Synthesis and Crystal Structures of N-Substituted Pyrazolines
Abstract
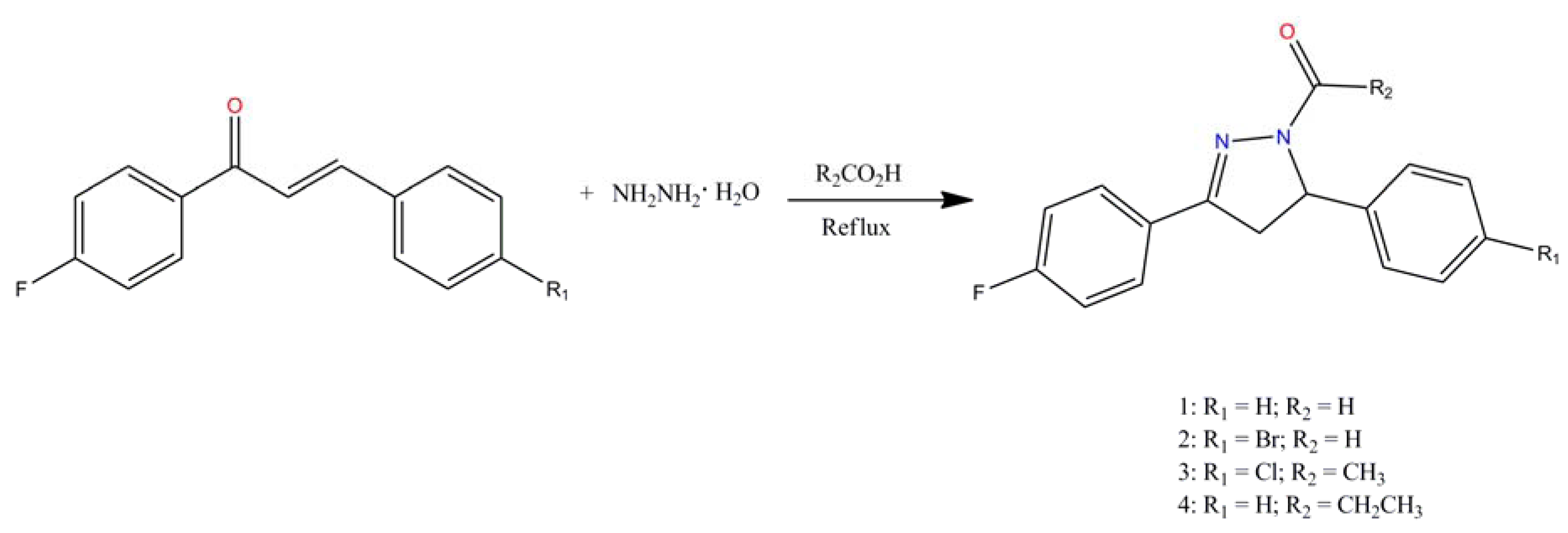
:1. Introduction
2. Results and Discussion
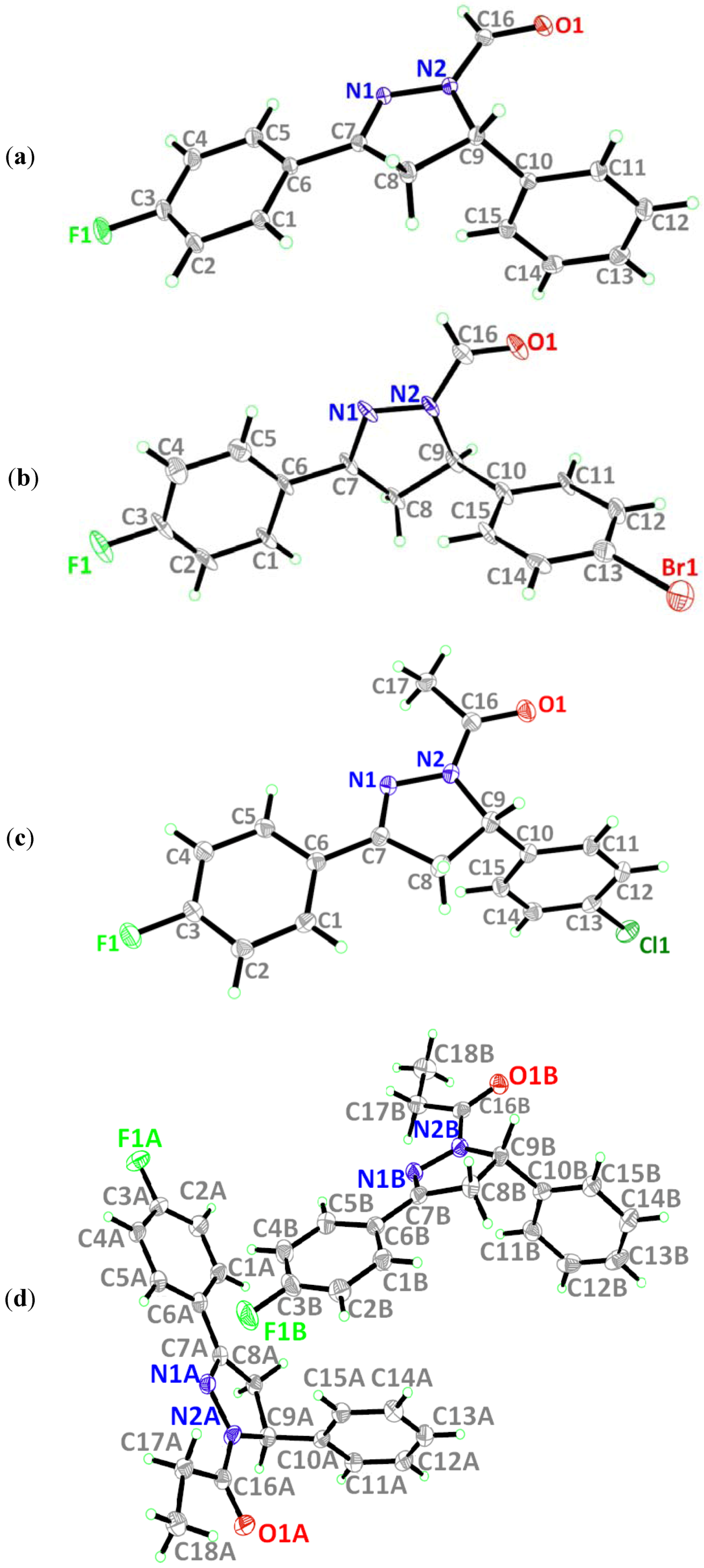
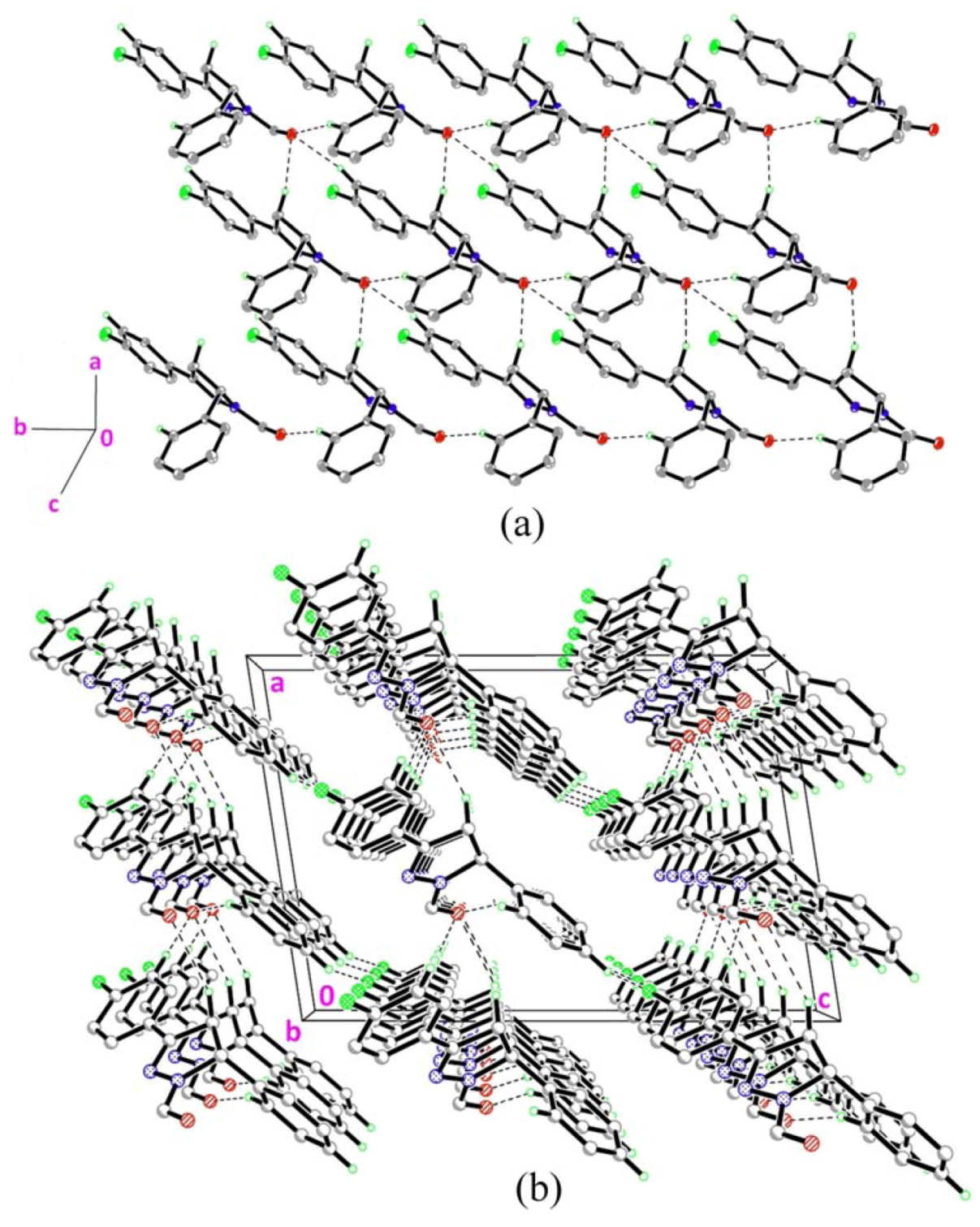
2.1. Crystal Structure Description of Compound 1
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| CCDC deposition numbers | 895315 | 895316 | 895317 | 895318 |
| Molecular formula | C16H13FN2O | C16H12BrFN2O | C17H14ClFN2O | C18H17FN2O |
| Molecular weight | 268.28 | 347.19 | 316.75 | 296.34 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | Cc | Cc | P21/c | P  |
| a/Å | 11.9069(7) | 6.2375(14) | 6.1087(4) | 9.3334(2) |
| b/Å | 6.2516(4) | 12.191(3) | 12.3725(9) | 13.2760(3) |
| c/Å | 17.3103(10) | 18.703(4) | 19.6142(14) | 13.2857(3) |
| α/° | 90 | 90 | 90 | 107.052(1) |
| β/° | 98.737(1) | 93.239(5) | 97.769(1) | 95.124(1) |
| γ/° | 90 | 90 | 90 | 96.629(1) |
| V/ Å3 | 1273.58(13) | 1419.9(6) | 1468.83(18) | 1550.14(6) |
| Z | 4 | 4 | 4 | 4 |
| Dcalc (g cm−3) | 1.399 | 1.624 | 1.432 | 1.270 |
| Crystal Dimensions (mm) | 0.19 × 0.32 × 0.46 | 0.13 × 0.29 × 0.41 | 0.17 × 0.17 × 0.29 | 0.11 × 0.17 × 0.29 |
| μ/mm−1 | 0.10 | 2.906 | 0.274 | 0.088 |
| Radiation λ (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Tmin/Tmax | 0.9559/0.9810 | 0.3792/0.7074 | 0.9238/0.9549 | 0.9753/0.9907 |
| Reflections measured | 7119 | 9227 | 16261 | 33624 |
| Ranges/indices (h, k, l) | −16, 16; −8, 8; −22, 24 | −8, 7; −15, 15; −24, 24 | −8, 8; −16, 17; −22, 27 | −13, 11; −18, 18; −18, 18 |
| θ limit (°) | 2.4-30.1 | 2.2-27.5 | 2.0-30.2 | 1.6-30.1 |
| Unique reflections | 1866 | 2748 | 4340 | 9058 |
| Observed reflections (I > 2σ(I)) | 1847 | 2600 | 3706 | 5096 |
| Parameters | 181 | 172 | 200 | 399 |
| Goodness of fit on F2 | 1.07 | 1.131 | 1.047 | 1.043 |
| R1, wR2 [I ≥ 2σ(I)] | 0.0267, 0.0748 | 0.0672, 0.1814 | 0.0341, 0.1010 | 0.0628, 0.1538 |
| D–H···A | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | Angle (D–H···A) (°) |
|---|---|---|---|---|
| (1) | ||||
| C2–H2A···O1 i | 0.95 | 2.36 | 3.2950(16) | 166 |
| C8–H8B···O1 ii | 0.99 | 2.60 | 3.5011(16) | 151 |
| C13–H13A···F1 iii | 0.95 | 2.53 | 3.1809(17) | 126 |
| C15–H15A···O1 iv | 0.95 | 2.36 | 3.2691(17) | 161 |
| (2) | ||||
| C2–H2A···O1v | 0.95 | 2.37 | 3.305(10) | 167 |
| C12–H12A···F1vi | 0.95 | 2.48 | 3.322(10) | 148 |
| C15–H15A···O1vii | 0.95 | 2.37 | 3.257(10) | 155 |
| (3) | ||||
| C2–H2A···O1viii | 0.93 | 2.43 | 3.2492(14) | 147 |
| C14–H14A···F1ix | 0.93 | 2.49 | 3.3462(14) | 154 |
| C15–H15A···O1 x | 0.93 | 2.52 | 3.4282(14) | 166 |
| (4) | ||||
| C1A–H1AA···O1B xi | 0.95 | 2.43 | 3.324(2) | 156 |
| C1B–H1BA···O1A xii | 0.95 | 2.43 | 3.223(2) | 141 |
| C9B–H9BA···O1A xiii | 1.00 | 2.56 | 3.345(2) | 135 |
| C15B–H15B···O1A iv | 0.95 | 2.48 | 3.349(2) | 151 |
2.2. Crystal Structure Description of Compound 2
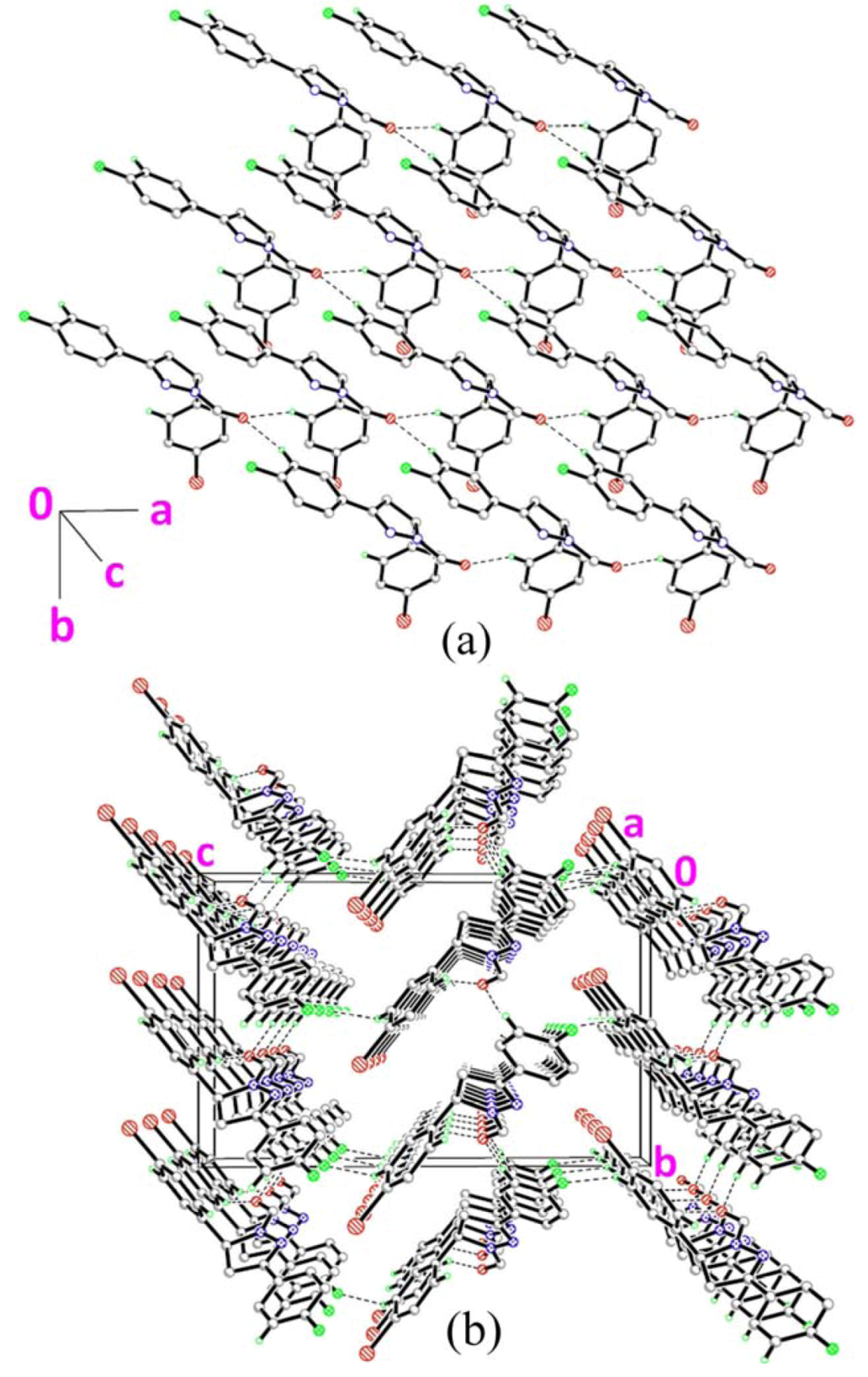
2.3. Crystal Structure Description of Compound 3
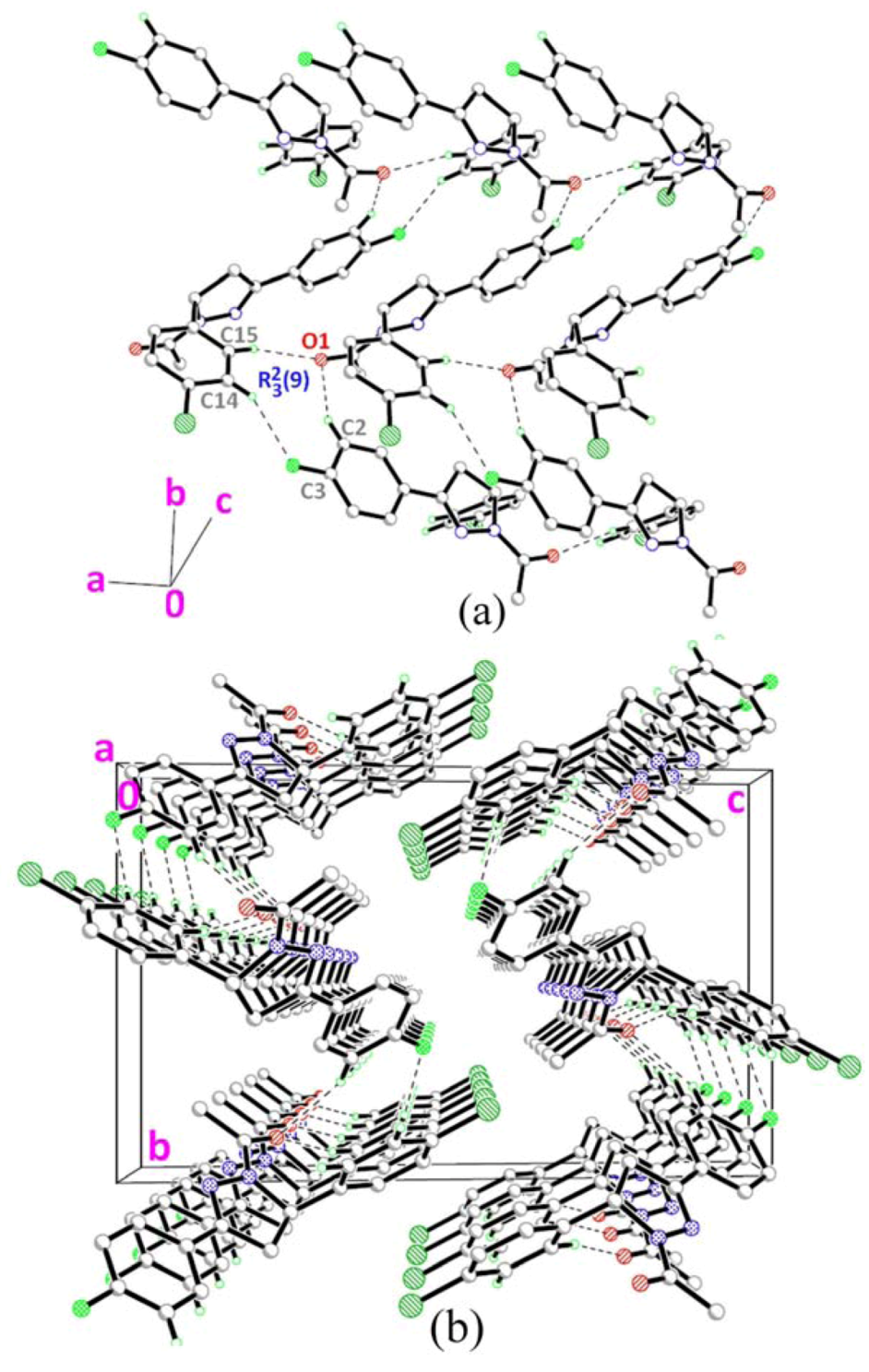
2.4. Crystal Structure Description of Compound 4

 ] as shown in Figure 5b via intermolecular C1A–H1AA•••O1B and C1B–H1BA•••O1A hydrogen bonds (Table 2).
] as shown in Figure 5b via intermolecular C1A–H1AA•••O1B and C1B–H1BA•••O1A hydrogen bonds (Table 2).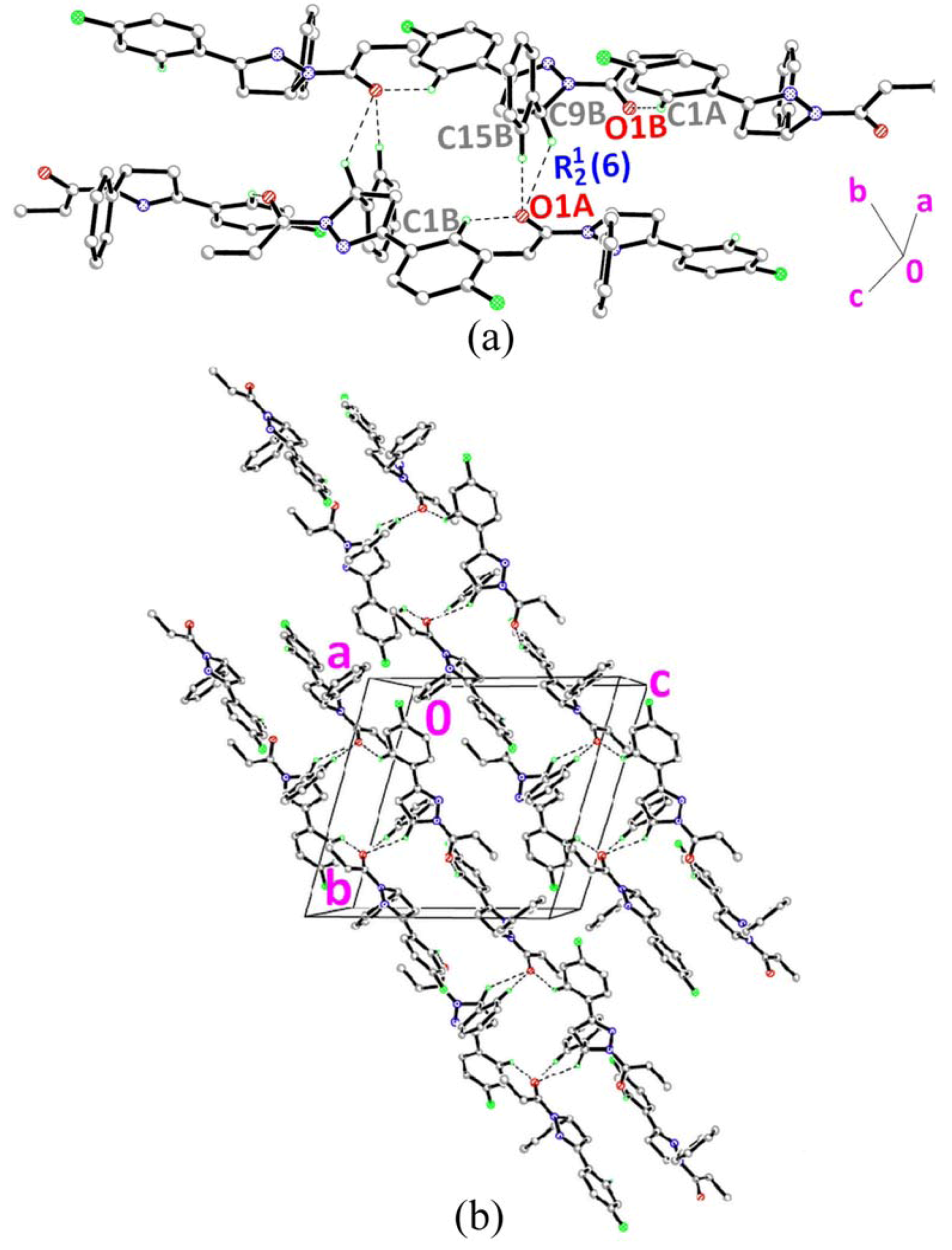
3. Experimental
3.1. Materials and Method
3.2. General Procedure for the Synthesis of N-Substituted Pyrazolines
3.3. X-ray Crystallographic Analysis
4. Conclusions
Acknowledgments
References
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Khan, M.T.H.; Yathirajan, H.S.; Raj, C.G.D.; Raghavendra, R. Antimicrobial, analgesic, DPPH scavenging activities and molecular docking study of some 1,3,5-triaryl-2-pyrazolines. Med. Chem. Res. 2012, 21, 2012–2022. [Google Scholar]
- Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Synthesis, characterization and crystal structures of 3,5-bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide. Crystals 2012, 2, 1108–1115. [Google Scholar] [CrossRef]
- Samshuddin, S.; Narayana, B.; Baktir, Z.; Akkurt, M.; Yathirajan, H.S. Synthesis, characterization and crystal structure of 1-[3,5-bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]propan-1-one. Der Pharma Chemica 2011, 3, 487–493. [Google Scholar]
- Azarifar, D.; Ghasemnejad, H. Microwave-assisted synthesis of some 3,5-arylated 2-pyrazolines. Molecules 2003, 8, 642–648. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis and antimicrobial evaluation of some pyrazole derivatives. Molecules 2012, 17, 4962–4971. [Google Scholar] [CrossRef]
- Kant, R.; Gupta, V.G.; Kapoor, K.; Sapnakumari, M.; Sarojini, B.K.; Narayana, B. (1Z)-1-[(2E)-3-(4-bromophenyl)-1-(4-fluorophenyl)prop-2-en-1-ylidene]-2-(2,4-dinitrophenyl)hydrazine. Acta Crystallogr. E 2012, 68, o2193. [Google Scholar]
- Amir, M.; Kumar, H.; Khan, S. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Hes, R.V.; Wellinga, K.; Grosscurt, A.C. 1-Phenylcarbamoyl-2-pyrazolines: A new class of insecticides. 2. Synthesis and insecticidal properties of 3,5-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J. Agric. Food Chem. 1978, 26, 915–918. [Google Scholar] [CrossRef]
- Rahman, M.A.; Siddiqui, A.A. Pyrazoline Derivatives: A Worthy insight into the recent advances and potential pharmacological activities. Int. J. Pharm. Sci. Drug Res. 2010, 2, 165–175. [Google Scholar]
- Wiley, R.H.; Jarboe, C.H.; Hayes, F.N.; Hansbury, E.; Nielsen, J.T.; Callahan, P.X.; Sellars, M. 1,3,5-Triaryl-2-pyrazolines for use as scintillation solutes. J. Org. Chem. 1958, 23, 732–738. [Google Scholar]
- Zhang, X.H.; Wu, S.K.; Gao, Z.Q.; Lee, C.S.; Lee, S.T.; Kwong, H.L. Pyrazoline derivatives for blue color emitter in organic electroluminescent devices. Thin Solid Films 2000, 371, 40–46. [Google Scholar] [CrossRef]
- Fun, H.K.; Ooi, C.W.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[3-(4-Fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Acta Crystallogr. E 2012, 68, o2634. [Google Scholar]
- Fun, H.K.; Loh, W.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Acta Crystallogr. E 2012, 68, o2586. [Google Scholar]
- Fun, H.K.; Loh, W.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]butan-1-one. Acta Crystallogr. E 2012, 68, o2655–o2656. [Google Scholar]
- Fun, H.K.; Chia, T.S.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. 5-(4-Bromophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole. Acta Crystallogr. E 2012, 68, o2680. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Edit. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loh, W.-S.; Quah, C.K.; Chia, T.S.; Fun, H.-K.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K. Synthesis and Crystal Structures of N-Substituted Pyrazolines. Molecules 2013, 18, 2386-2396. https://doi.org/10.3390/molecules18022386
Loh W-S, Quah CK, Chia TS, Fun H-K, Sapnakumari M, Narayana B, Sarojini BK. Synthesis and Crystal Structures of N-Substituted Pyrazolines. Molecules. 2013; 18(2):2386-2396. https://doi.org/10.3390/molecules18022386
Chicago/Turabian StyleLoh, Wan-Sin, Ching Kheng Quah, Tze Shyang Chia, Hoong-Kun Fun, Majal Sapnakumari, Badiadka Narayana, and Balladka Kunhanna Sarojini. 2013. "Synthesis and Crystal Structures of N-Substituted Pyrazolines" Molecules 18, no. 2: 2386-2396. https://doi.org/10.3390/molecules18022386
APA StyleLoh, W.-S., Quah, C. K., Chia, T. S., Fun, H.-K., Sapnakumari, M., Narayana, B., & Sarojini, B. K. (2013). Synthesis and Crystal Structures of N-Substituted Pyrazolines. Molecules, 18(2), 2386-2396. https://doi.org/10.3390/molecules18022386




