RETRACTED: Cytotoxicity and Anti-Inflammatory Activity of Methylsulfanyl-Triazoloquinazolines
Abstract
1. Introduction
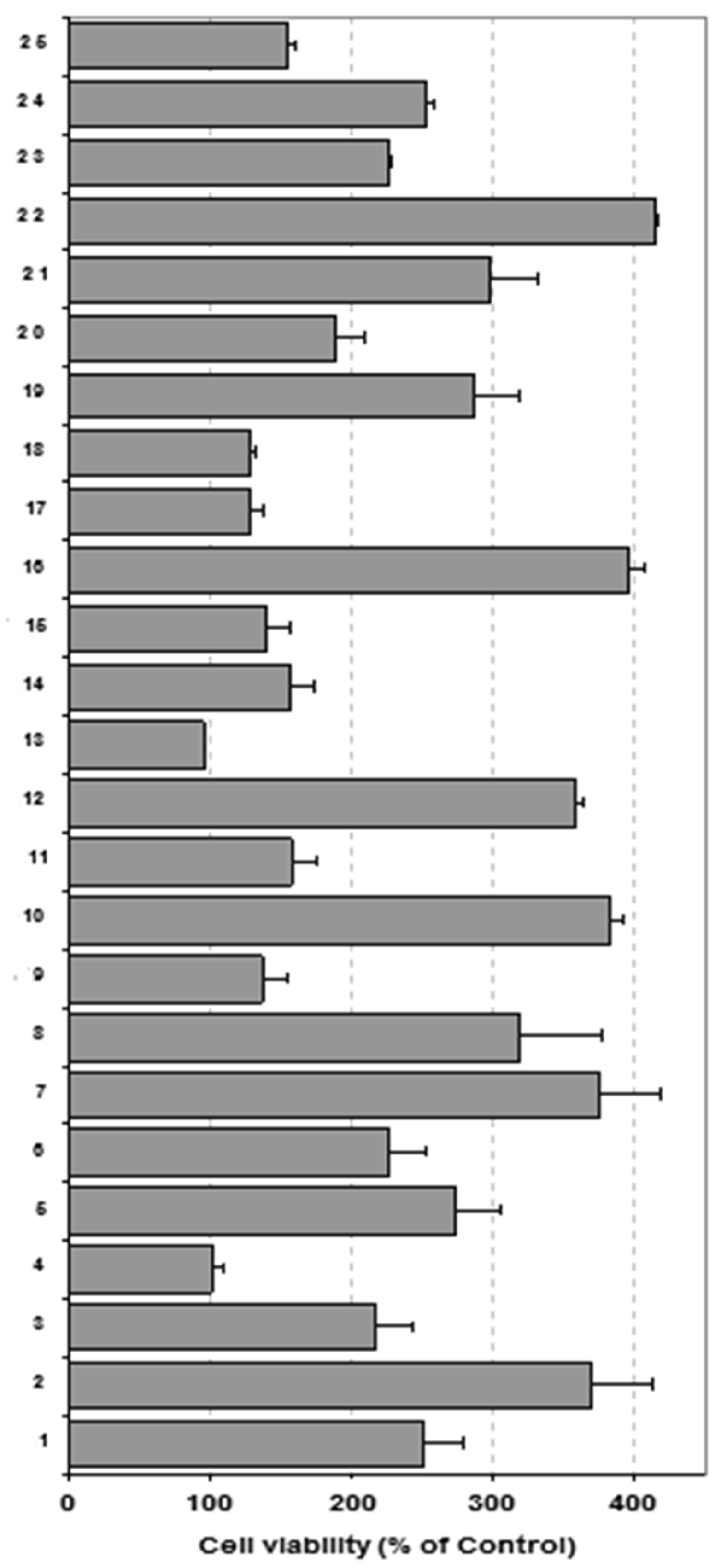
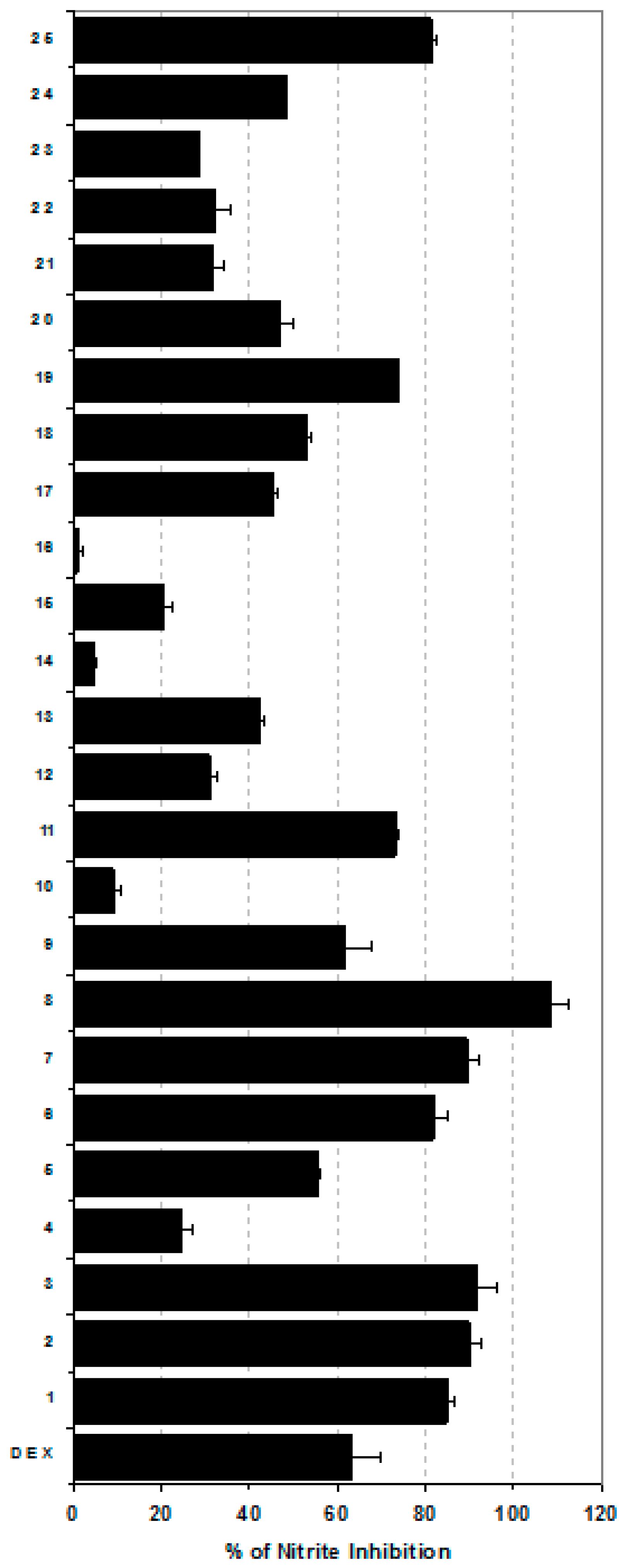
2. Results and Discussion
3. Experimental
3.1. Cell Culture
3.2. Cytotoxicity Assay
3.3. Macrphage Viability Assay
3.4. Nitrite Assay
3.5. Determination of Tumor Necrosis Factor-α and Prostaglandin E2
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Tajana, G.; Iuvone, T. Nuclear factor-kappaB regulates inflammatory cell apoptosis and phagocytosis in rat carrageenin-sponge implant model. Am. J. Pathol. 2004, 165, 115–126. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Hodge-Dufour, J.; Marino, M.W.; Horton, M.R. Inhibition of interferon gamma induced interleukin 12 production: A potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1998, 95, 13806–13811. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Wyllie, A.H.; Henson, J.E.; Walport, M.J.; Henson, P.M.; Haslett, C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 1989, 83, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Fadok, V.A. Corpse clearance defines the meaning of cell death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.P.; Fadok, V.A.; Bratton, D.; Henson, P.M. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J. Immunol. 1999, 163, 6164–6172. [Google Scholar] [PubMed]
- Huynh, M.L.N.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002, 109, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy, American Journal of Physiology. J. Am. Physiol. Gast. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C.; Austel, K.E. Paths to Better and Safer Drugs, Modern Drug Research; Marcel Dekker: New York, NY, USA, 1989; pp. 243–273. [Google Scholar]
- Roth, H.J.; Fenner, H. Arzneistoffe, 3rd, ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2000; p. 51. [Google Scholar]
- Harris, C.R.; Thorarensen, A. Advances in the discovery of novel antibacterial agents during the year 2002. Curr. Med. Chem. 2004, 11, 2213–2243. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Gohlmann, H.W.; Neefs, J.M.; Winkler, H.; van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Vangapandu, S.; Jain, M.; Jain, R.; Kaur, S.; Singh, P.P. Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorg. Med. Chem. 2004, 12, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Piras, S.; Palomba, M.; Jabes, D.; Molicotti, P.; Zanetti, S. Anti-mycobacterial activity of quinolones. Triazoloquinolones a new class of potent anti-mycobacterial agents. Anti-Infective Agents Med. Chem. 2008, 7, 134–147. [Google Scholar] [CrossRef]
- Padia, J.K.; Field, M.; Hilton, J.; Meecham, K.; Pablo, J.; Pinnock, R.; Roth, B.D.; Singh, L.; Suman-Chauhan, N.; Trivedi, B.K.; et al. Novel nonpeptide CCK-B antagonists: Design and development of quinazolinone derivatives as Potent, Selective, and orally active CCKB Antagonists. J. Med. Chem. 1998, 41, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.Y.; Hour, M.J.; Kuo, S.C.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Nampoothiri, P.; Hackl, T.; Hamel, E.; et al. Antitumor agents. Part 204: Synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg. Med. Chem. Lett. 2001, 11, 1193–1196. [Google Scholar] [CrossRef]
- Kenichi, O.; Yoshihisa, Y.; Toyonari, O.; Toru, I.; Yoshio, I. Studies on 4(1H)-quinazolinones. 5. synthesis and antiinflammatory activity of 4(1H)-quinazolinone derivatives. J. Med. Chem. 1985, 28, 568–576. [Google Scholar]
- Buchanan, J.G.; Sable, H.Z. Selective Organic Transformations; Thygarajan, B.S., Ed.; Wiley-Interscience: New York, NY, USA, 1972; Volume 2, pp. 1–95. [Google Scholar]
- Hamidian, H.; Tikdari, A.M.; Khabazzadeh, H. Synthesis of new 4(3H)-quinazolinone derivatives using 5(4H)-oxazolones. Molecules 2006, 11, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Birendra, N.G.; Jiban, C.S.K.; Jogendra, N.B. Quinoline-based fused heterocyclic systems are found as potential anticancer. J. Heterocycl. Chem. 1984, 21, 1225–1229. [Google Scholar]
- Kothari, P.J.; Mehlhoff, M.A.; Singh, S.P.; Parmar, S.S.; Stenberg, V.I. Synthesis of some new 5-methyl-2-benzoxazolinone derivatives and investigation on their analgesic-antiinflammatory activities. J. Heterocycl. Chem. 1980, 17, 1369–1372. [Google Scholar] [CrossRef]
- Sengupta, A.K.; Misra, H.K. Studies on potential pesticides 13 Synthesis and evaluation of s-(3-substituted-phenoxymethyl-4-aryl/cyclohexyl-4h-1,2,4-triazol-5-yl)-2-mercaptomethyl benzimidazoles for anti-bacterial and insecticidal activities. J. Indian Chem. Soc. 1981, 8, 508. [Google Scholar]
- Sarmah, S.C.; Bahel, S.C. Synthesis of aryloxy/aryl acetyl thiosemicarbazides, substituted 1,3,4-oxadiazoles, 1,3,4-thiadiazoles, 1,2,4-triazoles and related compounds as potential fungicides. J. Indian Chem. Soc. 1982, 59, 877–880. [Google Scholar]
- Francis, J.E.; Cash, W.D.; Psychoyos, S.; Ghai, G.; Wenk, P.; Friedmann, R.C.; Atkins, C.; Warren, V.; Furness, P.; Hyun, T.L.; et al. Structure-activity profile of a series of novel triazoloquinazoline adenosine antagonists. J. Med. Chem. 1988, 31, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; De Zwart, M.; Chang, L.; Moro, S.; Kuenzel, J.; Melman, N.; Jzerman, A.P.; Jacobson, K.A. Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) having high potency at the human A2B and A3 receptor subtypes. J. Med. Chem. 1998, 41, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Ongini, E.; Monoppoli, A.; Cacciari, B.; Baraldi, P.G. Selective adenosine A2A receptor antagonists. Il Farmaco 2001, 56, 87–90. [Google Scholar] [CrossRef]
- Francis, J.E.; Cash, W.D.; Barbaz, B.S.; Bernard, P.S.; Lovell, R.A.; Mazzenga, G.C.; Friedmann, R.C.; Hyun, J.L.; Braunwalder, A.F.; Loo, P.S.; et al. Synthesis and benzodiazepine binding activity of a series of novel [1,2,4]triazolo[1,5-c]quinazolin-5(6H)-ones. J. Med. Chem. 1991, 34, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Giridhar, R.; Yadav, M.R. Synthesis and pharmacological investigation of novel 1-substituted-4-phenyl-1,2,4-triazolo[4,3-a]quinazolin-5(4H)-ones as a new class of H1-antihistaminic agents. Bioorg. Med. Chem. Lett. 2005, 15, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Solomon, V.R.; Murugan, M. Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new class of H1-antihistaminic agents. Bioorg. Med. Chem. 2007, 15, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Geffken, D.; Koellner, M. A new series of 2-Alkoxy(aralkoxy)-[1,2,4]triazolo[1,5-a]quinazolin-5-ones as Adenosine Receptor Antagonists. Chem. Pharm. Bull. 2011, 59, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Geffken, D. Synthesis of novel 2-alkoxy(aralkoxy)-4H-[1,2,4]triazolo[1,5-a]quinazolin-5-ones starting with dialkyl-N-cyanoimidocarbonates. J. Heterocycl. Chem. 2011, 48, 656–662. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Geffken, D. Novel synthesis of 2-alkoxy(aralkoxy)-5-chloro[1,2,4]-triazolo[1,5-a]quinazoline and their derivatives. Heterocycles 2010, 81, 1843–1859. [Google Scholar] [CrossRef]
- Al-Salahi, R. Synthesis and reactivity of [1,2,4]triazolo-annelated quinazolines. Molecules 2010, 15, 7016–7034. [Google Scholar] [CrossRef]
- Jantova, S.; Ovadekova, R.; Letasiova, S.; Spirkova, K.; Stankovsky, S. Anti-microbial activity of some substituted triazoloquinazolines. Folia Microbiol. 2005, 50, 90–94. [Google Scholar] [CrossRef]
- Al-Omary, M.F.; Abou-zeid, L.A.; Nagi, M.N.; Habib, E.E.; Abdel-Aziz, A.; El-Azab, A.S.; Abdel-Hamide, S.G.; Al-Omar, M.A.; Al-Obaid, A.M.; El-Subbagh, H.I. Non-classical antifolates. Part 2: Synthesis, Biological evaluation, And molecular modeling study of some new 2,6-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2010, 18, 2849–2863. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Geffken, D. Synthesis of 2-methylsulfanyl-4H-[1,2,4]triazolo[1,5-a]quinazolin-5-one and derivatives. Synth. Comm. 2011, 41, 3512–3523. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S. The molecular biology of cancer. Mol. Asp. Med. 2000, 21, 167–223. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Rooseboom, M.; Commandeur, J.N.M.; Vermeulen, N.P.E. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004, 56, 53–102. [Google Scholar] [CrossRef] [PubMed]
- Alper, A.E.; Taurine, A. Thiazolo[3,2-a]benzimidazoles. Can. J. Chem. 1967, 45, 2903–2912. [Google Scholar] [CrossRef]
- Omiecinski, C.J.; Hassett, C.; Hosagrahara, V. Epoxide hydrolase-polymorphism and role in toxicology. Toxicol. Lett. 2000, 112–113, 365–370. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Meth. 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Gerhaeuser, C.; Elke Heiss, K.K.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.-Y.; Sitthimonchai, S.; Frank, N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003, 523–524, 163–172. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–25 are available from the authors. |

| Compounds | Cells | |||
|---|---|---|---|---|
| Hep-G2 | MCF-7 | HCT-116 | HeLa | |
| 1 | 29.88 ± 3.02 | >50 | 46.64 ± 0.62 | >50 |
| 2 | >50 | >50 | 29.62 ± 1.94 | >50 |
| 3 | >50 | >50 | 49.83 ± 2.27 | >50 |
| 4 | >50 | >50 | 31.19 ± 1.36 | >50 |
| 5 | 36.41 ± 3.07 | >50 | 46.58 ± 0.81 | >50 |
| 6 | >50 | >50 | >50 | >50 |
| 7 | 42.28 ± 4.69 | >50 | >50 | >50 |
| 8 | >50 | >50 | >50 | >50 |
| 9 | >50 | >50 | >50 | >50 |
| 10 | >50 | >50 | >50 | >50 |
| 11 | >50 | >50 | >50 | >50 |
| 12 | >50 | >50 | >50 | >50 |
| 13 | 9.34 ± 1.5 | >50 | 11.51 ± 2.87 | >50 |
| 14 | 31.22 ± 3.33 | >50 | 41.25 ± 1.93 | >50 |
| 15 | 22.73 ± 3.7 | >50 | >50 | >50 |
| 16 | 25.20 ± 1.96 | >50 | >50 | >50 |
| 17 | 19.22 ± 4.23 | >50 | 17.39 ± 0.15 | >50 |
| 18 | 22.69 ± 1.81 | >50 | >50 | >50 |
| 19 | 28.29 ± 3.42 | >50 | >50 | >50 |
| 20 | >50 | >50 | >50 | >50 |
| 21 | >50 | >50 | >50 | >50 |
| 22 | >50 | >50 | >50 | >50 |
| 23 | >50 | >50 | >50 | >50 |
| 24 | 26.93 ± 2.74 | >50 | >50 | >50 |
| 25 | 42.46 ± 4.11 | >50 | >50 | >50 |
| Paclitaxel | 0.51 ± 0.10 | 0.99 ± 0.20 | 0.46 ± 0.13 | 0.54 ± 0.08 |
| Sample | TNF-α (pg/mg protein) | PGE2 (pg/mg protein) |
|---|---|---|
| Control | 81.2 ± 11.64 | 34.4 ± 5.06 |
| LPS | 5740.6 ± 511.22 | 3101 ± 110.02 a) |
| LPS + 1 | 1345.8 ± 162.11 a) | 1345.8 ± 162.11 a) |
| LPS + 2 | 904.3 ± 84.45 a) | 1003.8 ± 183.58 a) |
| LPS + 3 | 215.8 ± 24.61 a) | 319.8 ± 34.81 a) |
| LPS + 4 | 5136.9 ± 498.36 | 3186.8 ± 140.60 |
| LPS + 5 | 2221.1 ± 325.52 | 2881.1 ± 323.32 |
| LPS + 6 | 1041.3 ± 194.21 a) | 1117.7 ± 293.19 |
| LPS + 7 | 503.2 ± 24.33 a) | 611.7 ± 62.24 a) |
| LPS + 8 | 195.1 ± 22.50 a) | 205.8 ± 23.52 a) |
| LPS + 9 | 3621.5 ± 225.24 | 3096.2 ± 208.03 |
| LPS + 10 | 5261.8 ± 488.47 | 3003. ± 294.21 |
| LPS + 11 | 809.2 ± 81.47 a) | 2903.7 ± 424.93 |
| LPS + 12 | 3869.4 ± 381.17 | 3191.3 ± 292.10 |
| LPS + 13 | 3009.6 ± 245.70 | 2548.8 ± 192.98 |
| LPS + 14 | 5016.7 ± 411.36 | 2145.1 ± 144.00 |
| LPS + 15 | 4300.8 ± 398.46 | 2877.5 ± 305.01 |
| LPS + 16 | 5096.2 ± 408.33 | 3221.4 ± 335.24 |
| LPS + 17 | 2043. ± 194.21 | 3361.9 ± 185.47 |
| LPS + 18 | 2403.5 ± 624.33 | 2804.2 ± 183.71 |
| LPS + 19 | 1196.3 ± 202.10 a) | 3069.5 ± 391.17 |
| LPS + 20 | 1546.2 ± 162.78 a) | 3019.4 ± 211.70 |
| LPS + 21 | 1147.1 ± 184.89 a) | 3016.7 ± 421.60 |
| LPS + 22 | 1877.7 ± 195.80 a) | 2708.8 ± 278.26 |
| LPS + 23 | 1500.4 ± 133.24 a) | 1111.5 ± 93.47 a) |
| LPS + 24 | 1666.3 ± 102.78 a) | 1676.2 ± 52.8 a) |
| LPS + 25 | 1167.2 ± 92.17 a) | 1266.4 ± 94.22 a) |
| LPS + DEX | 99.9 ± 12.55 a) | 87.11 ± 11.56 a) |
© 2013 by the author. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Salahi, R.A.; Gamal-Eldeen, A.M.; Alanazi, A.M.; Al-Omar, M.A.; Marzouk, M.A.; Fouda, M.M.G. RETRACTED: Cytotoxicity and Anti-Inflammatory Activity of Methylsulfanyl-Triazoloquinazolines. Molecules 2013, 18, 1434-1446. https://doi.org/10.3390/molecules18021434
Al-Salahi RA, Gamal-Eldeen AM, Alanazi AM, Al-Omar MA, Marzouk MA, Fouda MMG. RETRACTED: Cytotoxicity and Anti-Inflammatory Activity of Methylsulfanyl-Triazoloquinazolines. Molecules. 2013; 18(2):1434-1446. https://doi.org/10.3390/molecules18021434
Chicago/Turabian StyleAl-Salahi, Rashad A., Amira M. Gamal-Eldeen, Amer M. Alanazi, Mohamed A. Al-Omar, Mohamed A. Marzouk, and Moustafa M. G. Fouda. 2013. "RETRACTED: Cytotoxicity and Anti-Inflammatory Activity of Methylsulfanyl-Triazoloquinazolines" Molecules 18, no. 2: 1434-1446. https://doi.org/10.3390/molecules18021434
APA StyleAl-Salahi, R. A., Gamal-Eldeen, A. M., Alanazi, A. M., Al-Omar, M. A., Marzouk, M. A., & Fouda, M. M. G. (2013). RETRACTED: Cytotoxicity and Anti-Inflammatory Activity of Methylsulfanyl-Triazoloquinazolines. Molecules, 18(2), 1434-1446. https://doi.org/10.3390/molecules18021434




