahu, K.B.; Saha, P.; Banerjee, S.; Mondal, N.B. Novel route for the synthesis of structurally diverse pyrrolo[2,1-a]isoquinoline in aqueous micellar medium. Tetrahedron Lett. 2011, 52, 1527–1531. [Google Scholar]
- Yu, C.; Zhang, Y.; Zhang, S.; Li, H.; Wang, W. Cu(II) catalyzed oxidation-[3+2] cycloaddition-aromatization cascade: Efficient synthesis of pyrrolo [2,1-a]isoquinolines. Chem. Commun. 2011, 47, 1036–1038. [Google Scholar]
- Voskressensky, L.G.; Listratova, A.V.; Bolshov, A.V.; Bizhko, O.V.; Borisova, T.N.; Varlamov, A.V. A new approach towards the synthesis of pyrrolo[2,1-a]isoquinolines. Tetrahedron Lett. 2010, 51, 840–842. [Google Scholar]
- Najera, C.; Sansano, J.M. Azomethine ylides in organic synthesis. Curr. Org. Chem. 2003, 7, 1105–1150. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, S. Reactions of N-benzyl-pyridinium or -isoquinolinium ylides with ethyl 3-fluoro-3-(fluoroalkyl)acrylates to give fluoroalkyl-substituted indolizine and pyrrolo[2,1-a]isoquinoline derivatives. J. Chem. Soc. Perkin Trans 1 2001, 3204–3210. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H.-Y.; Liu, Q.-J.; Hu, H.-W.; Xu, J.-H. Synthesis of polycyclic indolizine derivatives via one-pot tandem reactions of N-ylides with dichloro substituted α,β-unsaturated carbonyl compounds. Tetrahedron 2007, 63, 2024–2033. [Google Scholar] [CrossRef]
- Farnum, D.G.; Alaimo, R.J.; Dunston, J.M. Synthesis of azaindenes. The benzo[c]pyrazolo[1,2-a]cinnolinium cation, a novel heteroaromatic cation. J. Org. Chem. 1967, 32, 1130. [Google Scholar] [CrossRef]
- Kutsuma, T.; Sekine, Y.; Fujiyama, K.; Kobayashi, Y. Studies on the reactions of heterocyclic compounds. IX. Syntheses of 1,10b-dihydropyrrolo[2,1-a]isoquinolines and 2,3-dihydropyrrolo[2,1-α]isoquinolines. Chem. Pharm. Bull. 1972, 20, 2701–2706. [Google Scholar]
- Dawood, K.M.; Ragab, E.A.; Khedr, N.A. Facile access to benzothiazole-containing pyrrolo[1,2-a]quinolines and pyrrolo[2,1-a]isoquinolines via nitrogen ylides. J. Chin. Chem. Soc. 2009, 56, 1180–1185. [Google Scholar]
- Bacu, E.; Samson-Belei, D.; Nowogrocki, G.; Couture, A.; Grandclaudon, P. Benzoindolizine derivatives of N-acylphenothiazine. Synthesis and characterization. Org. Biomol. Chem. 2003, 1, 2377–2382. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Georgescu, E.; Georgescu, F.; Draghici, C.; Popa, M.M. Generation of pyrrolo[2,1-a]isoquinoline derivatives from N-ylides: Synthetic control and structural characterization. Heteroat. Chem. 2011, 22, 723–729. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, L.; He, X.; Zhang, M. Novel syntheses of pyrrolo[2,1-a]isoquinolines via 1,3-dipolar cycloaddition between isoquinoliniums and alkynes. RSC Adv. 2012, 2, 7681–7688. [Google Scholar] [CrossRef]
- Caira, M.R.; Dumitrascu, F.; Draghici, C.; Dumitrescu, D.; Cristea, M. Synthesis and X-ray structure of a new pyrrolo[1,2-b]-pyridazine derivative. Molecules 2005, 10, 360–366. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Drăghici, B.; Căproiu, M.T.; Dumitrescu, D.G. A novel approach for the synthesis of highly fluorescent pyrrolo[1,2-b]pyridazines. Synlett 2008, 813–816. [Google Scholar]
- Georgescu, E.; Caira, M.R.; Georgescu, F.; Drăghici, B.; Popa, M.M.; Dumitrascu, F. One-pot, three-component synthesis of a library of new pyrrolo[1,2-a]quinoline derivatives. Synlett 2009, 1795–1799. [Google Scholar]
- Dumitrascu, F.; Caproiu, M.T.; Georgescu, F.; Draghici, B.; Popa, M.M.; Georgescu, E. New pyrrolo[2,1-a]phthalazine derivatives by one-pot three-component synthesis. Synlett 2010, 2407–2410. [Google Scholar]
- Caira, M.R.; Georgescu, E.; Georgescu, F.; Albota, F.; Dumitrascu, F. Contributions to syntheses of pyrrolo[2,1-a]phthalazines. Monatsh. Chem. 2011, 142, 743–748. [Google Scholar]
- Dumitrascu, F.; Georgescu, E.; Caira, M.R.; Georgescu, F.; Popa, M.; Draghici, B.; Dumitrescu, D.G. A novel approach for the synthesis of N-arylpyrroles. Synlett 2009, 3336–3340. [Google Scholar]
- Dömling, A. Multicomponent reactions. Chem. Rev. 2006, 106, 17–89. [Google Scholar]
- Eckert, H. Diversity oriented syntheses of conventional heterocycles by smart Multi Component Reactions (MCRs) of the last decade. Molecules 2012, 17, 1074–1102. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Xu, C.; Wang, Y.; Lin, W.; Zou, Y.; Shi, D. Ultrasound-promoted one-pot, three-component synthesis of spiro[indoline-3,1'-pyrazolo[1,2-b]phthalazine] derivatives. Molecules 2012, 17, 8674–8686. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–r are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M.M.; Dumitrescu, D. Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition. Molecules 2013, 18, 2635-2645. https://doi.org/10.3390/molecules18032635
Dumitrascu F, Georgescu E, Georgescu F, Popa MM, Dumitrescu D. Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition. Molecules. 2013; 18(3):2635-2645. https://doi.org/10.3390/molecules18032635
Chicago/Turabian StyleDumitrascu, Florea, Emilian Georgescu, Florentina Georgescu, Marcel Mirel Popa, and Denisa Dumitrescu. 2013. "Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition" Molecules 18, no. 3: 2635-2645. https://doi.org/10.3390/molecules18032635
APA StyleDumitrascu, F., Georgescu, E., Georgescu, F., Popa, M. M., & Dumitrescu, D. (2013). Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition. Molecules, 18(3), 2635-2645. https://doi.org/10.3390/molecules18032635




