Crystal Structures and Antifungal Activities of Fluorine-Containing Thioureido Complexes with Nickel(II)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characteristic
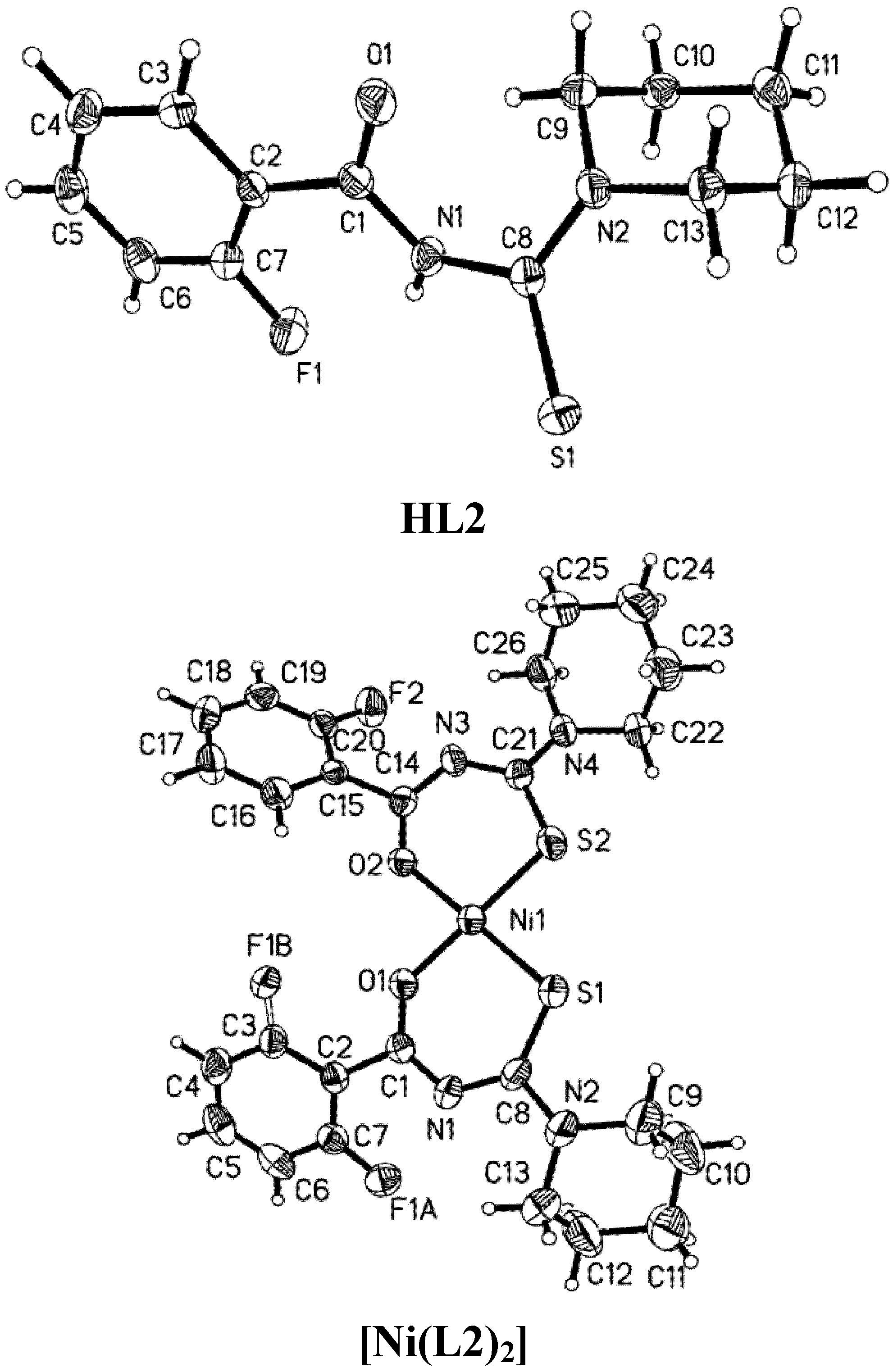
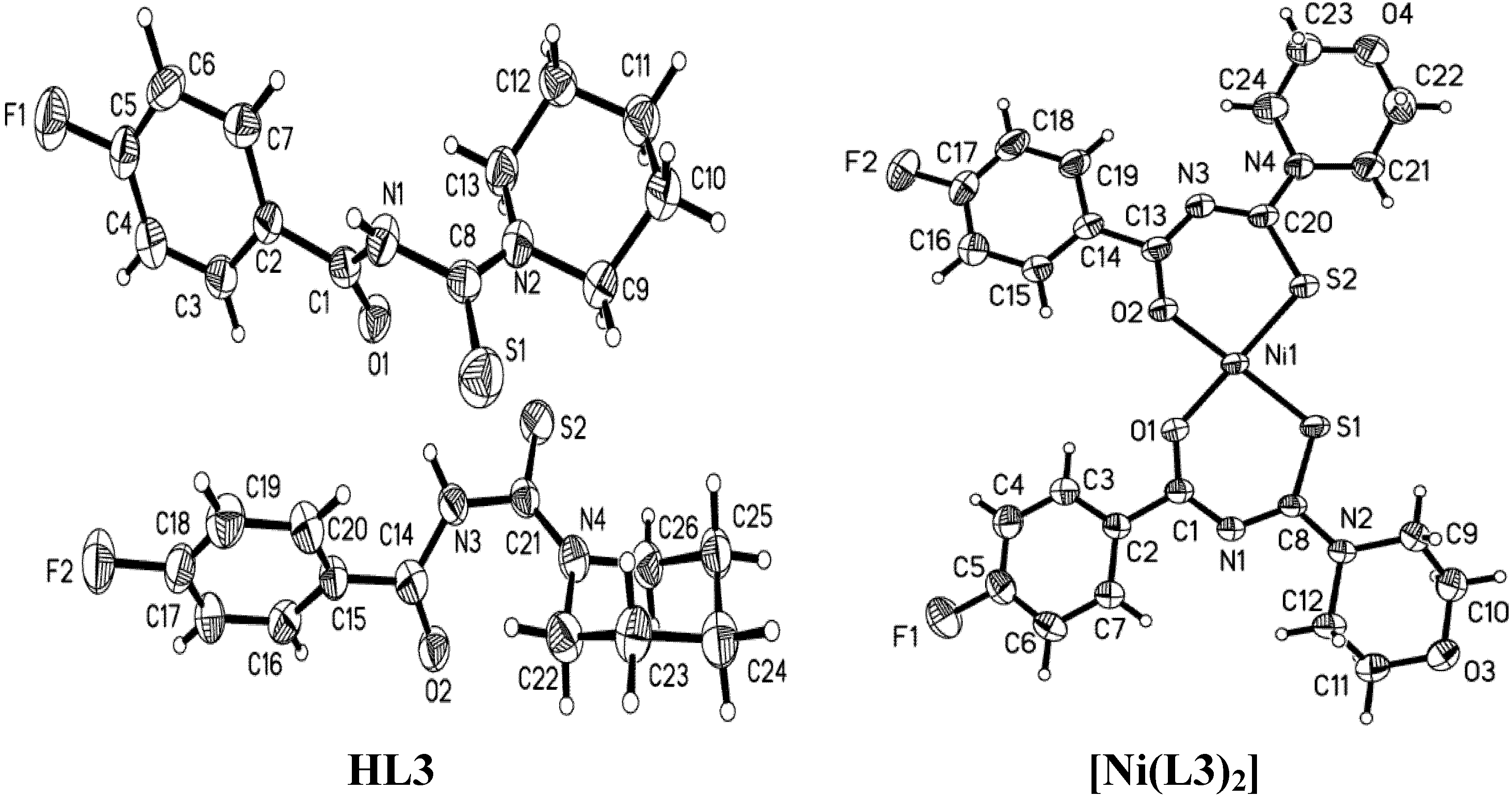
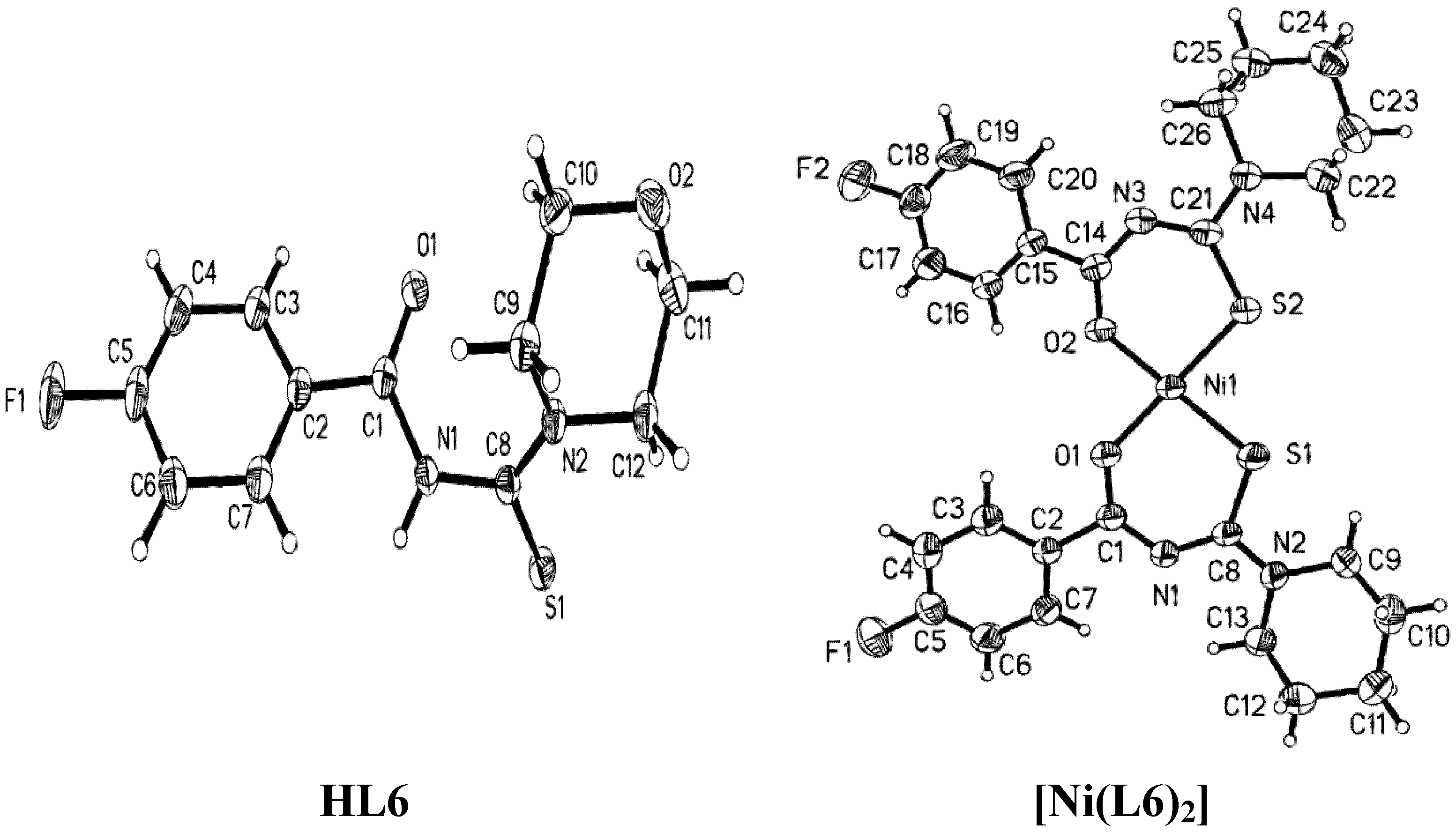
| Parameter | HL2 | [Ni(L2)2] | HL3 | [Ni(L3)2] | HL6 | [Ni(L6)2] |
|---|---|---|---|---|---|---|
| Empirical formula | C13H15FN2OS | C26H28F2N4NiO2S2 | C13H15FN2OS | C26H28F2N4NiO2S2 | C12H13FN2O2S | C24H24F2N4NiO4S2 |
| Formula weight | 266.33 | 589.35 | 266.33 | 589.35 | 268.3 | 593.3 |
| Crystal system | Monoclinic | Orthorhombic | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P 21/c | P b c a | P-1 | P-1 | C 2/c | P-1 |
| a (Å) | 11.843(3) | 11.6111(17) | 8.3759(15) | 9.5530(16) | 21.214(6) | 9.3383(6) |
| b (Å) | 13.282(3) | 9.8778(17) | 11.191(2) | 11.890(2) | 9.7391(16) | 11.5273(9) |
| c (Å) | 8.432(2) | 45.925(7) | 13.960(3) | 12.548(2) | 14.637(7) | 12.7230(8) |
| α (°) | 90 | 90 | 89.981(10) | 104.350(4) | 90 | 105.206(6) |
| β (°) | 108.474(5) | 90 | 89.969(10) | 100.377(3) | 125.91(3) | 101.420(5) |
| γ (°) | 90 | 90 | 79.699(9) | 97.110(4) | 90 | 96.112(6) |
| Z | 4 | 8 | 4 | 2 | 8 | 2 |
| Dcalc (g/cm3) | 1.406 | 1.486 | 1.374 | 1.464 | 1.455 | 1.543 |
| Radiation (MoKα) (Å) | 0.71075 | 0.7107 | 0.7107 | 0.7107 | 0.71075 | 0.71073 |
| μ(Mo Kα) (mm−1) | 0.259 | 0.941 | 0.253 | 0.927 | 0.272 | 0.977 |
| θ Range (°) | 3.03 to 27.50 | 3.02 to 25.35 | 3.17 to 25.34 | 3.01 to 25.35 | 3.34 to 27.50 | 3.03 to 26.37 |
| Reflections collected | 7538 | 34,645 | 12,652 | 12,987 | 10,790 | 13,024 |
| Independent reflections | 2850 | 4799 | 4678 | 4872 | 2773 | 5220 |
| R(int) | 0.022 | 0.0687 | 0.0719 | 0.0292 | 0.0514 | 0.0208 |
| Data | 2850 | 4799 | 4678 | 4872 | 2773 | 5220 |
| restraints | 0 | 0 | 2 | 0 | 0 | 17 |
| Parameters | 165 | 344 | 334 | 335 | 163 | 334 |
| GOF on F2 | 1.011 | 1.009 | 1.098 | 1.079 | 0.999 | 1.023 |
| R1 [I > 2sigma(I)] | 0.0436 | 0.0904 | 0.0838 | 0.0536 | 0.0422 | 0.0406 |
| wR2 [I > 2sigma(I)] | 0.1141 | 0.1785 | 0.1771 | 0.1242 | 0.1084 | 0.1002 |
| R1 [all data] | 0.0551 | 0.11 | 0.1575 | 0.0723 | 0.0636 | 0.0564 |
| wR2 [all data] | 0.1222 | 0.1892 | 0.2080 | 0.1353 | 0.1190 | 0.1105 |
| Largest difference peak (e.A−3) | 0.362 and −0.377 | 0.728 and −0.413 | 0.265 and −0.316 | 0.627 and −0.297 | 0.331 and −0.449 | 0.552 and −0.315 |
| Distances (Å) | HL2 | [Ni(L2)2] | HL3 | [Ni(L3)2] | HL6 | [Ni(L6)2] |
|---|---|---|---|---|---|---|
| Ni(1)-O(1) | 1.859 (4) | 1.864 (2) | 1.861 (18) | |||
| Ni(1)-O(2) | 1.846 (4) | 1.866 (2) | 1.864 (18) | |||
| Ni(1)-S(1) | 2.130 (2) | 2.140 (11) | 2.145 (8) | |||
| Ni(1) -S(2) | 2.147 (2) | 2.139 (11) | 2.142 (8) | |||
| S(1)-C(8) | 1.673 (15) | 1.728 (7) | 1.668 (5) | 1.742 (4) | 1.679 (2) | 1.737 (2) |
| S(2)-C(21) | 1.723 (6) | 1.666 (5) | 1.731 (4) | |||
| S(2)-C(20) | 1.731 (3) | |||||
| O(1)-C(1) | 1.215 (19) | 1.271 (7) | 1.233 (5) | 1.275 (4) | 1.219 (2) | 1.268 (3) |
| O(2)-C(14) | 1.269 (7) | 1.233 (6) | 1.268 (4) | |||
| O(2)-C(13) | 1.272 (3) | |||||
| N(1)-C(1) | 1.378 (18) | 1.319 (7) | 1.358 (6) | 1.312 (4) | 1.392 (2) | 1.321 (3) |
| N(3)-C(14) | 1.309 (7) | 1.358 (6) | 1.319 (5) | |||
| N(3)-C(13) | 1.307 (3) | |||||
| N(1)-C(8) | 1.413 (19) | 1.337 (8) | 1.434 (6) | 1.340 (5) | 1.397 (2) | 1.342 (3) |
| N(3)-C(21) | 1.341 (8) | 1.430 (6) | 1.334 (5) |
2.2. Antifungal Activities
| Samples | Botrytis cinerea | Trichoderma spp. | Myrothecium | Verticillium. spp. |
|---|---|---|---|---|
| Fluconazoleole | 7.0 ± 0.1 | 8.0 ± 0.1 | 7.5 ± 0.1 | 7.5 ± 0.1 |
| HL1 | 31.0 ± 0.0 | 22.5 ± 0.1 | 32.5 ± 0.1 | 34.0 ± 0.0 |
| [Ni(L1)2] | 33.0 ± 0.0 | 31.5 ± 0.1 | 36.0 ± 0.0 | 38.0 ± 0.1 |
| HL2 | 27.5 ± 0.0 | 29.0 ± 0.0 | 27.5 ± 0.1 | 28.0 ± 0.0 |
| [Ni(L2)2] | 31.0 ± 0.1 | 33.0 ± 0.1 | 31.0 ± 0.1 | 31.5 ± 0.1 |
| HL3 | 20.5 ± 0.1 | 18.0 ± 0.1 | 22.5 ± 0.1 | 25.0 ± 0.0 |
| [Ni(L3)2] | 22.5 ± 0.0 | 31.0 ± 0.0 | 29.0 ± 0.1 | 31.0 ± 0.0 |
| HL4 | 2.5 ± 0.1 | 2 ± 0.0 | 2.3 ± 0.0 | 0.8 ± 0.1 |
| [Ni(L4)2] | 29.0 ± 0.1 | 32.5 ± 0.1 | 35.5 ± 0.1 | 35.5 ± 0.1 |
| HL5 | 31.0 ± 0.1 | 27.5 ± 0.0 | 27.5 ± 0.0 | 31.0 ± 0.1 |
| [Ni(L5)2] | 32.5 ± 0.1 | 31.0 ± 0.1 | 31.0 ± 0.1 | 31.5 ± 0.0 |
| HL6 | 8.0 ± 0.0 | 8.5 ± 0.0 | 7.0 ± 0.1 | 8.5 ± 0.0 |
| [Ni(L6)2] | 31.0 ± 0.0 | 25.0 ± 0.0 | 27.5 ± 0.1 | 27.5 ± 0.0 |
2.3. Structure-Activity Relationships
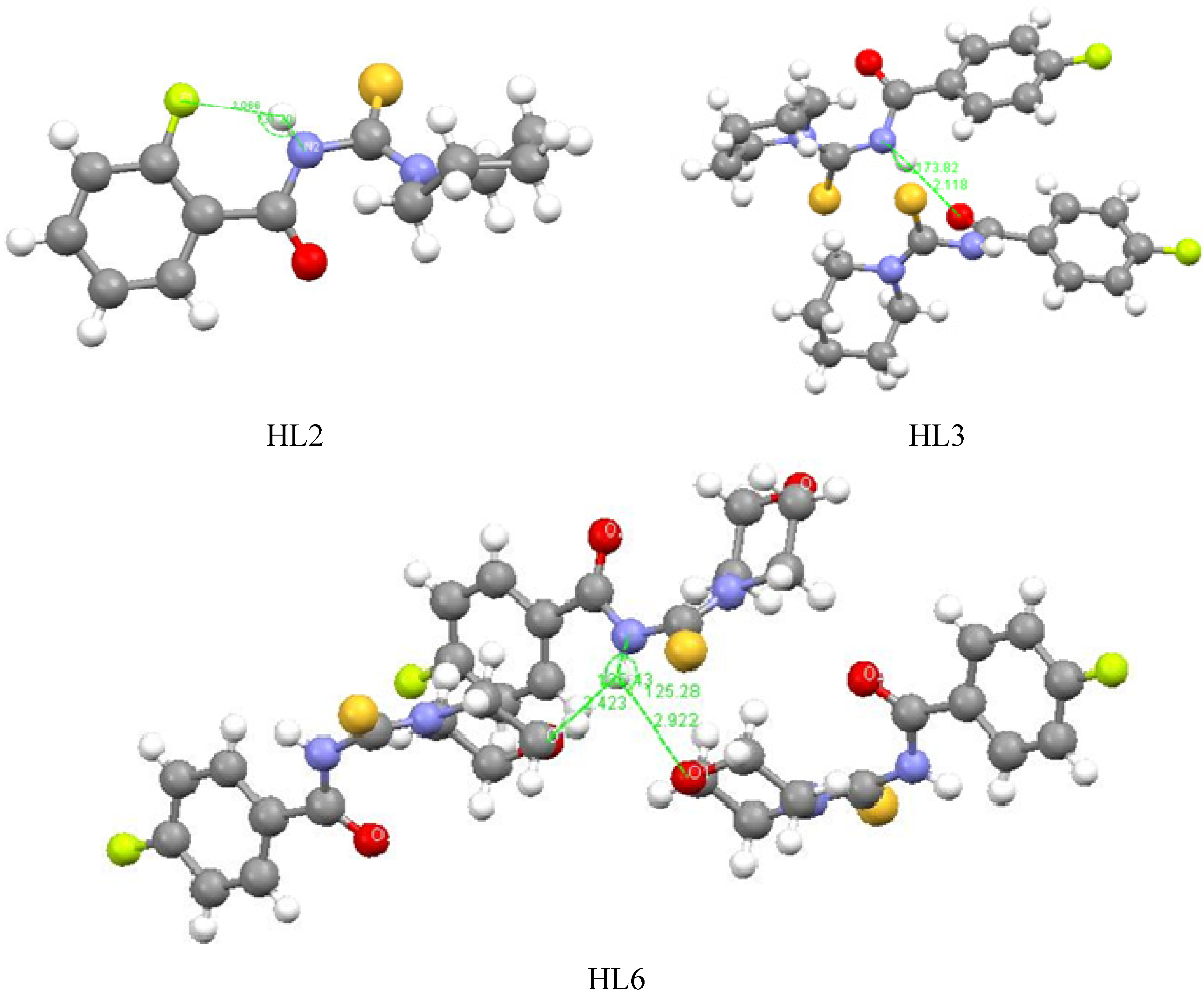
3. Experimental
3.1. Materials
3.2. Synthesis
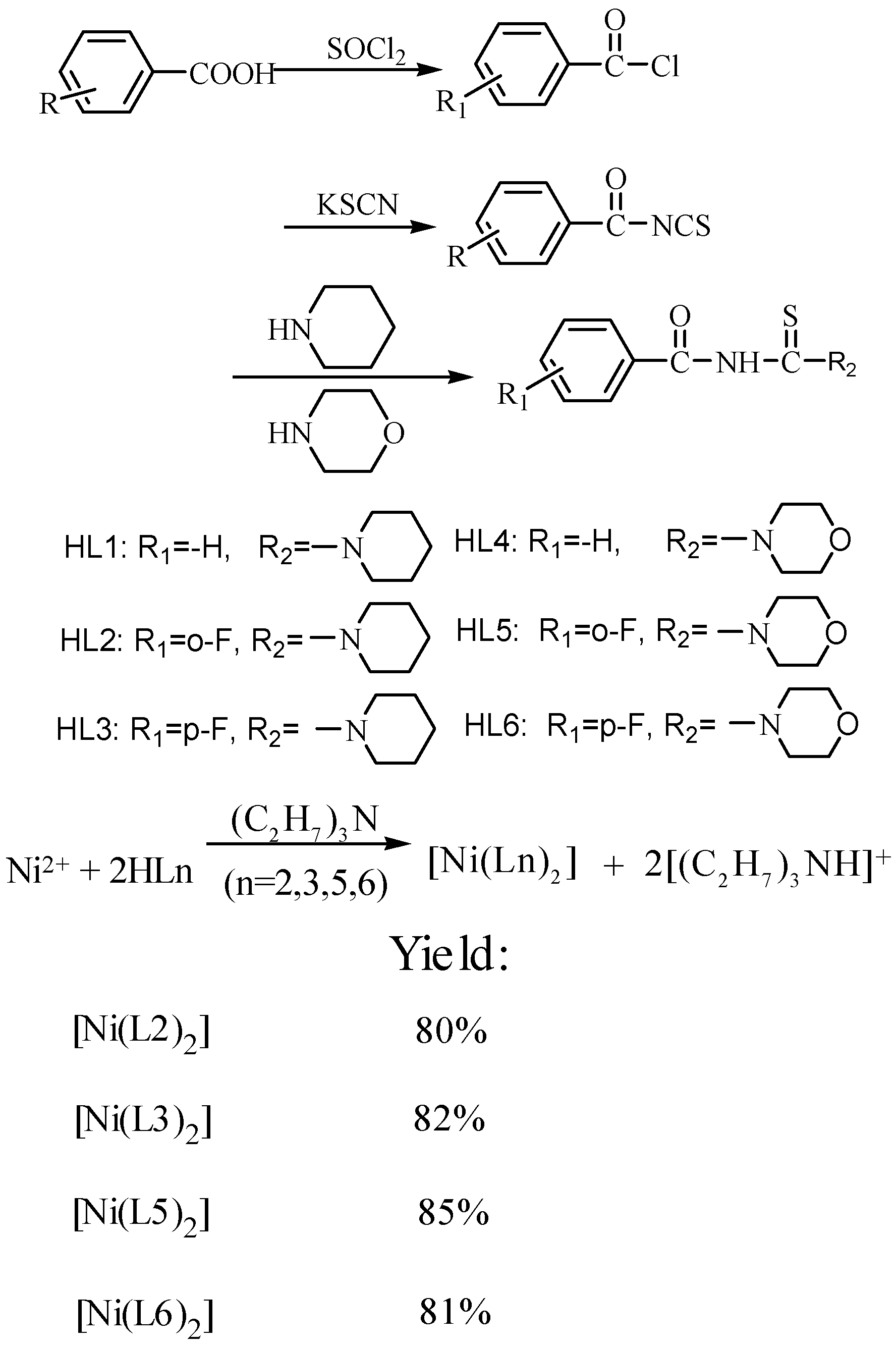
3.3. X-ray Structure Determination
3.4. Determination of the Minimum Inhibitory Concentration (MIC)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Krogul, A.; Cedrowski, J.; Wiktorska, K.; Oziminski, W.P.; Skupińska, J.; Litwinienko, G. Biological activity of Pd(II) complexes with mono- and disubstituted pyridines-Experimental and theoretical studies. Bioorg. Med. Chem. Lett. 2013, 23, 2765–2768. [Google Scholar] [CrossRef]
- Kim, B.Y.; Ahn, J.B.; Lee, H.W.; Kang, S.K.; Lee, J.H.; Shin, J.S.; Ahn, S.K.; Hong, C.; Yoon, S.S. Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione. Eur. J. Med. Chem. 2004, 39, 433–447. [Google Scholar] [CrossRef]
- Çolak, A.T.; Çolak, F.; Akduman, D.; Yeşilel, O.Z.; Büyükgüngör, O. Syntheses, crystal structures, spectral and thermal analysis and biological activities of copper(II)-pyridine-2,5-dicarboxylate complexes with 4-methylimidazole, imidazole, and 3,4-dimethylpyridine. Solid State Sci. 2009, 11, 1908–1918. [Google Scholar] [CrossRef]
- Yancheva, D.; Daskalova, L.; Cherneva, E.; Mikhova, B.; Djordjevic, A.; Smelcerovic, Z.; Smelcerovic, A. Synthesis, structure and antimicrobial activity of 6-(propan-2-yl)-3-methyl-morpholine-2,5-dione. J. Mol. Struct. 2012, 1016, 147–154. [Google Scholar]
- Kravchenko, D.V.; Kysil, V.M.; Tkachenko, S.E.; Maliarchouk, S.; Okun, I.M.; Ivachtchenko, A.V. Pyrrolo[3,4-c]quinoline-1,3-diones as potent caspase-3 inhibitors. Synthesis and SAR of 2-substituted 4-methyl-8-(morpholine-4-sulfonyl)-pyrrolo [3,4-c]quinoline-1,3-diones. Eur. J. Med. Chem. 2005, 40, 1377–1383. [Google Scholar] [CrossRef]
- Patel, N.B.; Purohit, A.C.; Rajani, D.P.; Moo-Puc, R.; Rivera, G. New 2-benzylsulfanyl-nicotinic acid based 1,3,4-oxadiazoles: Their synthesis and biological evaluation. Eur. J. Med. Chem. 2013, 62, 677–687. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Sudbeck, E.A.; Uckun, F.M. Regiospecific synthesis, X-ray crystal structure and biological activities of 5-bromothiophenethyl thioureas. Tetrahedron Lett. 2001, 42, 6629–6632. [Google Scholar] [CrossRef]
- Yao, J.W.; Chen, J.; He, Z.P.; Sun, W.; Xu, W.F. Design, synthesis and biological activities of thiourea containing sorafenibanalogs as antitumor agents. Bioorg. Med. Chem. 2012, 20, 2923–2929. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, spectroscopic characterization, crystal structure and pharmacological properties of some novel thiophene-thiourea core derivatives. Eur. J. Chem. 2010, 45, 1323–1331. [Google Scholar]
- Ventosa-Andrés, P.; Valdivielso, Á.M.; Pappos, I.; García-López, M.T.; Tsopanoglou, N.E.; Herranz, R. Design, synthesis and biological evaluation of new peptide-based ureas and thioureas as potential antagonists of the thrombin receptor PAR1. Eur. J. Med. Chem. 2012, 58, 98–111. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Kamel, M.M.; Kassem, E.M.M.; Abotaleb, N.; Abd El-moez, S.I.; Ahmed, M.F. Novel 6,8-dibromo-4(3H)quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur. J. Med. Chem. 2010, 45, 3311–3319. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Yang, W.; Xie, L.Q.; Cheng, X.C. N-Benzoyl-N'-dialkylthiourea derivatives and their Co(III) complexes: Structure, and antifungal. J. Inorg. Biochem. 2005, 99, 1314–1319. [Google Scholar] [CrossRef]
- Upadlgaya, J.S.; Srivastava, P.K. Potential antithyroid agents. Part V: Synthetic and pharmacological studies on some N-aryl-N'-benzoylthiocarbamides. J. Ind. Chem. Soc. 1982, 59, 767–769. [Google Scholar]
- Emen, M.F.; Arslan, H.; Kulcu, N.; Florke, U.; Duran, N. Synthesis, characterization and antimicrobial activities of some metal complexes with N'-(2-chloro-benzoyl)thiourea ligands: The crystal structure of fac-[CoL3] and cis-[PdL2]. Pol. J. Chem. 2005, 79, 1615–1626. [Google Scholar]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluorine Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Bonacorso, H.G.; Wentz, A.P.; Lourega, R.V.; Cechinel, C.A.; Moraes, T.S.; Coelho, H.S.; Zanatta, N.; Martins, M.A.P.; Hoerner, M.; Alves, S.H. Trifluoromethyl-containing pyrazolinyl (p-tolyl) sulfones: The synthesis and structure of promising antimicrobial agents. J. Fluorine Chem. 2006, 127, 1066–1072. [Google Scholar] [CrossRef]
- Jagodzinska, M.; Huguenot, F.; Candiani, G.; Zanda, M. Assessing the bioisosterism of the trifluoromethyl group with a protease probe. Chem. Med. Chem. 2009, 4, 49–51. [Google Scholar] [CrossRef]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef]
- Ismail, F.M.D. Important fluorinated drugs in experimental and clinical use. J. Fluorine Chem. 2002, 118, 27–33. [Google Scholar] [CrossRef]
- Yonetoku, Y.; Kubota, H.; Okamoto, Y.; Ishikawa, J.; Takeuchi, M.; Ohta, M.; Tsukamoto, S. Novel potent and selective calcium-release-activated calcium (CRAC) channel inhibitors. Part 2: Synthesis and inhibitory activity of aryl-3-trifluoromethylpyrazoles. Bioorg. Med. Chem. 2006, 14, 5370–5383. [Google Scholar] [CrossRef]
- Szymanski, P.; Karpinski, A.; Mikiciuk-Olasik, E. Synthesis, biological activity and HPLC validation of 1,2,3,4-tetrahydroacridine derivatives as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2011, 46, 3250–3257. [Google Scholar] [CrossRef]
- Saeed, A.; Shaheen, U.; Hameed, A.; Haider Naqvi, S.Z. Synthesis, characterization and antimicrobial activity of some new 1-(fluorobenzoyl)-3-(fluorophenyl)thioureas. J. Fluorine Chem. 2009, 130, 1028–1034. [Google Scholar] [CrossRef]
- Mendes, I.C.; Moreira, J.P.; Spez iali, N.L.; Mangrich, A.S.; Takahashi, J.A.; Beraldo, H. N(4)-tolyl-2-benzoylpyridine thiosemicarbazones and their copper(II) complexes with significant antifungal activity: Crystal structure of N(4)-para-tolyl-2-benzoylpyridine thiosemicarbazone. Braz.J. Chem. Soc. 2006, 17, 1571–1577. [Google Scholar] [CrossRef]
- Mendes, I.C.; Soares, M.A.; dos Santos, R.G.; Pinheiro, C.; Beraldo, H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones: Cytotoxic activity against malignant glioblastoma. Eur. J. Med. Chem. 2009, 44, 1870–1877. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Azzolini, L.S.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Increasing the antibacterial activity of gallium(III) against Pseudomonas aeruginosa upon coordination to pyridine-derived thiosemicarbazones. Polyhedron 2009, 28, 2301–2305. [Google Scholar] [CrossRef]
- Mendes, I.C.; Moreira, J.P.; Ardisson, J.D.; dos Santos, R.G.; da Silva, P.R.O.; Garcia, I.; Castiñeiras, A.; Beraldo, H. Organotin(IV) complexes of 2-pyridineformamide-derived thiosemicarbazones: Antimicrobial and cytotoxic effects. Eur. J. Med. Chem. 2008, 43, 1454–1461. [Google Scholar] [CrossRef]
- Reis, D.C.; Pinto, M.C.X.; Souza-Fagundes, E.M.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. 2-Acetylpyridine thiosemicarbazones: Cytotoxic activity in nanomolar doses against malignant gliomas. Eur. J. Med. Chem. 2010, 45, 3904–3910. [Google Scholar] [CrossRef]
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2008, 43, 2639–2649. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.H.; Li, M.Y.; Wang, F.; Zhou, W.Q.; Fan, J.F. Synthesis, structures and antibacterial activities of benzoylthiourea derivatives and their complexes with cobalt. J. Inorg. Biochem. 2012, 116, 97–105. [Google Scholar] [CrossRef]
- Alomar, K.; Gaumet, V.; Allain, M.; Bouet, G.; Landreau, A. Synthesis, crystal structure, characterisation, and antifungal activity of 3-thiophene aldehyde semicarbazone (3STCH), 2,3-thiophene dicarboxaldehyde bis(semicarbazone) (2,3BSTCH2) and their nickel (II) complexes. J. Inorg. Biochem. 2012, 115, 36–43. [Google Scholar] [CrossRef]
- Alves, L.C.; Rubinger, M.M.M.; Lindemann, R.H.; Perpétuo, G.J.; Janczak, J.; Miranda, L.D.L.; Zambolim, L.; Oliveira, M.R.L. Syntheses, crystal structure, spectroscopic characterization and antifungal activity of new N-R-sulfonyldithiocarbimate metal complexes. J. Inorg. Biochem. 2009, 103, 1045–1053. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Zhu, L.M.; Yong, Z.G.; Yu, Z.F.; Lu, L.; Yang, X.J. Structure and vibrational spectra of the thiourea derivative and its complex with Ni(II). Vib. Spectrosc. 2004, 36, 73–78. [Google Scholar] [CrossRef]
- Hernández, W.; Spodine, E.; Richter, R.; Griebel, J.; Kirmsec, R.; Schröder, U.; Beyer, L. Cis-trans isomerism in Copper(II) complexes with N-acyl thiourea ligands. Z. Anorg. Allg. Chem. 2004, 630, 1381–1386. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Ramasamy, K.; Malik, M.A.; O’Brien, P.; Haigh, S.J.; Raftery, J. New routes to copper sulfide nanostructures and thin films. J. Mater. Chem. 2011, 21, 17888–17895. [Google Scholar] [CrossRef]
- Banks, R.E.; Smart, B.E.; Tatlow, J.C. Organofluorine Chemistry, Principles and Commercial Applications; Plenum Press: New York, NY, USA, 1994; pp. 57–88. [Google Scholar]
- Sheldrick, G.M. SHELXS97 and SHELXL97. University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Version 5.1; Bruker AXS Inc.: Madison, WI, USA, 1998.
- CrystalStructure 3.7.0, Single Crystal Structure Analysis Software; Rigaku, Inc.: The Woodlands, TX, USA, 2005–2007.
- Sheldrick, G.M. CRYSTRCLEAR, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sample Availability: Samples of the HL2, HL3, HL6, [Ni(L2)2], [Ni(L3)2] and [Ni(L6)2] are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, C.; Yang, W.; Liu, H.; Li, M.; Zhou, W.; Xie, J. Crystal Structures and Antifungal Activities of Fluorine-Containing Thioureido Complexes with Nickel(II). Molecules 2013, 18, 15737-15749. https://doi.org/10.3390/molecules181215737
Li C, Yang W, Liu H, Li M, Zhou W, Xie J. Crystal Structures and Antifungal Activities of Fluorine-Containing Thioureido Complexes with Nickel(II). Molecules. 2013; 18(12):15737-15749. https://doi.org/10.3390/molecules181215737
Chicago/Turabian StyleLi, Chun, Wen Yang, Huanhuan Liu, Mengying Li, Weiqun Zhou, and Juan Xie. 2013. "Crystal Structures and Antifungal Activities of Fluorine-Containing Thioureido Complexes with Nickel(II)" Molecules 18, no. 12: 15737-15749. https://doi.org/10.3390/molecules181215737
APA StyleLi, C., Yang, W., Liu, H., Li, M., Zhou, W., & Xie, J. (2013). Crystal Structures and Antifungal Activities of Fluorine-Containing Thioureido Complexes with Nickel(II). Molecules, 18(12), 15737-15749. https://doi.org/10.3390/molecules181215737




