Abstract
The enantioselective addition of phenylethynylzinc to aldehydes catalyzed by a series of cyclopropane-based amino alcohol ligands 7 was investigated. The reactions afforded chiral propargylic alcohols in high yields (up to 96%) and with excellent enantioselectivities (up to 98% ee) under mild conditions. Furthermore, studies on the structural relationship show that the matching of the chiral center configuration is crucial to obtain the high enantioselectivity.
1. Introduction
The catalytic enantioselective addition of alkynylzinc to aldehydes is one of the most useful carbon-carbon bond-forming reactions because the resulting propargylic alcohols are versatile, useful building blocks and important precursors for fine chemicals, pharmaceuticals, and natural products [1,2,3,4,5,6,7]. In 1994, Hoshino reported the first example of the addition of alkynylzinc reagents to cyclohexanecarbaldehyde and benzaldehyde using the ligand 1, which afforded the corresponding products with high enantioselectivity [8]. Subsequently, various other catalytic system were reported, including (+)- or (–)-N-methylephedrine 2 by Carreira [9,10,11,12,13,14], (R)- or (S)-BINOL 3 and their derivatives by Pu [15,16,17,18,19,20,21,22,23,24], amino alcohols 4, and β-sulfonamide alcohols by Chan [25,26,27,28], sulfonamide alcohols 5 and a bifunctional catalyst by Wang [29,30,31,32,33,34,35,36], and ProPhenol 6 by Trost [37,38,39,40] (Figure 1).
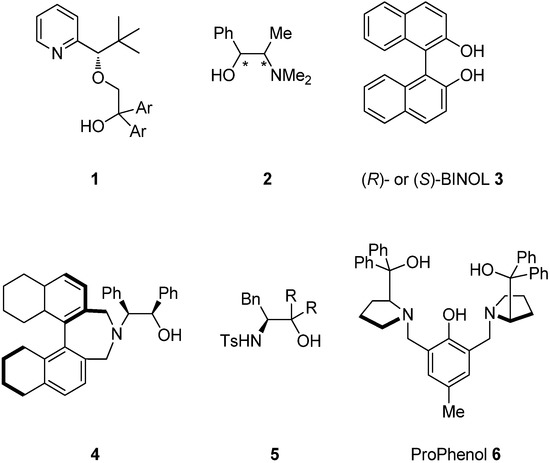
Figure 1.
Chiral ligands for the enantioselective addition of alkynylzinc to aldehydes.
Recently, we have developed a series of chiral cyclopropane-based ligands bearing amino alcohols, bisoxazolines, and amide alcohols (Figure 2). These ligands were proven to be very effective in some stereoselective reactions, including dialkylzinc addition to aldehydes and ketoesters, nitroaldol (Henry) reaction, Diels-Alder additions [41,42,43,44,45,46,47]. In this study, we focused on the structural relationship of our cyclopropane-based ligands amino alcohol 7 in the phenylethynylzinc addition to various aldehydes. It is noteworthy that the desired chiral propargylic alcohols were achieved with high to excellent yield (80%–96%). Importantly, high enantioselectivities (84%–98%) and broad substrate tolerance are also observed without any additives.
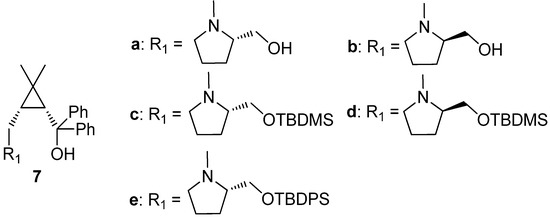
Figure 2.
Cyclopropane amino alcohol 7a–e.
2. Results and Discussion
The chiral ligands 7 were easily synthesised from commercially available (+)-cis-methyl chrysanthemate and (R) or (S)-prolinol according to a previously reported procedure [44]. The absolute configuration of ligands 7 was (1R, 3S), as confirmed by X-ray crystallography analysis of ligand 7e (Figure 3). This configuration is identical to that of the starting material, (+)-cis-methyl chrysanthemate.
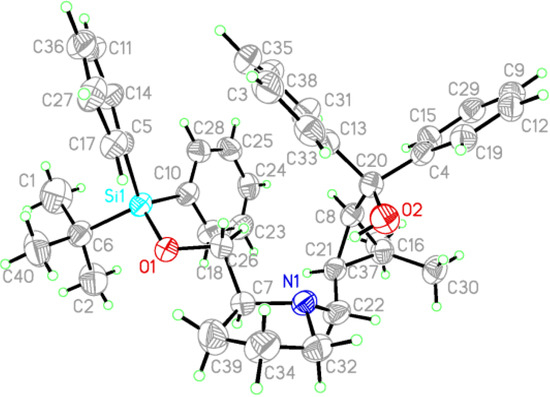
Figure 3.
X-ray crystallographic structure of ligand 7e.
An initial study on the structural relationship of the cyclopropane-based ligands 7 in the addition of phenylethynylzinc to benzaldehyde was performed (Table 1).

Table 1.
Ligand survey for the addition of phenylethynylzinc to benzaldehyde a. 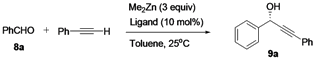
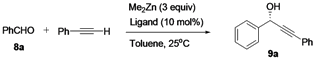
| Entry | Ligand | Time (h) | Yield (%) b | Ee (%) c | Config. d |
|---|---|---|---|---|---|
| 1 | 7a | 20 | 89 | 10 | S |
| 2 | 7b | 20 | 90 | 16 | S |
| 3 | 7c | 20 | 94 | 80 | S |
| 4 | 7d | 20 | 90 | 22 | S |
| 5 | 7e | 20 | 91 | 79 | S |
a All reactions were run on a 1 mmol scale; b Isolated yields after chromatographic purification; c Enantiomeric excess determined by HPLC on a Chiracel OD-H column; d Absolute configuration assigned by comparing their specific rotations or the HPLC elution order with literature data.
The results indicated that varying the substitution on the pyrrolidine ring of the ligands significantly affected the enantioselectivity of the reaction. The ee value was significantly increased when the hydroxyl group in the prolinol of ligand 7a was protected with a tert-butyldimethylsilyl chloride (TBDMSCl; ligand 7c) or tert-butyldiphenylsilyl chloride (TBDPSCl; ligand 7e) moiety (entry 1 vs. entries 3 and 5). Furthermore, the cyclopropane-based amino alcohol 7d, which was synthesized from (R)-prolinol, afforded the corresponding (S)-propargyl alcohol with only 22% ee, whereas ligand 7c, which was prepared from (S)-prolinol, exhibited a higher ee (80%). This result showed that the match of the cyclopropane configuration with the additional chiral center on the pyrrolidine was crucial to achieve high enantioselectivity. Therefore, the cyclopropane-based amino alcohol 7c was the ligand of choice, providing the propargylic alcohol product with 80% ee (entry 3).
Attempts were made to optimize the reaction conditions by employing the addition of phenylethynylzinc to benzaldehyde. (Table 2). Early optimization showed that temperature significantly affected the ee value. A decrease in the reaction temperature from room temperature to 0 °C increased the ee values (entry 1 vs. entry 2). However, a further decrease in the temperature to −10 and −20 °C reduced both the enantioselectivity and yield (entries 3 and 4). It was interesting to note that the results were almost equally good when the amount of ligand was increased to 20 mol% (entry 6 vs. entry 2). Moreover, both the yield and enantioselectivity of the reaction decreased when the amount of ligand was reduced to 5 mol% (entry 7 vs. entry 2). Although previous studies [47] showed that the addition of polyethylene glycol dimethyl ether (DiMPEG) can significantly promote asymmetric induction, our additive to this reaction only slightly reduced the enantioselectivity (entry 5 vs. entry 2), because the DiMPEG would impact the generation of our unique catalyst system. Finally, the effects of the solvent on this reaction were investigated. Reaction in heptane gave lower enantioselectivity than in toluene (entry 8 vs. entry 2), this may be due to the poor solubility of Zn-amino alcohol complexes. Finally, the optimized reaction conditions were considered as following: 8a (0.5 mmol) with phenylacetylene (1.5 mmol) and Me2Zn (1.5 mmol) in toluene at 0 °C for 48 h (Table 2, entry 2).

Table 2.
Reaction optimization for the addition of phenylethynylzinc to benzaldehyde a. 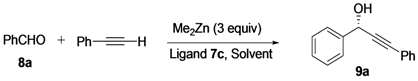
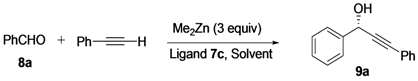
| Entry | Ligand (mol %) | Solvent | Time (h) | Temp (°C) | Yield (%) b | Ee (%) c |
|---|---|---|---|---|---|---|
| 1 | 10 | Toluene | 20 | 25 | 94 | 80 |
| 2 | 10 | Toluene | 48 | 0 | 91 | 93 |
| 3 | 10 | Toluene | 48 | −10 | 83 | 86 |
| 4 | 10 | Toluene | 48 | −20 | 40 | 79 |
| 5d | 10 | Toluene | 48 | 0 | 95 | 90 |
| 6 | 20 | Toluene | 48 | 0 | 97 | 94 |
| 7 | 5 | Toluene | 48 | 0 | 75 | 85 |
| 8 | 10 | Heptane | 48 | 0 | 80 | 83 |
a All reactions were run on a 1 mmol scale; b Isolated yields after chromatographic purification; c Enantiomeric excess determined by HPLC on a Chiracel OD-H column; d With the addition of 10 mol % DiMPEG.
With the optimal condition in hand, we continued to explore the scope of this reaction. The addition of phenylethynylzinc to various aldehydes was investigated (Table 3). The results revealed that ligand 7c was a highly enantioselective catalyst for the addition of alkynylzinc to aldehydes. Ortho-, meta-, and para-substituted benzaldehydes containing either electron-donating or electron-withdrawing substituents gave uniformly high ee (90%–98%, entries 1 to 13). In particular, the result (98% ee) obtained from 2-methylbenzaldehyde was remarkable (entry 5). High enantioselectivity was also observed for the addition to other aromatic aldehydes such as 1-naphthaldehyde and 2-naphthaldehyde (entries 14 and 15). A favorable result (entries 16 and 17) was also obtained when the aliphatic aldehydes 8p and 8q were used as substrates.

Table 3.
Substrate scope for the addition of phenylethynylzinc to aldehydes a. 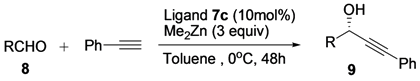
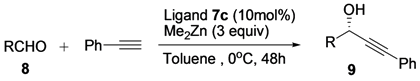
| Entry | R | Product | Yield (%) b | Ee (%) c |
|---|---|---|---|---|
| 1 | Ph | 9a | 91 | 93 |
| 2 | p-FC6H4 | 9b | 90 | 94 |
| 3 | o-BrC6H4 | 9c | 96 | 94 |
| 4 | p-NO2C6H4 | 9d | 92 | 93 |
| 5 | o-CH3C6H4 | 9e | 91 | 98 |
| 6 | m-CH3C6H4 | 9f | 89 | 95 |
| 7 | p-CH3C6H4 | 9g | 90 | 95 |
| 8 | o-CH3OC6H4 | 9h | 81 | 96 |
| 9 | m-CH3OC6H4 | 9i | 80 | 94 |
| 10 | p-CH3OC6H4 | 9j | 85 | 97 |
| 11 | o-ClC6H4 | 9k | 95 | 90 |
| 12 | m-ClC6H4 | 9l | 93 | 93 |
| 13 | p-ClC6H4 | 9m | 92 | 93 |
| 14 | 1-Naphthyl | 9n | 91 | 98 |
| 15 | 2-Naphthyl | 9o | 80 | 92 |
| 16 | Cyclohexyl | 9p | 92 | 84 |
| 17 | Isopropyl | 9q | 91 | 88 |
a All reactions were run on a 0.5 mmol scale; b Isolated yields after chromatographic purification; c Enantiomeric excess determined by HPLC on a Chiracel OD-H column.
3. Experimental
3.1. General Methods and Materials
All reactions were performed under a nitrogen atmosphere. Solvents were dried according to standard procedures and were then distilled prior to use. All reagents were purchased commercially and used without further purification, unless stated otherwise. 1H- and 13C-NMR spectra were recorded using a Bruker DP-X300 MHz spectrometer (Bruker, Fallanden, Switzerland), and referenced internally to Me4Si. High-resolution mass spectra were obtained on an Agilent MS using the time-of-flight mass spectrometry technique (Agilent Technologies, Waldbroon, Germany). The optical rotations were determined on a Perkin-Elmer PE-341 polarimeter (Perkin-Elmer, Waltham, MA, USA). Crystallographic data were obtained using a Rigaku RAPID-S image plate X-Ray diffractometer (Rigaku Denki Co., Ltd, Tokyo, Japan). Enantiomeric excesses (ee) were determined on an Agilent 1100 HPLC system using a chiral Chiralcel OD-H column (Daicel Chiral Technologies (China) Co., Ltd., Shanghai, China) and isopropanol-hexanes as the eluent.
3.2. X-Ray Crystallographic Data of the Ligand 7e
CCDC 808539 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). This text may be included in the General subsection of the Experimental or as a suitably referenced endnote.
3.3. General Procedure for the Asymmetric Alkynylation of Aldehydes
Phenylacetylene (0.165 mL, 1.5 mmol, 3 equiv) was added to a solution of Me2Zn (1.25 mL, 1.2 M in toluene, 1.5 mmol, 3 equiv) in dry toluene (1.75 mL) at room temperature under a nitrogen atmosphere. The mixture was stirred for 30 min, then was transferred via syringe to another Schlenk tube containing neat ligand 7 (0.05 mmol, 0.1 equiv). After stirring for 30 min, an aldehyde (0.5 mmol) was added at 0 °C. The reaction mixture was stirred at 0 °C for 48 h and then quenched with saturated aqueous NH4Cl (5 mL). The organic phase was separated, and the aqueous phase was extracted with Et2O. The combined organic layers were dried over anhydrous Na2SO4. The solvents were removed under reduced pressure. Flash chromatography (silica gel, 10% ether in hexanes) afforded the pure propargylic alcohols. The enantiomeric excess was determined by HPLC on a Chiralcel OD-H column. The absolute configurations of the products were assigned by comparing their specific rotations or their HPLC elution order with literature data.
(S)-1,3-Diphenylprop-2-yn-1-ol (9a). 91% yield. [α]D20 = −3.8 (c = 1.52, CHCl3). 1H-NMR (CDCl3): δ 7.80–7.76 (m, 2H), 7.65–7.62 (m, 2H), 7.57–7.46 (m, 6H), 5.85 (d, J = 5.8 Hz, 1H), 2.47 (d, J = 5.9 Hz, 1H). 13C-NMR (CDCl3): δ 140.6, 131.7, 128.6, 128.5, 128.3, 128.2, 126.7, 122.3, 88.7, 86.6, 65.0. HRMS (TOF) calcd. for C15H12NaO [M+Na]+: 231.0786; found: 231.0776. 93% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 8.13 min, tmajor = 10.27 min.
(S)-1-(4-Fluorophenyl)-3-phenylprop-2-yn-1-ol (9b). 90% yield. [α]D20 = −4.0 (c = 1.50, CHCl3). 1H-NMR (CDCl3): δ 7.62–7.57 (m, 2H), 7.49–7.45 (m, 2H), 7.35–7.32 (m, 3H), 7.11–7.05 (m, 2H), 5.67 (d, J = 6.0 Hz, 1H), 2.34 (d, J = 6.1 Hz, 1H). 13C-NMR (CDCl3): δ 164.2, 136.4, 131.6, 128.6, 128.4, 128.3, 122.1, 115.4 88.5, 86.7, 64.2. HRMS (TOF) calcd. for C15H11FNaO [M+Na]+: 249.0692; found: 249.0685. 94% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 6.45 min, tmajor = 12.47 min.
(S)-1-(2-Bromophenyl)-3-phenylprop-2-yn-1-ol (9c). 96% yield. [α]D20 = +71.9 (c = 1.01, CHCl3). 1H-NMR (CDCl3): δ 7.85 (dd, J = 1.7, 7.7 Hz, 1H), 7.59 (dd, J =1.2, 8.0 Hz, 1H), 7.49–7.21 (m, 7H), 6.02 (d, J = 5.5 Hz, 1H), 2.56 (d, J = 5.6 Hz, 1H). 13C-NMR (CDCl3): δ 139.4, 132.9, 131.7, 129.8, 128.6, 128.2, 127.8, 122.7, 122.2, 87.6, 86.6, 64.5. HRMS (TOF) calcd. for C15H11BrNaO [M+Na]+: 308.9891; found: 308.9894. 94% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tmajor = 6.44 min, tminor = 6.92 min.
(S)-1-(4-Nitrophenyl)-3-phenylprop-2-yn-1-ol (9d). 92% yield. [α]D20 = −12.8 (c = 1.00, CHCl3). 1H-NMR (CDCl3): δ 8.27 (dd, J = 2.0, 6.8 Hz, 1H), 7.82–7.78 (m, 2H), 7.49–7.45 (m, 2H), 7.37–7.34 (m, 3H), 5.80 (d, J = 5.6 Hz, 1H), 2.45 (d, J = 5.7 Hz, 1H). 13C-NMR (CDCl3): δ 147.9, 147.4, 131.8, 129.1, 128.4, 127.4, 123.8, 121.7, 87.7, 87.4, 64.1. HRMS (TOF) calcd for C15H12NO3 [M+H]+: 254.0817; found: 254.0813. 93% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 9.50 min, tmajor = 27.68 min.
(S)-1-(2-Methylphenyl)-3-phenylprop-2-yn-1-ol (9e). 91% yield. [α]D20 = +13.6 (c = 0.73, CHCl3). 1H-NMR (CDCl3): δ 7.74–7.71 (m, 1H), 7.48–7.45 (m, 2H), 7.33–7.30 (m, 3H), 7.27–7.20 (m, 3H), 5.84 (s, 1H), 2.50 (s, 3H), 2.18 (br, 1H). 13C-NMR (CDCl3): δ 138.3, 136.0, 131.7, 130.8, 128.5, 128.4, 128.2, 126.5, 126.2, 122.5, 88.5, 86.4, 62.9, 19.0. HRMS (TOF) calcd. for C16H14NaO [M+Na]+: 245.0942; found: 245.0938. 98% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 6.29 min, tmajor = 9.72 min.
(S)-1-(3-Methylphenyl)-3-phenylprop-2-yn-1-ol (9f). 89% yield. [α]D20 = −6.8 (c = 1.11, CHCl3). 1H-NMR (CDCl3): δ 7.49–7.40 (m, 4H), 7.33–7.30 (m, 4H), 7.17 (d, J = 7.6 Hz, 1H), 5.66 (d, J = 5.8 Hz, 1H), 2.39 (s, 3H), 2.23 (d, J = 6.1 Hz, 1H). 13C-NMR (CDCl3): δ 140.5, 138.4, 131.7, 129.2, 128.55, 128.53, 128.3, 127.4, 123.7, 122.4, 88.8, 86.5, 65.1, 21.4. HRMS (TOF) calcd. for C16H14NaO [M+Na]+: 245.0942; found: 245.0938. 95% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 7.12 min, tmajor = 11.27 min.
(S)-1-(4-Methylphenyl)-3-phenylprop-2-yn-1-ol (9g). 90% yield. [α]D20 = −5.9 (c = 0.76, CHCl3). 1H-NMR (CDCl3): δ 7.52–7.45 (m, 4H), 7.33–7.20 (m, 5H), 5.66 (s, 1H), 2.37 (s, 3H), 2.26 (br, 1H). 13C-NMR (CDCl3): δ 138.2, 137.8, 131.7, 129.3, 128.5, 128.3, 126.7, 122.5, 88.9, 86.4, 64.9, 21.1. HRMS (TOF) calcd. for C16H14NaO [M+Na]+: 245.0942; found: 245.0948. 95% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 6.53 min, tmajor = 9.69 min.
(S)-1-(2-Methoxyphenyl)-3-phenylprop-2-yn-1-ol (9h). 81% yield. [α]D20 = +12.3 (c = 2.03, CHCl3). 1H-NMR (CDCl3): δ 7.65 (dd, J = 1.8, 7.6 Hz, 1H), 7.49–7.46 (m, 2H), 7.33–7.29 (m, 4H), 7.00–6.92 (m, 2H), 5.93 (s, 1H), 3.91(s, 3H), 3.07 (br, 1H). 13C-NMR (CDCl3): δ 156.8, 131.7, 129.6, 128.9, 128.3, 128.1, 127.9, 122.7, 120.8, 110.9, 88.5, 85.9, 61.5, 55.5. HRMS (TOF) calcd. for C16H14NaO2 [M+Na]+: 261.0891; found: 261.0895. 96% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 9.19 min, tmajor = 10.16 min.
(S)-1-(3-Methoxyphenyl)-3-phenylprop-2-yn-1-ol (9i). 80% yield. [α]D20 = −12.9 (c = 1.04, CHCl3). 1H-NMR (CDCl3): δ 7.49–7.45 (m, 2H), 7.33–7.29 (m, 4H), 7.21–7.18 (m, 2H), 6.91–6.88 (m, 1H), 5.66 (s, 1H), 3.83 (s, 3H), 2.27 (br, 1H). 13C-NMR (CDCl3): δ 159.7, 142.2, 131.7, 129.6, 128.5, 128.2, 122.3, 118.9, 114.0, 112.1, 88.7, 86.4, 64.8, 55.2. HRMS (TOF) calcd. for C16H14NaO2 [M+Na]+: 261.0891; found: 261.0885. 94% ee (80:20n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 11.43 min, tmajor = 14.51 min.
(S)-1-(4-Methoxyphenyl)-3-phenylprop-2-yn-1-ol (9j). 85% yield. [α]D20 = −5.3 (c = 1.16, CHCl3). 1H-NMR (CDCl3): δ 7.55 (dd, J = 2.1, 6.7 Hz, 2H), 7.49–7.46 (m, 2H), 7.33–7.31 (m, 3H), 6.93 (dd, J = 2.0, 6.7 Hz, 2H), 5.65 (d, J = 6.0 Hz, 1H), 3.83 (s, 3H), 2.18 (d, J = 6.2 Hz, 1H). 13C-NMR (CDCl3): δ 159.7, 133.0, 131.7, 128.5, 128.3, 128.1, 122.5, 114.0, 89.0, 86.5, 64.7, 55.3. HRMS (TOF) calcd. for C16H14NaO2 [M+Na]+: 261.0891; found: 261.0887. 97% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 10.05 min, tmajor = 14.41 min.
(S)-1-(2-Chlorophenyl)-3-phenylprop-2-yn-1-ol (9k). 95% yield. [α]D20 = +12.1 (c = 1.20, CHCl3). 1H-NMR (CDCl3): δ 7.85–7.82 (m, 1H), 7.49–7.26 (m, 8H), 6.05 (d, J = 4.5Hz, 1H), 2.53 (d, J = 5.1Hz, 1H). 13C-NMR (CDCl3): δ 137.9, 132.8, 131.7, 129.75, 129.67, 128.6, 128.4, 128.3, 127.2, 122.3, 87.6, 86.6, 62.4. HRMS (TOF) calcd. for C15H11ClNaO [M+Na]+: 265.0396; found: 265.0396. 90% ee (97:3 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 29.93 min, tmajor = 34.49 min.
(S)-1-(3-Chlorophenyl)-3-phenylprop-2-yn-1-ol (9l). 93% yield. [α]D20 = −8.6 (c = 1.54, CHCl3). 1H-NMR (CDCl3): δ 7.56 (t, J = 0.5 Hz, 1H), 7.44–7.41 (m, 3H), 7.29–7.24 (m, 5H), 5.60 (s, 1H), 3.10 (s, 1H). 13C-NMR (CDCl3): δ 142.4, 134.3, 131.7, 129.8, 128.7, 128.4, 128.2, 126.8, 124.7, 122.0, 88.0, 86.9, 64.2. HRMS (TOF) calcd. for C15H11ClNaO [M+Na]+: 265.0396; found: 265.0393. 93% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 6.05 min, tmajor = 13.35 min.
(S)-1-(4-Chlorophenyl)-3-phenylprop-2-yn-1-ol (9m). 92% yield. [α]D20 = −9.0 (c = 1.01, CHCl3). 1H-NMR (CDCl3): δ 7.57–7.32 (m, 9H), 5.67 (s, 1H), 2.30 (br, 1H). 13C-NMR (CDCl3): δ 139.0, 134.1, 131.7, 128.7, 128.6, 128.3, 128.0, 122.0, 88.2, 86.8, 64.2. HRMS (TOF) calcd. for C15H11ClNaO [M+Na]+: 265.0396; found: 265.0390. 93% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 5.96 min, tmajor = 12.43 min.
(S)-1-(1-Naphthyl)-3-phenylprop-2-yl-1-ol (9n). 91% yield. [α]D20 = +35.3 (c = 1.00, CHCl3). 1H-NMR (CDCl3): δ 8.36 (d, J = 8.4, 2H), 7.92–7.84 (m, 3H), 7.58–7.46 (m, 5H), 7.32–7.29 (m, 3H), 6.34 (s, 1H), 2.45 (br, 1H). 13C-NMR (CDCl3): δ 135.5, 133.8, 131.6, 130.4, 129.2, 128.6, 128.4, 128.1, 126.3, 125.7, 125.1, 124.5, 123.9, 122.3, 88.6, 87.1, 63.1. HRMS (TOF) calcd. for C19H14NaO [M+Na]+: 281.0942; found: 281.0938. 98% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 9.46 min, tmajor = 15.77 min.
(S)-1-(2-Naphthyl)-3-phenylprop-2-yl-1-ol (9o). 80% yield. [α]D20 = +8.6 (c = 0.70, CHCl3). 1H-NMR (CDCl3): δ 8.06 (s, 1H), 7.91–7.87 (m, 3H), 7.73 (dd, J = 1.7, 8.4 Hz, 1H), 7.52–7.49 (m, 4H), 7.35–7.33 (m, 3H), 5.87 (d, J = 6.2 Hz, 1H), 2.35 (d, J = 6.2 Hz, 1H). 13C-NMR (CDCl3): δ 138.0, 133.3, 133.2, 131.8, 128.7, 128.6, 128.3, 128.2, 127.7, 126.3, 125.5, 124.6, 122.4, 88.7, 87.0, 65.3. HRMS (TOF) calcd. for C19H14NaO [M+Na]+: 281.0942; found: 281.0938. 92% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 9.34 min, tmajor = 21.95 min.
(S)-1-Cyclohexyl-3-phenylprop-2-yn-1-ol (9p). 92% yield. [α]D20 = +7.9 (c = 0.71, CHCl3). 1H-NMR (CDCl3): δ 7.45–7.41 (m, 2H), 7.33–7.28 (m, 3H), 4.38 (t, J = 5.9, 1H), 1.95–1.90 (m, 2H), 1.86–1.78 (m, 3H), 1.72–1.64 (m, 2H), 1.32–1.11 (m, 5H). 13C-NMR (CDCl3): δ 131.7, 128.27, 128.24, 122.8, 89.3, 85.7, 67.7, 44.3, 28.6, 28.2, 26.4, 25.92, 25.90. HRMS (TOF) calcd. for C15H18NaO [M+Na]+: 237.1255; found: 237.1250. 84% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 4.35 min, tmajor = 6.32 min.
(S)-4-Methyl-1-phenylpent-1-yl-3-ol (9q). 91% yield. [α]D20 = +1.6 (c = 1.35, CHCl3). 1H-NMR (CDCl3): δ 7.45–7.42 (m, 2H), 7.33–7.28 (m, 3H), 4.40 (d, J = 5.6, 1H), 2.01–1.95 (m, 1H), 1.87 (br, 1H), 1.07 (t, J = 6.7, 6H). 13C-NMR (CDCl3): δ 131.7, 128.28, 128.23, 122.7, 88.9, 85.6, 68.4, 34.7, 18.1, 17.5. HRMS (TOF) calcd. for C12H14NaO [M+Na]+: 197.0942; found: 197.0941. 88% ee (80:20 n-hexane-2-propanol, 1.0 mL/min, 254 nm). Retention time: tminor = 4.22 min, tmajor = 5.71 min.
4. Conclusions
The cyclopropane-based amino alcohol 7c successfully promotes the enantioselective alkynylation of aldehydes and affords chiral propargylic alcohols in high yields and high enantiomeric excess (up to 98% ee) without requiring any additives. In addition, studies on the structural relationship show that the matching of the cyclopropane configuration with the additional chiral center on pyrrolidine is crucial to obtain high enantioselectivity.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/12/15422/s1.
Acknowledgments
We thank the National Basic Research Program of China (81102340) and the National Key Technology Research and Development Program (2012BAK25B03-3) for the financial support that they provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trost, B.M.; Quintard, A. Asymmetric catalytic alkynylation of acetaldehyde: Application to the synthesis of (+)-tetrahydropyrenophorol. Angew. Chem. Int. Ed. Engl. 2012, 51, 6704–6708. [Google Scholar] [CrossRef]
- Chinkov, N.; Warm, A.; Carreira, E.M. Asymmetric autocatalysis enables an improved synthesis of efavirenz. Angew. Chem. Int. Ed. 2011, 50, 2957–2961. [Google Scholar] [CrossRef]
- Tan, L.; Chen, C.; Tillyer, R.D.; Grabowski, E.J.J.; Reider, P.J. A novel highly enantioselective ketone alkynylation reaction mediated by chiral zinc aminoalkoxides. Angew. Chem. Int. Ed. 1999, 38, 711–713. [Google Scholar] [CrossRef]
- Reber, S.; Knöpfel, T.F.; Carreira, E.M. Enantioselective total synthesis of (R)-strongylodiols A and B. Tetrahedron 2003, 59, 6813–6817. [Google Scholar] [CrossRef]
- Escorihuela, J.; Altava, B. C2 symmetrical nickel complexes derived from α-amino amides as efficient catalysts for the enantioselective addition of dialkylzinc reagents to aldehydes. Tetrahedron 2013, 69, 551–558. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzynski, S. Aziridine ring-containing chiral ligands as highly efficient catalysts in asymmetric synthesis. Tetrahedron 2013, 24, 421–425. [Google Scholar]
- Turlington, M.; Du, Y.; Ostrum, S.G.; Santosh, V.; Wren, K.; Lin, T.; Sabat, M.; Pu, L. From highly enantioselective catalytic reaction of 1,3-diynes with aldehydes to facile asymmetric synthesis of polycyclic compounds. J. Am. Chem. Soc. 2011, 133, 11780–11794. [Google Scholar]
- Ishizaki, M.; Hoshino, O. Efficient ligands, chiral 2-[2,2-dimethyl-1-(2-pyridyl)propoxy]-1, 1-diarylethanols for highly enantioselective addition of alkynylzinc reagents to various aldehydes. Tetrahedron 1994, 5, 1901–1904. [Google Scholar]
- Frantz, D.E.; Fässler, R.; Carreira, E.M. Facile enantioselective synthesis of propargylic alcohols by direct addition of terminal alkynes to aldehydes. J. Am. Chem. Soc. 2000, 122, 1806–1807. [Google Scholar] [CrossRef]
- Anand, N.K.; Carreira, E.M. A simple, mild, catalytic, enantioselective addition of terminal acetylenes to aldehydes. J. Am. Chem. Soc. 2001, 123, 9687–9688. [Google Scholar] [CrossRef]
- Sasaki, H.; Boyall, D.; Carreira, E.M. Facile, asymmetric addition of acetylene to aldehydes: In Situ generation of reactive zinc acetylide. Helv. Chim. Acta 2001, 84, 964–971. [Google Scholar] [CrossRef]
- Boyall, D.; Frantz, D.E.; Carreira, E.M. Efficient enantioselective additions of terminal alkynes and aldehydes under operationally convenient conditions. Org. Lett. 2002, 4, 2605–2606. [Google Scholar] [CrossRef]
- El-Sayed, E.; Anand, N.K.; Carreira, E.M. Asymmetric synthesis of γ-hydroxy α,β-unsaturated aldehydes via enantioselective direct addition of propargyl acetate to aldehydes. Org. Lett. 2001, 3, 3017–3020. [Google Scholar] [CrossRef]
- Boyall, D.; López, F.; Sasaki, H.; Frantz, D.; Carreira, E.M. Enantioselective addition of 2-methyl-3-butyn-2-ol to aldehydes: Preparation of 3-hydroxy-1-butynes. Org. Lett. 2000, 2, 4233–4236. [Google Scholar] [CrossRef]
- Gao, G.; Moore, D.; Xie, R.-G.; Pu, L. Highly enantioselective phenylacetylene additions to both aliphatic and aromatic aldehydes. Org. Lett. 2002, 4, 4143–4146. [Google Scholar] [CrossRef]
- Moore, D.; Pu, L. BINOL-catalyzed highly enantioselective terminal alkyne additions to aromatic aldehydes. Org. Lett. 2002, 4, 1855–1857. [Google Scholar] [CrossRef]
- Xu, M.-H.; Pu, L. A new 1,1'-binaphthyl-based catalyst for the enantioselective phenylacetylene addition to aromatic aldehydes without using a titanium complex. Org. Lett. 2002, 4, 4555–4557. [Google Scholar] [CrossRef]
- Moore, D.; Huang, W.-S.; Xu, M.-H.; Pu, L. Greatly enhanced enantioselectivity by an apparently remote steric effect in the 1,1'-binaphthyl-catalyzed alkynylzinc addition to aldehydes. Tetrahedron Lett. 2002, 43, 8831–8834. [Google Scholar] [CrossRef]
- Liu, L.; Pu, L. 3,3'-Functionalized octahydro-BINOL: A facile synthesis and its highenantioselectivity in the alkyne addition to aldehydes. Tetrahedron 2004, 60, 7427–7430. [Google Scholar] [CrossRef]
- Li, Z.-B.; Pu, L. BINOL-salen-catalyzed highly enantioselective alkyne additions to aromatic aldehydes. Org. Lett. 2004, 6, 1065–1068. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.-Y.; Yu, X.-Q.; Pu, L. 1,1-Binaphthyl ligands with bulky 3,3-thtertiaryalkyl substituents for the asymmetric alkyne addition to aromatic aldehydes. Tetrahedron 2007, 63, 4422–4428. [Google Scholar] [CrossRef]
- Yue, Y.; Turlington, M.; Yu, X.-Q.; Pu, L. 3,3'-Anisyl-substituted BINOL, H4BINOL, and H8BINOL ligands: Asymmetric synthesis of diverse propargylic alcohols and their ring-closing metathesis to chiral cycloalkenes. J. Org. Chem. 2009, 74, 8681–8689. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Wang, L.; Yu, X.-Q.; Huang, D.-S.; Pu, L. Synthesis of a C1 symmetric BINOL-terpyridine ligand and highly enantioselective methyl propiolate addition to aromatic aldehydes. Tetrahedron 2010, 66, 1990–1993. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Q.; Yu, X.-Q.; Xie, R.-G.; Pu, L. Highly enantioselective synthesis of γ-hydroxy-α, β-acetylenic esters by asymmetric alkyne addition to aldehydes. Angew. Chem. Int. Ed. Engl. 2006, 45, 122–125. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Chan, W.L.; Chan, A.S.C. Titanium-catalyzed enantioselective alkynylation of aldehydes. Chem. Commun. 2002, 21, 172–173. [Google Scholar]
- Li, X.; Lu, G.; Kwok, W.H.; Chan, A.S.C. Highly enantioselective alkynylzinc addition to aromatic aldehydes catalyzed by self-assembled titanium catalysts. J. Am. Chem. Soc. 2002, 124, 12636–12637. [Google Scholar]
- Lu, G.; Li, X.; Zhou, Z.; Chan, W.L.; Chan, A.S.C. Enantioselective alkynylation of aromatic aldehydes catalyzed by new chiral amino alcohol-based ligands. Tetrahedron 2001, 12, 2147–2152. [Google Scholar]
- Ruan, J.; Lu, G.; Xu, L.; Li, Y.-M.; Chan, A.S.C. Catalytic asymmetric alkynylation and arylation of aldehydes by an H8-binaphthyl-based amino alcohol ligand. Adv. Synth. Catal. 2008, 350, 76–84. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R.; Xu, J.; Da, C.-S.; Yan, W.-J.; Chen, C. Highly enantioselective addition of phenylacetylene to aldehydes catalyzed by a β-sulfonamide alcohol–titanium complex. Angew. Chem. Int. Ed. 2003, 42, 5747–5749. [Google Scholar]
- Kang, Y.-F.; Liu, L.; Wang, R.; Yan, W.-J.; Zhou, Y.-F. The use of bifunctional catalyst systems in the asymmetric addition of alkynylzinc to aldehydes. Tetrahedron 2004, 15, 3155–3159. [Google Scholar]
- Xu, Z.; Chen, C.; Xu, J.; Miao, M.; Yan, W.; Wang, R. Highly enantioselective addition of phenylacetylene to aldehydes catalyzed by a camphor sulfonamide ligand. Org. Lett. 2004, 6, 1193–1195. [Google Scholar] [CrossRef]
- Han, Z.-J.; Da, C.-S.; Xu, Z.-Q.; Ni, M.; Wang, R. The asymmetric addition of phenylacetylene to aldehydes catalyzed by l-leucine derived chiral sulfonamide alcohol ligands. J. Mol. Cat. A 2005, 236, 32–37. [Google Scholar] [CrossRef]
- Ni, M.; Wang, R.; Han, Z.-J.; Mao, B.; Da, C.-S.; Liu, L.; Chen, C. Synthesis of new C2-symmetrical bissulfonamide ligands and application in the enantioselective addition of alkynylzinc to aldehydes and ketones. Adv. Synth. Catal. 2005, 347, 1659–1665. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, L.; Xu, J.; Yan, W.; Wang, R. Asymmetric addition of phenylacetylene to aldehydes catalyzed by β-sulfonamide alcohol-titanium complex. Adv. Synth. Catal. 2006, 348, 506–514. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Liu, W.; Qiu, L.; Xu, Z.; Xu, J.; Chan, A.S.C.; Wang, R. Highly enantioselective synthesis of γ-hydroxy-α, β-acetylenic esters catalyzed by a β-sulfonamide alcohol. Org. Lett. 2007, 9, 2329–2332. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Q.; Lin, L.; Liu, X.D.; Jiang, X.X.; Zhao, Q.Y.; Hu, G.W.; Wang, R. Highly enantioselective addition of terminal alkynes to aldehydes catalyzed by a new chiral β-sulfonamide alcohol/Ti(OiPr)4/Et2Zn/R3N catalyst system. Chirality 2009, 21, 316–323. [Google Scholar] [CrossRef]
- Trost, B.M.; Weiss, A.H.; von Wangelin, A.J. Dinuclear Zn-catalyzed asymmetric alkynylation of unsaturated aldehydes. J. Am. Chem. Soc. 2006, 128, 8–9. [Google Scholar]
- Trost, B.M.; Sieber, J.D.; Qian, W.; Dhawan, R.; Ball, Z.T. Asymmetric total synthesis of soraphen A: A flexible alkyne strategy. Angew. Chem. Int. Ed.Engl. 2009, 48, 5478–5481. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, V.S.; Yamamoto, D. Enantioselective prophenol-catalyzed addition of 1,3-diynes to aldehydes to generate synthetically versatile building blocks and diyne natural products. J. Am. Chem. Soc. 2010, 132, 5186–5192. [Google Scholar] [CrossRef]
- Trost, B.M.; O’Boyle, B.M. Exploiting orthogonally reactive functionality: Synthesis and stereochemical assignment of (−)-ushikulide A. J. Am. Chem. Soc. 2008, 130, 16190–16192. [Google Scholar] [CrossRef]
- Zhong, J.C.; Wang, M.A.; Guo, H.C.; Bian, Q.H.; Wang, M. Design and synthesis of 1,4-amino alcohol ligands with a chiral cyclopropane backbone for asymmetric diethylzinc addition to aromatic aldehydes. Synlett 2006, 11, 1667–1670. [Google Scholar]
- Zhong, J.C.; Wang, M.A.; Guo, H.C.; Yin, M.M.; Wang, M. Asymmetric diethylzinc addition and phenyl transfer to aldehydes using chiral cis-cyclopropane-based amino alcohols. Tetrahedron Asymmetry 2007, 18, 734–741. [Google Scholar] [CrossRef]
- Zhong, J.C.; Hou, S.C.; Bian, Q.H.; Yin, M.M.; Na, R.S.; Zheng, B.; Li, Z.Y.; Liu, S.Z.; Wang, M. Highly enantioselective zinc/amino alcohol-catalyzed alkynylation of aldehydes. Chem. Eur. J. 2009, 15, 3069–3071. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, M.; Bian, Q.H.; Zheng, B.; Mao, J.Y.; Li, S.N.; Liu, S.Z.; Wang, M.A.; Zhong, J.C.; Guo, H.C. Highly enantioselective addition of trimethylsilylacetylene to aldehydes catalyzed by a zinc-amino-alcohol complex. Chem. Eur. J. 2011, 17, 5782–5786. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, M.; Li, Z.Y.; Bian, Q.H.; Mao, J.Y.; Li, S.N.; Liu, S.Z.; Zhong, J.C.; Guo, H.C. Asymmetric henry reaction catalyzed by a Zn-amino alcohol system. Tetrahedron Asymmetry 2011, 22, 1156–1160. [Google Scholar] [CrossRef]
- Mao, J.Y.; Nie, X.; Wang, M.; Wang, Q.; Zheng, B.; Bian, Q.H.; Zhong, J.C. Catalytic asymmetric nitroaldol (Henry) reactions with copper(II)/cyclopropane-based bisoxazoline complexes. Tetrahedron Asymmetry 2012, 23, 965–971. [Google Scholar] [CrossRef]
- Dahmen, S. Enantioselective alkynylation of aldehydes catalyzed by [2.2]paracyclophane-based ligands. Org. Lett. 2004, 6, 2113–2116. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7a–d are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).