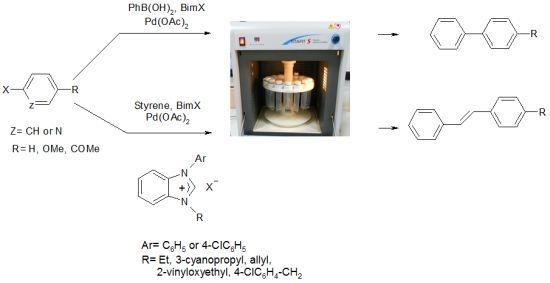
Synthesis, Characterization and Microwave-Promoted Catalytic Activity of Novel N-phenylbenzimidazolium Salts in Heck-Mizoroki and Suzuki-Miyaura Cross-Coupling Reactions under Mild Conditions
Abstract
:1. Introduction
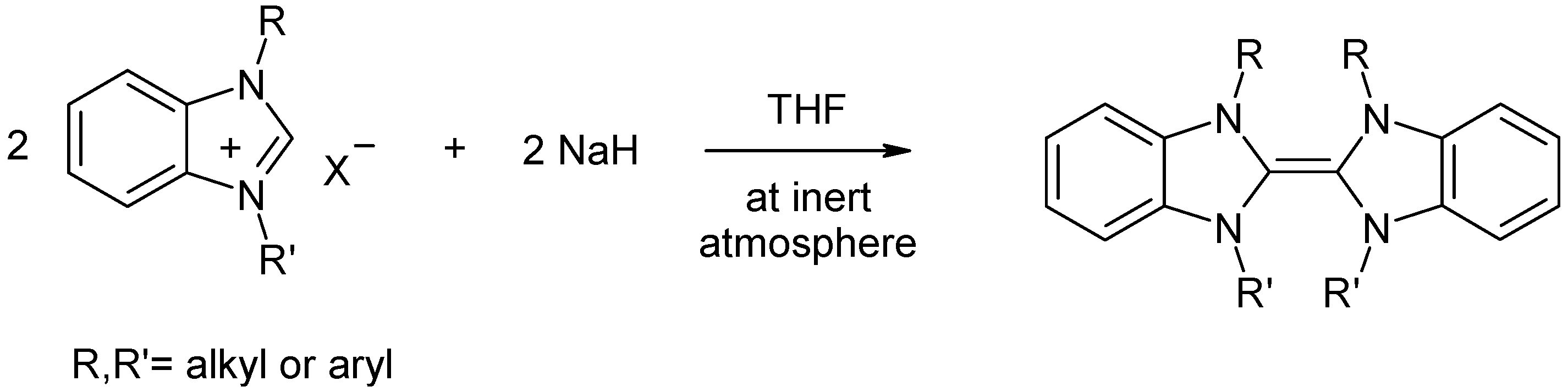
2. Results and Discussion
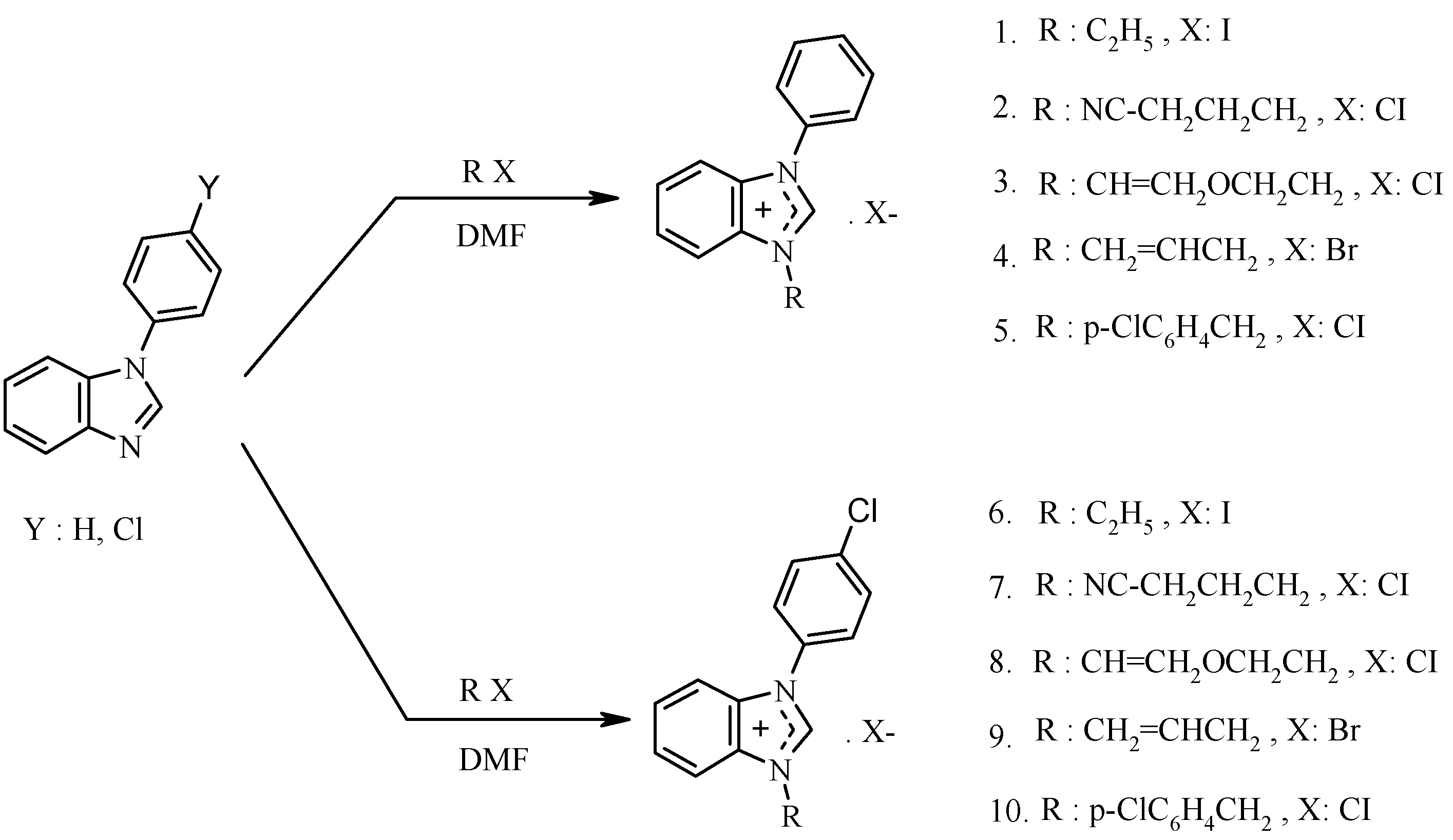
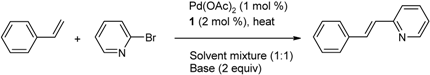
2.1. The Heck-Mizoroki Coupling Reaction
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Ligand | Base | Solvent | Time (min) | Thermal | heating | Microwave | heating |
| °C | Yield,% | °C (300 W) | Yield,% | |||||
| 1 | 1 | K2CO3 | DMF/H2O | 5 | 60 | n.d. | 60 | 33 |
| 2 | 1 | K2CO3 | DMF/H2O | 10 | 60 | n.d. | 60 | 49 |
| 3 | 1 | K2CO3 | DMF/H2O | 20 | 60 | 17 | 60 | 52 |
| 4 | 1 | K2CO3 | DMF/H2O | 5 | 80 | 03 | 80 | 46 |
| 5 | 1 | K2CO3 | DMF/H2O | 10 | 80 | 09 | 80 | 63 |
| 6 | 1 | K2CO3 | DMF/H2O | 20 | 80 | 13 | 80 | 64 |
| 7 | 1 | K2CO3 | DMF/H2O | 10 | 100 | 65 | ||
| 8 | 1 | CsCO3 | DMF/H2O | 10 | 80 | 62 | ||
| 9 | 1 | CsCO3 | EtOH/H2O | 10 | 80 | 43 | ||
| 10 | 1 | Et3N | DMF/H2O | 10 | 80 | 57 | ||
| 11 | 1 | Et3N | EtOH/H2O | 10 | 80 | 52 | ||
| 12 | 1 | DBU | DMF/H2O | 10 | 80 | 59 | ||
| 13 | 1 | DBU | EtOH/H2O | 10 | 80 | 54 | ||
| 14 | 1 | K2CO3 | C2H4(OH)2/H2O | 10 | 80 | 51 | ||
| 15 | 1 | K2CO3 | DMA | 10 | 80 | 32 | ||
| 16 | no | K2CO3 | DMF/H2O | 10 | 80 | 16 | ||
| 17 | no | DBU | EtOH/H2O | 10 | 80 | 13 | ||
| 18 | 1 | K2CO3 | DMF/H2O | 10 | 80 | 63 k | ||
| 19 | 1 | K2CO3 | DMF/H2O | 10 | 80 | n.d. l | ||
 | |||||
|---|---|---|---|---|---|
| Entry | R | Z | X | Salt | Conversion a (%) |
| 1 | H | N | Br | 1 | 46 b |
| 2 | H | N | Br | 1 | 63 c |
| 3 | H | N | Br | 1 | 64 d |
| 4 | H | N | Br | 1 | 03 e |
| 5 | H | N | Br | 1 | 09 f |
| 6 | H | N | Br | no | 16 g |
| 7 | H | CH | I | 1 | 98 |
| 8 | H | CH | I | 2 | 98 |
| 9 | H | CH | I | 3 | 97 |
| 10 | H | CH | I | 4 | 96 |
| 11 | H | CH | I | 5 | 96 |
| 12 | H | CH | I | 6 | 93 91 i |
| 13 | H | CH | I | 7 | 90 i |
| 14 | H | CH | I | 8 | 89 i |
| 15 | H | CH | I | 9 | 87 i |
| 16 | H | CH | I | 10 | 88 i |
| 17 | OCH3 | CH | I | 1 | 99 |
| 18 | OCH3 | CH | I | 2 | 98 |
| 19 | OCH3 | CH | I | 3 | 98 |
| 20 | OCH3 | CH | I | 4 | 98 |
| 21 | OCH3 | CH | I | 5 | 97 |
| 22 | OCH3 | CH | I | 6 | 95i |
| 23 | OCH3 | CH | I | 7 | 94 89 i |
| 24 | OCH3 | CH | I | 8 | 90 i |
| 25 | OCH3 | CH | I | 9 | 91 i |
| 26 | OCH3 | CH | I | 10 | 90i |
| 27 | COCH3 | CH | Br | 1 | 99 |
| 28 | COCH3 | CH | Br | 2 | 99 |
| 29 | COCH3 | CH | Br | 3 | 98 |
| 30 | COCH3 | CH | Br | 4 | 95 |
| 31 | COCH3 | CH | Br | 5 | 96 |
| 32 | COCH3 | CH | Br | 6 | 94 i |
| 33 | COCH3 | CH | Br | 7 | 96 92 i |
| 34 | COCH3 | CH | Br | 8 | 90 i |
| 35 | COCH3 | CH | Br | 9 | 87 i |
| 36 | COCH3 | CH | Br | 10 | 86 i |
| 37 | H | N | Br | 2 | 62 |
| 38 | H | N | Br | 3 | 62 |
| 39 | H | N | Br | 4 | 60 |
| 40 | H | N | Br | 5 | 61 |
| 41 | H | N | Br | 6 | 59 |
| 42 | H | N | Br | 7 | 58 |
| 43 | H | N | Br | 8 | 56 |
| 44 | H | N | Br | 9 | 57 |
| 45 | H | N | Br | 10 | 58 |
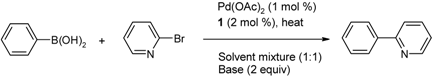
2.2. The Suzuki-Miyaura Coupling Reaction
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Ligand | Base | Solvent | Time (min) | Thermal | heating | Microwave | heating |
| °C | Yield,% | °C (300 W) | Conver. a, % | |||||
| 1 | 1 | K2CO3 | DMF/H2O | 5 | 60 | 0 | 60 | 47 |
| 2 | 1 | K2CO3 | DMF/H2O | 10 | 60 | 4 | 60 | 53 |
| 3 | 1 | K2CO3 | DMF/H2O | 20 | 60 | 9 | 60 | 55 |
| 4 | 1 | K2CO3 | DMF/H2O | 5 | 80 | 6 | 80 | 67 |
| 5 | 1 | K2CO3 | DMF/H2O | 10 | 80 | 11 | 80 | 75 |
| 6 | 1 | K2CO3 | DMF/H2O | 20 | 80 | 13 | 80 | 76 |
| 7 | 1 | K2CO3 | DMF/H2O | 5 | 100 | 7 | 100 | 67 |
| 8 | 1 | K2CO3 | DMF/H2O | 10 | 100 | 11 | 100 | 76 |
| 9 | 1 | K2CO3 | DMF/H2O | 20 | 100 | 14 | 100 | 77 |
| 10 | 1 | CsCO3 | DMF/H2O | 10 | 80 | 75 | ||
| 11 | 1 | CsCO3 | EtOH/H2O | 10 | 80 | 66 | ||
| 12 | 1 | K2CO3 | H2O | 10 | 80 | 39 | ||
| 13 | 1 | K2CO3 | C2H4(OH)2/H2O | 10 | 80 | 56 | ||
| 14 | 1 | K2CO3 | DMA | 10 | 80 | 47 | ||
| 15 | 1 | DBU | DMF/H2O | 10 | 80 | 64 | ||
| 16 | 1 | DBU | EtOH/H2O | 10 | 80 | 66 | ||
| 17 | 1 | K2CO3 | Glycerine/H2O | 10 | 80 | 58 | ||
| 18 | no | K2CO3 | DMF/H2O | 10 | 10 | 32 | ||
| 19 | 1 | K2CO3 | DMF/H2O | 10 | 10 | 74 m | ||
| 20 | 1 | K2CO3 | DMF/H2O | 10 | 10 | n.d. n | ||
| 21 | 1 | K2CO3 | DMF/H2O | 10 | 10 | 77 p | ||
| 22 | 1 | K2CO3 | DMF/H2O | 10 | 10 | 65 r | ||
 | |||||
|---|---|---|---|---|---|
| Entry | R | Z | X | Salt | Conversion a (%) |
| 1 | H | N | Br | 1 | 67 b |
| 2 | H | N | Br | 1 | 75 c |
| 3 | H | N | Br | 1 | 76 d |
| 4 | H | N | Br | 1 | 06 e |
| 5 | H | N | Br | 1 | 11 f |
| 6 | H | N | Br | no | 32 g |
| 7 | H | CH | I | 1 | 98 |
| 8 | H | CH | I | 2 | 98 |
| 9 | H | CH | I | 3 | 97 |
| 10 | H | CH | I | 4 | 97 |
| 11 | H | CH | I | 5 | 96 |
| 12 | H | CH | I | 6 | 97 95 i |
| 13 | H | CH | I | 7 | 94i |
| 14 | H | CH | I | 8 | 91i |
| 15 | H | CH | I | 9 | 90i |
| 16 | H | CH | I | 10 | 90i |
| 17 | OCH3 | CH | I | 1 | 99 |
| 18 | OCH3 | CH | I | 2 | 99 |
| 19 | OCH3 | CH | I | 3 | 97 |
| 20 | OCH3 | CH | I | 4 | 98 |
| 21 | OCH3 | CH | I | 5 | 98 |
| 22 | OCH3 | CH | I | 6 | 97 95 i |
| 23 | OCH3 | CH | I | 7 | 95 i |
| 24 | OCH3 | CH | I | 8 | 94 i |
| 25 | OCH3 | CH | I | 9 | 94 i |
| 26 | OCH3 | CH | I | 10 | 93 i |
| 27 | COCH3 | CH | Br | 1 | 99 |
| 28 | COCH3 | CH | Br | 2 | 99 |
| 29 | COCH3 | CH | Br | 3 | 99 |
| 30 | COCH3 | CH | Br | 4 | 99 |
| 31 | COCH3 | CH | Br | 5 | 98 |
| 32 | COCH3 | CH | Br | 6 | 98 96 i |
| 33 | COCH3 | CH | Br | 7 | 96 i |
| 34 | COCH3 | CH | Br | 8 | 95 i |
| 35 | COCH3 | CH | Br | 9 | 95 i |
| 36 | COCH3 | CH | Br | 10 | 95 i |
| 37 | H | N | Br | 2 | 72 |
| 38 | H | N | Br | 3 | 69 |
| 39 | H | N | Br | 4 | 70 |
| 40 | H | N | Br | 5 | 63 |
| 41 | H | N | Br | 6 | 70 |
| 42 | H | N | Br | 7 | 67 |
| 43 | H | N | Br | 8 | 68 |
| 44 | H | N | Br | 9 | 70 |
| 45 | H | N | Br | 10 | 70 |
3. Experimental
3.1. General Chemical Procedure
3.2. General Procedure for the Heck-Mizoroki Reactions
3.3. General Procedure for the Suzuki Reactions
4. Conclusions
Acknowledgments
Supplementary Materials
References
- Firouzabadi, H.; Iranpoor, N.; Kaazemi, F. Carbon-carbon bond formation via homocoupling reaction of substrates with a broad diversity in water using Pd(OAc)2 and agarose hydrogel as a bioorganic ligand, support and reductant. J. Mol. Cat. A: Chem. 2011, 348, 94–99. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 2009, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Carbon-carbon bonding made easy. Chem. Commun. 2005, 4759–4763. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Beletskaya, I.P. Toward the ideal catalyst: From atomic centers to a “Cocktail” of catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar]
- Dawood, K.M.; Fayed, M.S.; Elkhalea, M.M. Heck and Suzuki cross-couplings of aryl and heteroaryl bromides in water using a new palladium(II)-complex. ARKIVOC 2009, xiii, 324–341. [Google Scholar]
- Alonso, D.A.; Najera, C. Oxime-derived palladacycles as source of palladium nanoparticles. Chem. Soc. Rev. 2010, 39, 2891–2902. [Google Scholar] [CrossRef]
- Li, C.-J.; Chen, L. Organic chemistry in water. Chem. Rev. 2006, 35, 68–82. [Google Scholar]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic synthesis “On Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, F.; Shi, M. Elimination of an alkyl group from imidazolium salts: Imidazole-Coordinated dinuclear monodentate NHC-Palladium complexes driven by self-assembly and their application in the Heck reaction. Eur. J. Inorg. Chem. 2009, 2009, 1723–1728. [Google Scholar] [CrossRef]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—A synthetic chemist’s perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Linninger, C.S.; Herdtweck, E.; Hoffmann, S.D.; Herrmann, W.A. A new palladium(II) complex of a functionalized N-heterocyclic carbene: Synthesis, characterization and application in Suzuki-miyaura cross-coupling reactions. J. Mol. Struct. 2008, 890, 192–197. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic Carbenes-Palladium(II) precatalysts for cross-coupling reactions. Account. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Mercan, D.; Çetinkaya, E.; Çetinkaya, B. Influence of CH3 substituents on tetrahydropyrimidin-2-ylidene: Sigma-donating properties and in situ catalytic activities of precursor salts/Pd(OAc)2 system for C–C coupling reactions. J. Organomet. Chem. 2011, 696, 1359–1366. [Google Scholar]
- Korolev, D.N.; Bumagin, N.A. An improved protocol for ligandless Suzuki-Miyaura coupling in water. Tetrahedron Lett. 2006, 47, 4225–4229. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Chu, C.-Y.; Zhu, L.; Qian, B.; Shao, L.X. N-Heterocyclic carbene-Pd(II) complex derived from proline for the Mizoroki-Heck reaction in water. Tetrahedron 2011, 67, 9479–9483. [Google Scholar] [CrossRef]
- Liu, C.; Ni, Q.; Hu, P.; Qui, J. Oxygen-promoted PdCl2-catalyzed ligand-free Suzuki reaction in aqueous media. Org. Biomol. Chem. 2011, 9, 1054–1060. [Google Scholar] [CrossRef]
- Wu, X.-M.; Gu, Y.-B. TBAF-assisted palladium-catalyzed suzuki reaction in water under the ligand and base-free conditions. Lett. Org. Chem. 2012, 9, 396–400. [Google Scholar] [CrossRef]
- Chanthavong, F.; Leadbeater, N.E. The application of organic bases in microwave-promoted Suzuki coupling reactions in water. Tetrahedron Lett. 2006, 47, 1909–1912. [Google Scholar] [CrossRef]
- hrngren, P.; Fardost, A.; Russo, F.; Schanche, J.-S.; Fagrell, M.; Larhed, M. Evaluation of a nonresonant microwave applicator for continuous-flow chemistry applications. Org. Process Res. Dev. 2012, 16, 1053–1063. [Google Scholar] [CrossRef]
- Larhed, M.; Hallberg, A. Microwave-assisted high speed chemistry: A new technique in drug discovery. Drug Discov. Today 2001, 6, 406–416. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Avery, K.B.; Devine, W.G.; Kormos, C.M.; Leadbeater, N.E. Use of a silicon carbide multi-well plate in conjunction with microwave heating for rapid ligand synthesis, formation of palladium complexes, and catalyst screening in a Suzuki coupling. Tetrahedron Lett. 2009, 50, 2851–2853. [Google Scholar] [CrossRef]
- Prokopciva, H.; Ramirez, J.; Fernandez, E.; Kappe, C.O. Microwave-assisted one-pot diboration/Suzuki cross-couplings. A rapid route to tetrasubstituted alkenes. Tetrahedron Lett. 2008, 49, 4831–4835. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Findenig, S.; Kappe, C.O. Heterogeneous versus homogeneous palladium catalysts for ligandless Mizoroki-Heck reactions: A comparison of batch/microwave and continuous-flow processing. Chem. Eur. J. 2009, 15, 1001–1010. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Marco, M. Ligand-free palladium catalysis of the Suzuki reaction in water using microwave heating. Org. Lett. 2002, 4, 2973–2976. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E. Suzuki coupling of aryl chlorides with phenylboronic acid in water, using microwave heating with simultaneous cooling. Org. Lett. 2005, 7, 2101–2104. [Google Scholar] [CrossRef]
- Appukkuttan, P.; van der Eycken, E. Recent developments in microwave-assisted, transition-metal-catalysed C–C and C–N bond-forming reactions. Eur. J. Org. Chem. 2008, 2008, 1133–1155. [Google Scholar] [CrossRef]
- Dawood, K.M. Microwave-assisted Suzuki-Miyaura and Heck-Mizoroki cross-coupling reactions of aryl chlorides and bromides in water using stable benzothiazole-based palladium(II) precatalysts. Tetrahedron 2007, 63, 9642–9651. [Google Scholar] [CrossRef]
- Larhed, M.; Hallberg, A. Microwave-promoted palladium-catalyzed coupling reactions. J. Org. Chem. 1996, 61, 9582–9584. [Google Scholar] [CrossRef]
- Nilsson, P.; Ofsson, K.; Larhed, M. Microwave-assisted and metal-catalyzed coupling reactions. Top. Curr. Chem. 2006, 266, 103–144. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Şireci, N.; Deniz, S.; Küçükbay, H. Synthesis and microwave-assisted catalytic activity of novel bis-benzimidazole salts bearing furfuryl and thenyl moieties in Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2010, 24, 414–420. [Google Scholar]
- Küçükbay, H.; Şireci, N.; Yılmaz, Ü.; Akkurt, M.; Yalçın, Ş.P.; Tahir, M.N.; Ott, H. Synthesis, characterization and microwave-assisted catalytic activity of novel benzimidazole salts bearing piperidine and morpholine moieties in Heck cross-coupling reactions. Appl. Organomet. Chem. 2011, 25, 255–261. [Google Scholar]
- Yılmaz, Ü.; Küçükbay, H.; Şireci, N.; Akkurt, M.; Günal, S.; Durmaz, R.; Tahir, M.N. Synthesis, microwave-promoted catalytic activity in Suzuki-Miyaura cross-coupling reactions and antimicrobial properties of novel benzimidazole salts bearing trimethylsilyl group. Appl. Organomet. Chem. 2011, 25, 366–373. [Google Scholar]
- Şireci, N.; Yılmaz, Ü.; Küçükbay, H. Microwave assisted catalytic activity of some bis-5(6)-nitrobenzimidazole salts for Heck and Suzuki cross-coupling reactions. Asian J. Chem. 2010, 22, 7153–7158. [Google Scholar]
- Küçükbay, H.; Şireci, N.; Yılmaz, Ü.; Deniz, S.; Akkurt, M.; Baktır, Z.; Büyükgüngör, O. Synthesis, characterization, and microwave-promoted catalytic activity of novel benzimidazole salts bearing silicon-containing substituents in Heck-Mizoroki and Suzuki-Miyaura cross-coupling reactions under aerobic conditions. Turk. J. Chem. 2012, 36, 201–217. [Google Scholar]
- Phillips, M.A. The formation of 2-substituted benzimidazoles. J. Chem. Soc. 1928, 13, 2393–2399. [Google Scholar] [CrossRef]
- Şireci, N.; Yılmaz, Ü.; Küçükbay, H.; Akkurt, M.; Baktır, Z.; Türktekin, S.; Büyükgüngör, O. Synthesis of 1-substituted benzimidazole metal complexes and structural characterization of dichlorobis(1-phenyl-1H-benzimidazole-κN3)cobalt(II) and dichlorobis(1-phenyl-1H-benzimidazole-κN3)zinc(II). J. Coord. Chem. 2011, 64, 1894–1902. [Google Scholar] [CrossRef]
- Çetinkaya, E.; Hitchcock, P.B.; Küçükbay, H.; Lappert, M.F.; Al-Juaid, S. XXIV*. Preparation of two enetetramine-derived carbenerhodium(I) chloride complexes RhCl(LR)3 and [RhCl(COD)LR]{LR=CN(Me)((CH)4CNMe-o} and the preparation and X-ray structures of the enetetramine L2R and its salt [L2R][BF4]2. J. Organomet. Chem. 1994, 481, 89–95. [Google Scholar]
- Çetinkaya, B.; Çetinkaya, E.; Chamizo, J.A.; Hitchcock, P.B.; Jasim, H.A.; Küçükbay, H.; Lappert, M.F. Synthesis and structures of 1,3,1',3'-tetrabenzyl-2,2'-biimidazolidinylidenes (electron-rich alkenes), their aminal intermediates and their degradation products. J. Chem. Soc. Perkin. Trans. 1 1998, 1998, 2047–2054. [Google Scholar]
- Yılmaz, Ü.; Küçükbay, H. Synthis and properties of new endotricyclic electron-rich olefins and their some derivatives. Asian J. Chem. 2009, 21, 6149–6155. [Google Scholar]
- Rosa, G.R.; Rosa, D.S. NCP pincer palladacycle as a phosphine-free catalyst precursor for the Heck-Mizoroki coupling of aryl halides. RSC Adv. 2012, 2, 5080–5083. [Google Scholar] [CrossRef]
- Higgs, G. Novel treatments for asthma. Chem. Ind. 1997, 827–830. [Google Scholar]
- De Vries, J.G. The Heck reaction in the production of fine chemicals. Can. J. Chem. 2001, 79, 1086–1092. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.-H.; Yan, W.-B. Palladium-catalyzed cross-coupling reaction of aryl(trialkyl)silanes with aryl nitrile. Tetrahedron Lett. 2012, 53, 6743–6746. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006; pp. 55–70. [Google Scholar]
- Huang, W.; Guo, J.; Xiao, Y.; Zhu, M.; Zou, G.; Tang, J. Palladium-benzimidazolium salt catalyst systems for Suzuki coupling: Development of a practical and highly active palladium catalyst system for coupling of aromatic halides with arylboronic acids. Tetrahedron 2005, 61, 9783–9790. [Google Scholar] [CrossRef]
- Gök, Y.; Gürbüz, N.; Özdemir, İ.; Çetinkaya, B.; Çetinkaya, E. Benzimidazolin-2-ylidene-palladium-catalysed coupling reactions of aryl halides. Appl. Organomet. Chem. 2005, 19, 870–874. [Google Scholar]
- Singh, R.; Viciu, M.S.; Kramareva, N.; Navarro, O.; Nolan, S.P. Simple (imidazol-2-ylidene)-Pd-acetate complexes as effective precatalysts for sterically hindered Suzuki-Miyaura couplings. Org. Lett. 2005, 7, 1829–1832. [Google Scholar]
- Li, H.Y.; Cai, C. A novel system for the Suzuki cross-coupling reaction catalyzed with light fluorous palladium-NHC-complex. J. Fluorine Chem. 2012, 144, 143–146. [Google Scholar] [CrossRef]
- Borja, G.; Monge-Marcet, A.; Pleixats, R.; Parella, T.; Cattoen, X.; Man, M.M.C. Recyclable hybrid silica-based catalysts derived from Pd-NHC complexes for Suzuki, Heck, Sonogashira reactions. Eur. J. Org. Chem. 2012, 19, 3625–3635. [Google Scholar]
- Lee, J.-Y.; Cheng, P.-Y.; Tsai, Y.-H.; Lin, G.-R.; Liu, S.-P.; Sie, M.-H.; Lee, H.M. Efficient heck reactions catalyzed by Palladium(0) and -(II) complexes bearing N-heterocyclic carbene and amide functionalities. Organometallics 2010, 29, 3901–3911. [Google Scholar] [CrossRef]
- Durap, F.; Metin, Ö.; Aydemir, M.; Özkar, S. New route to synthesis of PVP-stabilized palladium(0) nanoclusters and their enhanced catalytic activity in Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2009, 23, 498–503. [Google Scholar]
- Akba, O.; Durap, F.; Aydemir, M.; Baysal, A.; Gümgüm, B.; Özkar, S. Synthesis and characterizations of N,N,N',N'-tetrakis (diphenylphosphino)ethylendiamine derivatives: Use of palladium(II) complex as pre-catalyst in Suzuki coupling and Heck reactions. J. Organomet. Chem. 2009, 694, 731–736. [Google Scholar]
- Xi, C.; Wu, Y.; Yan, X. cis-Fashioned palladium (II) complexes of 2-phenylbenzimidazole ligands: Synthesis, characterization, and catalytic behavior towards Suzuki-Miyaura reaction. J. Organomet. Chem. 2008, 693, 3842–3846. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the all compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yılmaz, Ü.; Küçükbay, H.; Deniz, S.; Şireci, N. Synthesis, Characterization and Microwave-Promoted Catalytic Activity of Novel N-phenylbenzimidazolium Salts in Heck-Mizoroki and Suzuki-Miyaura Cross-Coupling Reactions under Mild Conditions. Molecules 2013, 18, 2501-2517. https://doi.org/10.3390/molecules18032501
Yılmaz Ü, Küçükbay H, Deniz S, Şireci N. Synthesis, Characterization and Microwave-Promoted Catalytic Activity of Novel N-phenylbenzimidazolium Salts in Heck-Mizoroki and Suzuki-Miyaura Cross-Coupling Reactions under Mild Conditions. Molecules. 2013; 18(3):2501-2517. https://doi.org/10.3390/molecules18032501
Chicago/Turabian StyleYılmaz, Ülkü, Hasan Küçükbay, Selma Deniz, and Nihat Şireci. 2013. "Synthesis, Characterization and Microwave-Promoted Catalytic Activity of Novel N-phenylbenzimidazolium Salts in Heck-Mizoroki and Suzuki-Miyaura Cross-Coupling Reactions under Mild Conditions" Molecules 18, no. 3: 2501-2517. https://doi.org/10.3390/molecules18032501
APA StyleYılmaz, Ü., Küçükbay, H., Deniz, S., & Şireci, N. (2013). Synthesis, Characterization and Microwave-Promoted Catalytic Activity of Novel N-phenylbenzimidazolium Salts in Heck-Mizoroki and Suzuki-Miyaura Cross-Coupling Reactions under Mild Conditions. Molecules, 18(3), 2501-2517. https://doi.org/10.3390/molecules18032501