Euphorbia formosana Root Extract Induces Apoptosis by Caspase-Dependent Cell Death via Fas and Mitochondrial Pathway in THP-1 Human Leukemic Cells
Abstract
:1. Introduction
2. Results
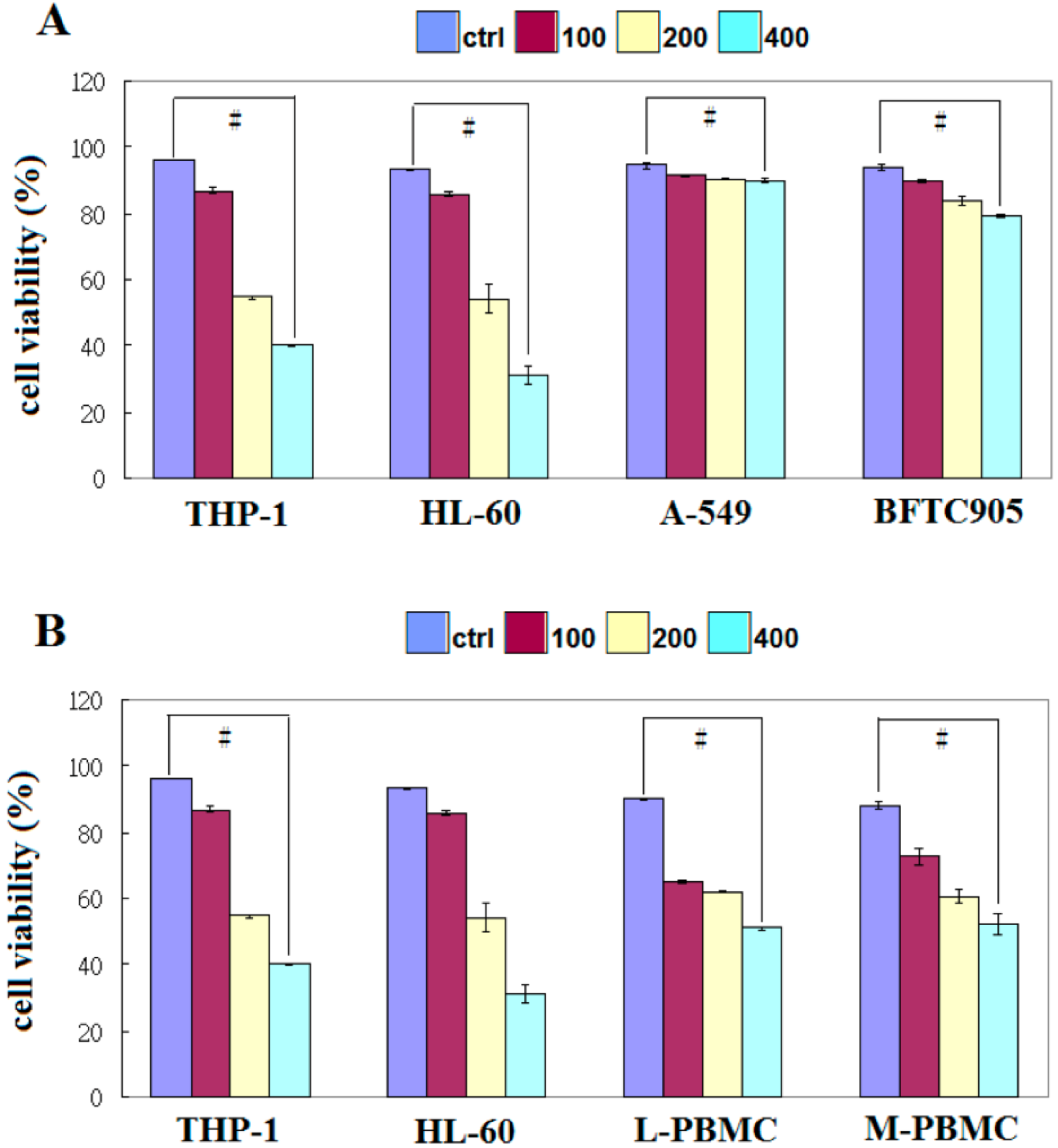
2.1. EFW Specifically Inhibits the Growth of Leukemic Cancer Cells
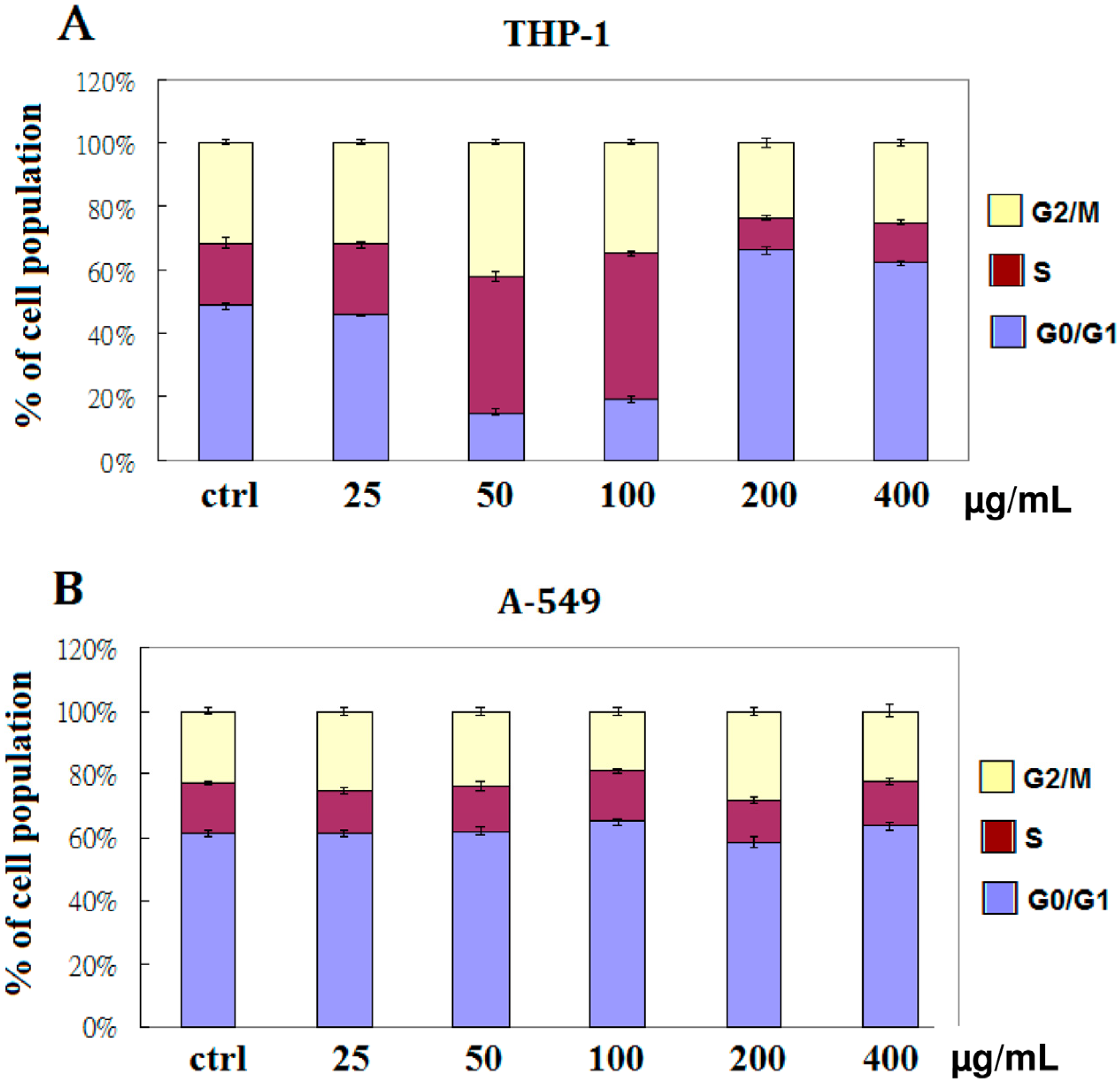
2.2. EFW Specifically Induces Cell Cycle Arrest
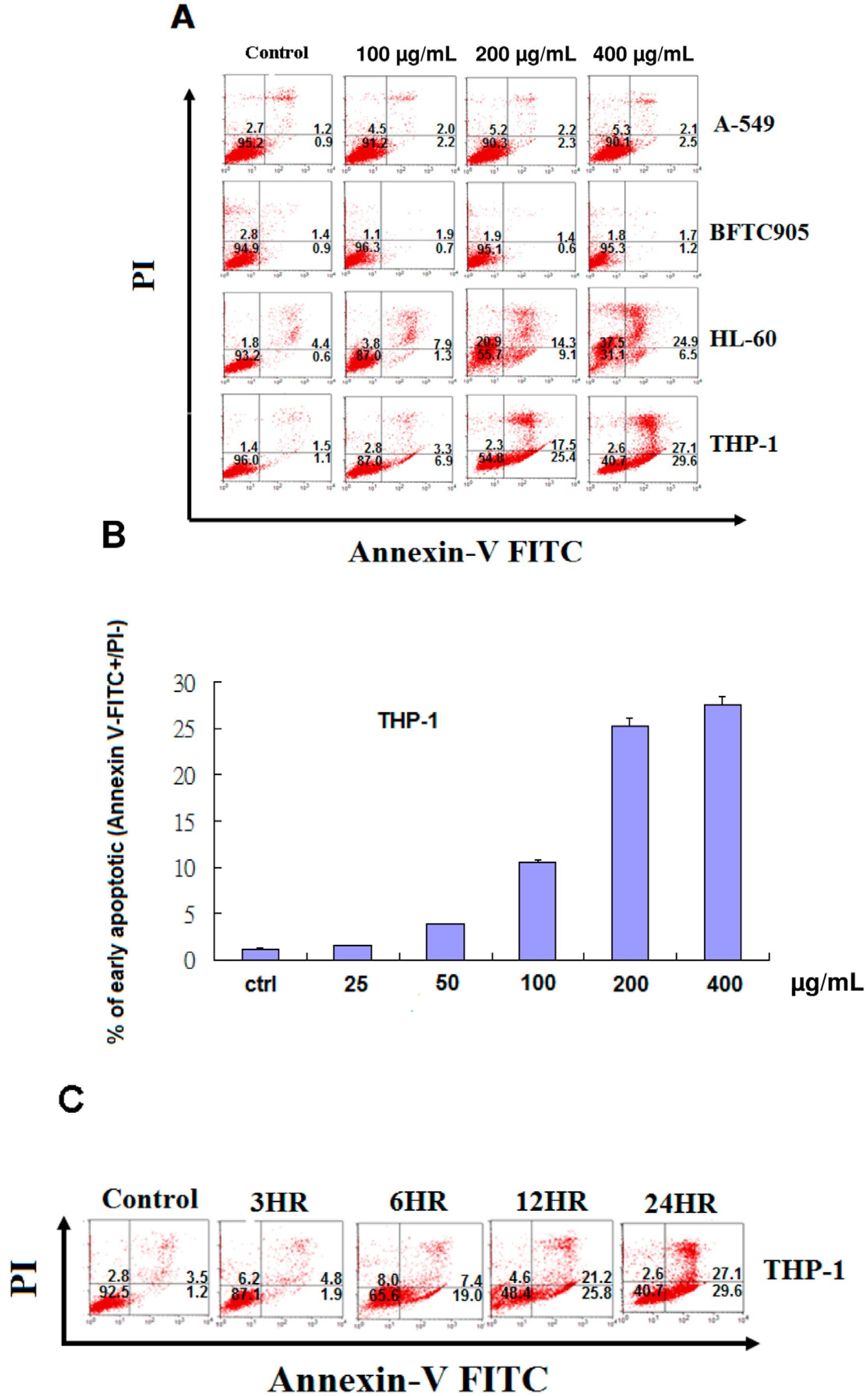
2.3. EFW Selectively Promoted Apoptosis for Leukemic Cells but not for Solid Human Cancer Cell Lines
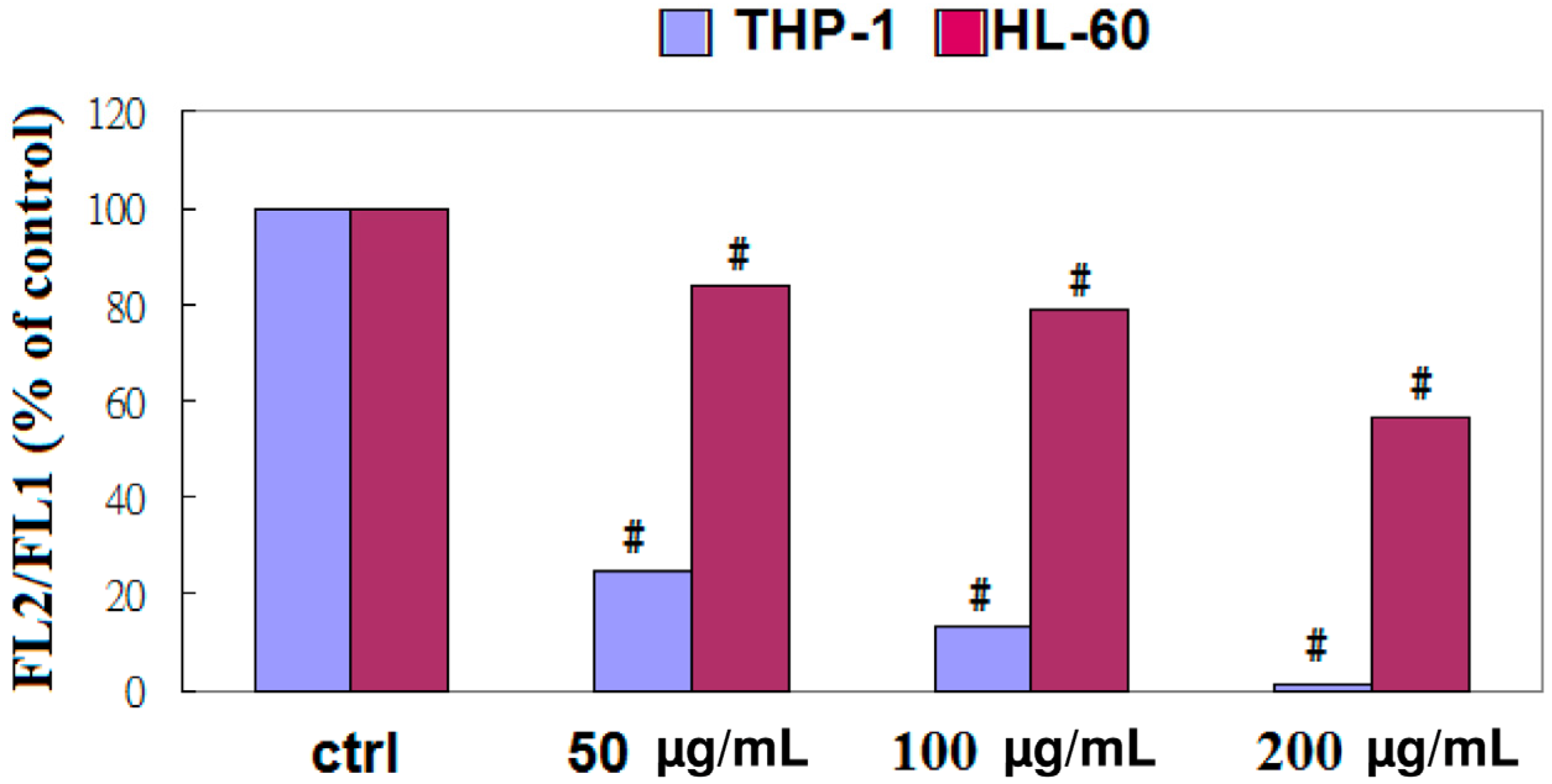
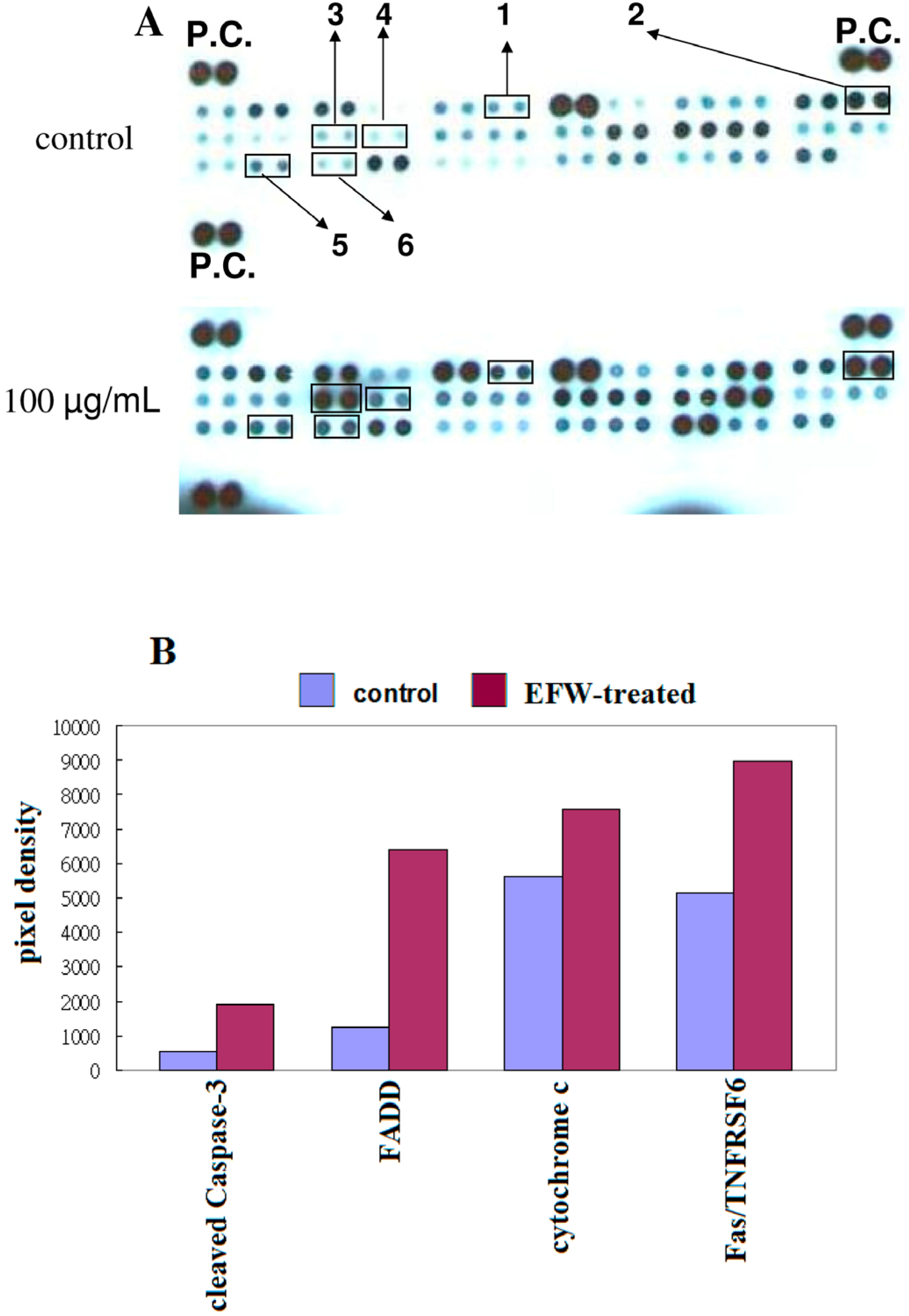
2.4. Apoptosis of THP-1 Cells by EFW via a Mitochondrial Pathway
2.5. Apoptotic Pathways in THP-1 Cells Induced by EFW
3. Discussion
4. Experimental
4.1. Preparing Water Extracts from Euphorbia formosana
4.2. Cell Lines and Culture
4.3. Reagents
4.4. Cell Viability Assay
4.5. Cell Cycle Analysis
4.6. Human Apoptosis Antibody Array
4.7. Isolating PBMCs and Purification of Lymphocytes and Monocytes
4.8. Detecting Apoptotic Cells with Annexin V-FITC and Propidium Iodide Staining
4.9. Assessment of Mitochondrial Membrane Potential (ΔΨm)
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the european leukemianet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Ait-Mohamed, O.; Battisti, V.; Joliot, V.; Fritsch, L.; Pontis, J.; Medjkane, S.; Redeuilh, C.; Lamouri, A.; Fahy, C.; Rholam, M.; et al. Acetonic extract of buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One 2011, 6, e24537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in mcf-7 cells via a mechanism involving the ros-dependent jnk activation and mitochondria-mediated pathways. PLoS One 2011, 6, e27441. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by leaf extract of ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.B.; Raychoudhuri, A.; Steele, R.; Nerurkar, P. Bitter melon (momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 2010, 70, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W.; et al. Inhibition of cancer cell proliferation and prostaglandin e2 synthesis by scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar] [PubMed]
- Scheck, A.C.; Perry, K.; Hank, N.C.; Clark, W.D. Anticancer activity of extracts derived from the mature roots of scutellaria baicalensis on human malignant brain tumor cells. BMC Complement. Altern. Med. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.T.; Wang, C.J.; Hung, G.U.; Wu, C.H.; Lee, H.J. Aqueous extract of shi-liu-wei-liu-qi-yin induces g2/m phase arrest and apoptosis in human bladder carcinoma cells via fas and mitochondrial pathway. Evid. Based Complement. Alternat. Med. 2011, 2011, nep016. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, K.I.; Park, H.S.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Hah, Y.S.; Chung, H.J.; et al. Flavonoids isolated from korea citrus aurantium l. Induce g2/m phase arrest and apoptosis in human gastric cancer ags cells. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.A.; Timotijevic, G.S.; Miljkovic, D.M.; Radovic, J.M.; Maksimovic-Ivanic, D.D.; Dekanski, D.P.; Stosic-Grujicic, S.D. Multiple antimelanoma potential of dry olive leaf extract. Int. J. Cancer 2011, 128, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Hsieh, C.R.; Hsiao, G.; Chen, P.Y.; Chang, M.L.; Yin, H.W.; Lee, T.H.; Lee, C.K. Regulated expressions of mmp-2, -9 by diterpenoids from euphorbia formosana hayata. Molecules 2012, 17, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the swedish acute leukemia registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Rollig, C.; Thiede, C.; Gramatzki, M.; Aulitzky, W.; Bodenstein, H.; Bornhauser, M.; Platzbecker, U.; Stuhlmann, R.; Schuler, U.; Soucek, S.; et al. A novel prognostic model in elderly patients with acute myeloid leukemia: Results of 909 patients entered into the prospective aml96 trial. Blood 2010, 116, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.E. Apoptosis in cancer therapy: Crossing the threshold. Cell 1994, 78, 539–542. [Google Scholar] [CrossRef]
- Bose, R.; Verheij, M.; Haimovitz-Friedman, A.; Scotto, K.; Fuks, Z.; Kolesnick, R. Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell 1995, 82, 405–414. [Google Scholar] [CrossRef]
- Barry, M.A.; Behnke, C.A.; Eastman, A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990, 40, 2353–2362. [Google Scholar] [CrossRef]
- Hannun, Y.A. Apoptosis and the dilemma of cancer chemotherapy. Blood 1997, 89, 1845–1853. [Google Scholar] [PubMed]
- Friesen, C.; Fulda, S.; Debatin, K.M. Cytotoxic drugs and the cd95 pathway. Leukemia 1999, 13, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000, 102, 1–4. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [PubMed]
- Boesen-de Cock, J.G.; de Vries, E.; Williams, G.T.; Borst, J. The anti-cancer drug etoposide can induce caspase-8 processing and apoptosis in the absence of cd95 receptor-ligand interaction. Apoptosis 1998, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; Tepper, C.G.; Seldin, M.F.; O’Rourke, K.; Kischkel, F.C.; Hellbardt, S.; Krammer, P.H.; Peter, M.E.; Dixit, V.M. Fadd/mort1 is a common mediator of cd95 (fas/apo-1) and tumor necrosis factor receptor-induced apoptosis. J. Boil. Chem. 1996, 271, 4961–4965. [Google Scholar]
- Boldin, M.P.; Goncharov, T.M.; Goltsev, Y.V.; Wallach, D. Involvement of mach, a novel mort1/fadd-interacting protease, in fas/apo-1- and tnf receptor-induced cell death. Cell 1996, 85, 803–815. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; O’Rourke, K.; Tewari, M.; Dixit, V.M. Fadd, a novel death domain-containing protein, interacts with the death domain of fas and initiates apoptosis. Cell 1995, 81, 505–512. [Google Scholar] [CrossRef]
- Muzio, M.; Chinnaiyan, A.M.; Kischkel, F.C.; O’Rourke, K.; Shevchenko, A.; Ni, J.; Scaffidi, C.; Bretz, J.D.; Zhang, M.; Gentz, R.; et al. Flice, a novel fadd-homologous ice/ced-3-like protease, is recruited to the cd95 (fas/apo-1) death-inducing signaling complex. Cell 1996, 85, 817–827. [Google Scholar] [CrossRef]
- Hirata, H.; Takahashi, A.; Kobayashi, S.; Yonehara, S.; Sawai, H.; Okazaki, T.; Yamamoto, K.; Sasada, M. Caspases are activated in a branched protease cascade and control distinct downstream processes in fas-induced apoptosis. J. Exp. Med. 1998, 187, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Solary, E.; Hammann, A.; Martin, F.; Dimanche-Boitrel, M.T. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J. Natl. Cancer Inst. 1997, 89, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Wilhelm, D.; Bohler, T.; Angel, P.; Debatin, K.M. Activation of cd95 (apo-1/fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 1997, 16, 6200–6208. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Solary, E.; Hammann, A.; Dimanche-Boitrel, M.T. Fas ligand-independent, fadd-mediated activation of the fas death pathway by anticancer drugs. J. Biol. Chem. 1999, 274, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Merhi, F.; Bauvois, B. Mechanistic insights into the antileukemic activity of hyperforin. Curr. Cancer Drug Targets 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zaher, M.; Mirshahi, M.; Nuraliev, Y.; Sharifova, M.; Bombarda, I.; Marie, J.P.; Billard, C. The bh3-only protein noxa is stimulated during apoptosis of chronic lymphocytic leukemia cells triggered by m2yn, a new plant-derived extract. Int. J. Oncol. 2011, 39, 965–972. [Google Scholar] [PubMed]
Sample Availability: Not available. |
| I. Polyphenols | ||
| Ellagic acid | 3,3'-Di-O-methylellagic acid | 3,3',4,4'-Tetra-O-methylellagic acid |
| 3,3'-Di-O-methylellagic acid-4'-O-b-xylopyranoside | 3,3'-Di-O-methylellagic acid-4'-O-b-glucoside | 3,3'-Di-O-methylellagic acid-4'-O-b-arabinopyranoside |
| 3'-O-methyl-3,4-methylenedioxyellagic acid | Gallic acid | Methyl gallate |
| Methyl brevifolincarboxylate | Brevifolin | Phyllanthusiin E |
| Dehydrochebulic acid trimethyl ester | Octacosyl ferulate | |
| II. Steroids | ||
| β-Sitosterol | β-Sitosteryl-3-O-glucoside | β-Sitostenone |
| Ergosterol peroxide | ||
| III. Peptide | ||
| Aurantiamide acetate | ||
| IV. Furan | ||
| 5-Hydroxymethylfurfural | ||
| V. Coumarins | ||
| Scopoletin | Euoniside | 6-Methoxy-7,8-methylenedioxycoumarin |
| VI. Diterpenes | ||
| Helioscopinolide E | Isopimara-7,15-dien-3-one | Epi-manool |
| Larixol | ||
| VII. Triterpenes | ||
| Euphol | Glutinone | Cycloart-23-ene-3b,25-diol |
| Tirucalla-8,25-diene-3,24-diol | ||
| VIII. Flavonoids | ||
| Quercetin-3-O-α-L-rhamnoside | Kaempferol-3-O-α-l-rhamnoside | |
| IX. Others | ||
| 4-Methyl-5,6-dihydropyran-2-one | 1,3,4,6-tetra-O-galloyl-β-d-glucopyranose | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsieh, Y.-J.; Chang, C.-J.; Wan, C.-F.; Chen, C.-P.; Chiu, Y.-H.; Leu, Y.-L.; Peng, K.-C. Euphorbia formosana Root Extract Induces Apoptosis by Caspase-Dependent Cell Death via Fas and Mitochondrial Pathway in THP-1 Human Leukemic Cells. Molecules 2013, 18, 1949-1962. https://doi.org/10.3390/molecules18021949
Hsieh Y-J, Chang C-J, Wan C-F, Chen C-P, Chiu Y-H, Leu Y-L, Peng K-C. Euphorbia formosana Root Extract Induces Apoptosis by Caspase-Dependent Cell Death via Fas and Mitochondrial Pathway in THP-1 Human Leukemic Cells. Molecules. 2013; 18(2):1949-1962. https://doi.org/10.3390/molecules18021949
Chicago/Turabian StyleHsieh, Yi-Jen, Chih-Jui Chang, Chin-Feng Wan, Chin-Piao Chen, Yi-Han Chiu, Yann-Lii Leu, and Kou-Cheng Peng. 2013. "Euphorbia formosana Root Extract Induces Apoptosis by Caspase-Dependent Cell Death via Fas and Mitochondrial Pathway in THP-1 Human Leukemic Cells" Molecules 18, no. 2: 1949-1962. https://doi.org/10.3390/molecules18021949
APA StyleHsieh, Y.-J., Chang, C.-J., Wan, C.-F., Chen, C.-P., Chiu, Y.-H., Leu, Y.-L., & Peng, K.-C. (2013). Euphorbia formosana Root Extract Induces Apoptosis by Caspase-Dependent Cell Death via Fas and Mitochondrial Pathway in THP-1 Human Leukemic Cells. Molecules, 18(2), 1949-1962. https://doi.org/10.3390/molecules18021949





