Astataricusones A–D and Astataricusol A, Five New Anti-HBV Shionane-Type Triterpenes from Aster tataricus L. f.
Abstract
:1. Introduction
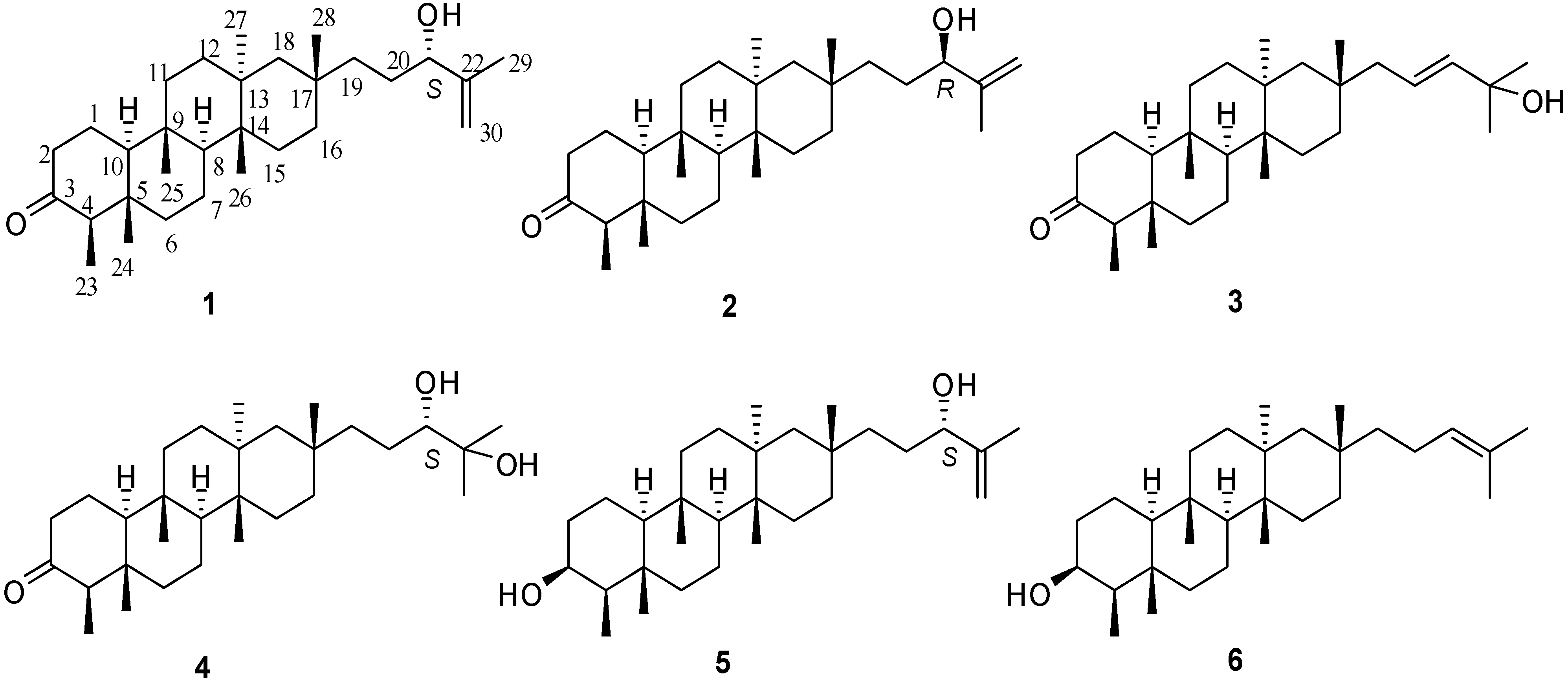
2. Results and Discussion
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 22.3, CH2 | 22.2, CH2 | 22.3, CH2 | 22.3, CH2 | 15.8, CH2 |
| 2 | 41.4, CH2 | 41.4, CH2 | 41.4, CH2 | 41.4, CH2 | 35.2, CH2 |
| 3 | 213.3, C | 213.1, C | 213.2, C | 213.3, C | 72.7, CH |
| 4 | 58.1, CH | 58.1, CH | 58.1, CH | 58.2, CH | 49.1, CH |
| 5 | 42.1, C | 42.1, C | 42.1, C | 42.1, C | 37.8, C |
| 6 | 41.0, CH2 | 41.1, CH2 | 41.0, CH2 | 41.1, CH2 | 41.6, CH2 |
| 7 | 17.8, CH2 | 17.8, CH2 | 17.9, CH2 | 17.9, CH2 | 17.2, CH2 |
| 8 | 49.8, CH | 49.8, CH | 49.8, CH | 49.9, CH | 49.9, CH |
| 9 | 38.4, C | 38.4, C | 38.4, C | 38.4, C | 38.1, C |
| 10 | 59.5, CH | 59.6, CH | 59.5, CH | 59.6, CH | 61.5, CH |
| 11 | 35.2, CH2 | 35.2, CH2 | 35.2, CH2 | 35.2, CH2 | 35.1, CH2 |
| 12 | 32.2, CH2 | 32.2, CH2 | 32.1, CH2 | 32.2, CH2 | 32.4, CH2 |
| 13 | 36.8, C | 36.8, C | 36.9, C | 36.9, C | 36.8, C |
| 14 | 38.5, C | 38.5, C | 38.4, C | 38.6, C | 38.6, C |
| 15 | 29.1, CH2 | 29.1, CH2 | 29.2, CH2 | 29.1, CH2 | 29.1, CH2 |
| 16 | 34.7, CH2 | 34.5, CH2 | 33.8, CH2 | 34.7, CH2 | 34.7, CH2 |
| 17 | 31.3, C | 31.4, C | 32.1, C | 31.6, C | 31.4, C |
| 18 | 44.4, CH2 | 44.4, CH2 | 45.3, CH2 | 44.5, CH2 | 44.5, CH2 |
| 19 | 38.9, CH2 | 38.8, CH2 | 45.8, CH2 | 26.5, CH2 | 38.9, CH2 |
| 20 | 29.7, CH2 | 25.6, CH2 | 124.2, CH | 40.5, CH2 | 29.8, CH2 |
| 21 | 76.9, CH | 90.5, CH | 140.7, CH | 79.5, CH | 77.0, CH |
| 22 | 147.4, C | 143.5, C | 70.8, C | 73.2, C | 147.4, C |
| 23 | 6.8, CH3 | 6.8, CH3 | 6.8, CH3 | 6.8, CH3 | 11.6, CH3 |
| 24 | 14.6, CH3 | 14.6, CH3 | 14.6, CH3 | 14.6, CH3 | 16.4, CH3 |
| 25 | 19.6, CH3 | 19.5, CH3 | 19.6, CH3 | 19.6, CH3 | 20.0, CH3 |
| 26 | 15.1, CH3 | 15.1, CH3 | 15.1, CH3 | 15.2, CH3 | 15.0, CH3 |
| 27 | 20.5, CH3 | 20.5, CH3 | 21.0, CH3 | 20.6, CH3 | 20.6, CH3 |
| 28 | 33.0, CH3 | 32.7, CH3 | 32.9, CH3 | 32.9, CH3 | 33.0, CH3 |
| 29 | 17.3, CH3 | 17.0, CH3 | 29.9, CH3 | 23.2, CH3 | 17.3, CH3 |
| 30 | 111.2, CH2 | 114.6, CH2 | 29.9, CH3 | 26.5, CH3 | 111.2, CH2 |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1a | 1.95, m | 1.98, m | 1.98, overlap | 1.98, m | 1.58, overlap |
| 1b | 1.67, overlap | 1.69, overlap | 1.69, overlap | 1.69, overlap | 1.46, overlap |
| 2a | 2.36, m | 2.39, m | 2.38, m | 2.39, m | 1.90, m |
| 2b | 2.27, m | 2.32, m | 2.30, overlap | 2.30, m | − |
| 3 | − | − | − | − | 3.73, m |
| 4 | 2.21, q (6.6) | 2.25, q (6.6) | 2.25, q (6.4) | 2.25, q (6.7) | 1.25, overlap |
| 6a | 1.69, overlap | 1.74, overlap | 1.72, overlap | 1.72, overlap | 1.72, overlap |
| 6b | 1.20, overlap | 1.25, overlap | 1.26, overlap | 1.25, overlap | 0.92, overlap |
| 7a | 1.47, overlap | 1.47, m | 1.49, m | 1.50, overlap | − |
| 7b | 1.30, overlap | 1.32, overlap | 1.32, overlap | 1.32, overlap | 1.37, overlap |
| 8 | 1.30, overlap | 1.32, overlap | 1.32, overlap | 1.32, overlap | 1.25, overlap |
| 10 | 1.54, overlap | 1.60, overlap | 1.60, overlap | 1.60, overlap | 0.94, overlap |
| 11a | 1.51, overlap | 1.53, overlap | 1.53, overlap | 1.50, overlap | 1.58, overlap |
| 11b | 1.39, m | 1.42, overlap | 1.40, overlap | 1.41, overlap | 1.37, overlap |
| 12a | 1.54, overlap | 1.53, overlap | 1.53, overlap | 1.60, overlap | 1.56, overlap |
| 12b | 0.89, overlap | 0.90, overlap | 0.91, overlap | 0.91, overlap | 0.87, overlap |
| 15 | 1.27, m | 1.25, overlap | 1.32, overlap | 1.32, overlap | 1.25, overlap |
| 16a | 1.61, overlap | 1.60, overlap | 1.60, overlap | 1.66, overlap | 1.61, overlap |
| 16b | 1.35, m | 1.32, overlap | 1.40, overlap | 1.41, overlap | 1.37, overlap |
| 18a | 1.17, overlap | 1.21, d (14.4) | 1.21, d (14.7) | 1.25, overlap | 1.19, overlap |
| 18b | 1.08, overlap | 1.08, d (14.4) | 1.09, d (14.7) | 1.07, overlap | 1.08, overlap |
| 19a | 1.57, overlap | 1.78, m | 2.33, overlap | 1.54, overlap | 1.59, overlap |
| 19b | 1.20, overlap | 1.11, overlap | 1.98, overlap | 1.23, overlap | 1.19, overlap |
| 20a | 1.57, overlap | 1.60, overlap | 5.66, m | 1.66, overlap | 1.61, overlap |
| 20b | 1.44, overlap | 1.39, overlap | − | 1.54, overlap | 1.46, overlap |
| 21 | 3.97, t (6.1) | 4.26, t (6.8) | 5.58, d (15.7) | 3.28, d (9.7) | 3.99, t (6.2) |
| 23 | 0.84, d (6.6) | 0.86, d (6.6) | 0.87, d (6.4) | 0.87, d (6.7) | 0.93, d (7.3) |
| 24 | 0.68, s | 0.71, s | 0.71, s | 0.71, s | 0.94, s |
| 25 | 0.89, s | 0.91, s | 0.91, s | 0.92, s | 0.90, s |
| 26 | 0.86, overlap | 0.88, s | 0.88, overlap | 0.88, s | 0.87, s |
| 27 | 1.08, s | 1.11, s | 1.13, s | 1.11, s | 1.08, s |
| 28 | 0.86, overlap | 0.88, s | 0.88, overlap | 0.89, s | 0.87, s |
| 29 | 1.69, s | 1.74, s | 1.32, overlap | 1.22, s | 1.72, s |
| 30a | 4.90, s | 5.05, s | 1.32, overlap | 1.17, s | 4.92, s |
| 30b | 4.81, s | 5.02, s | − | − | 4.83, s |
 ) and HMBC (H
) and HMBC (H  C) correlations of 1–5.
C) correlations of 1–5.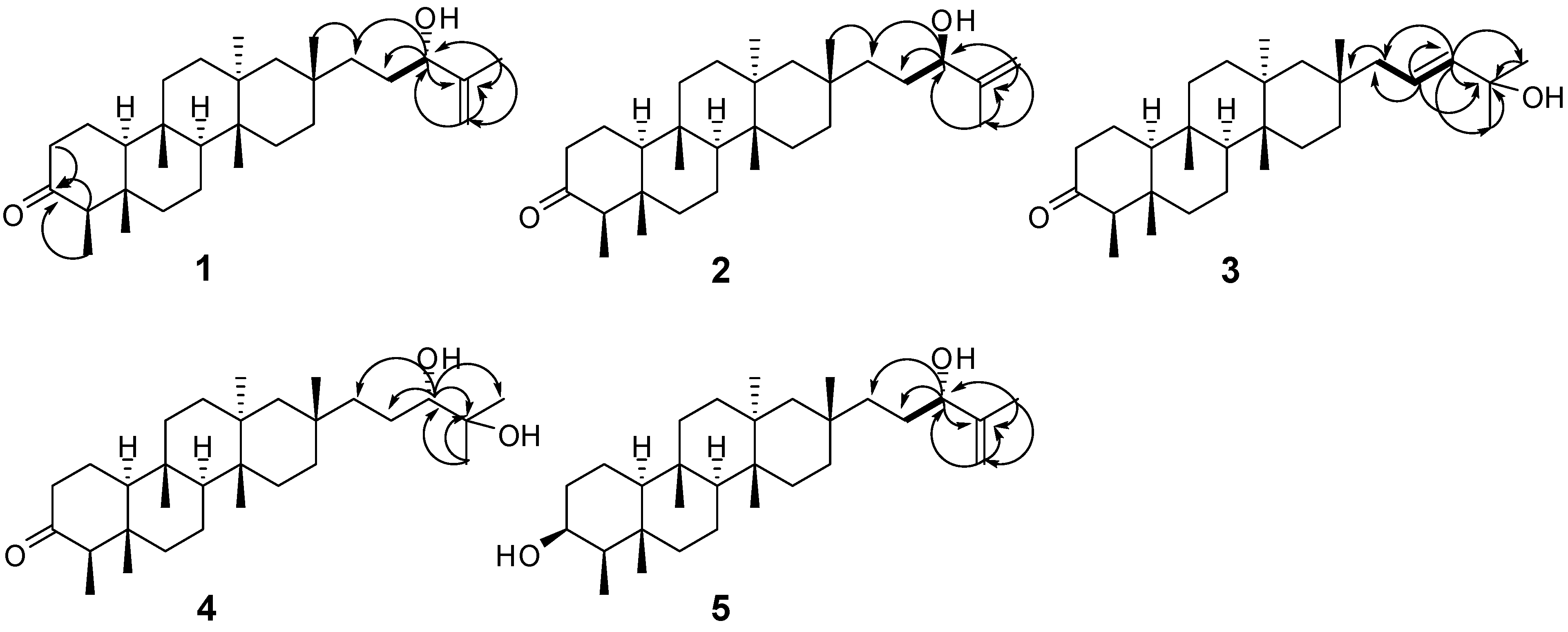
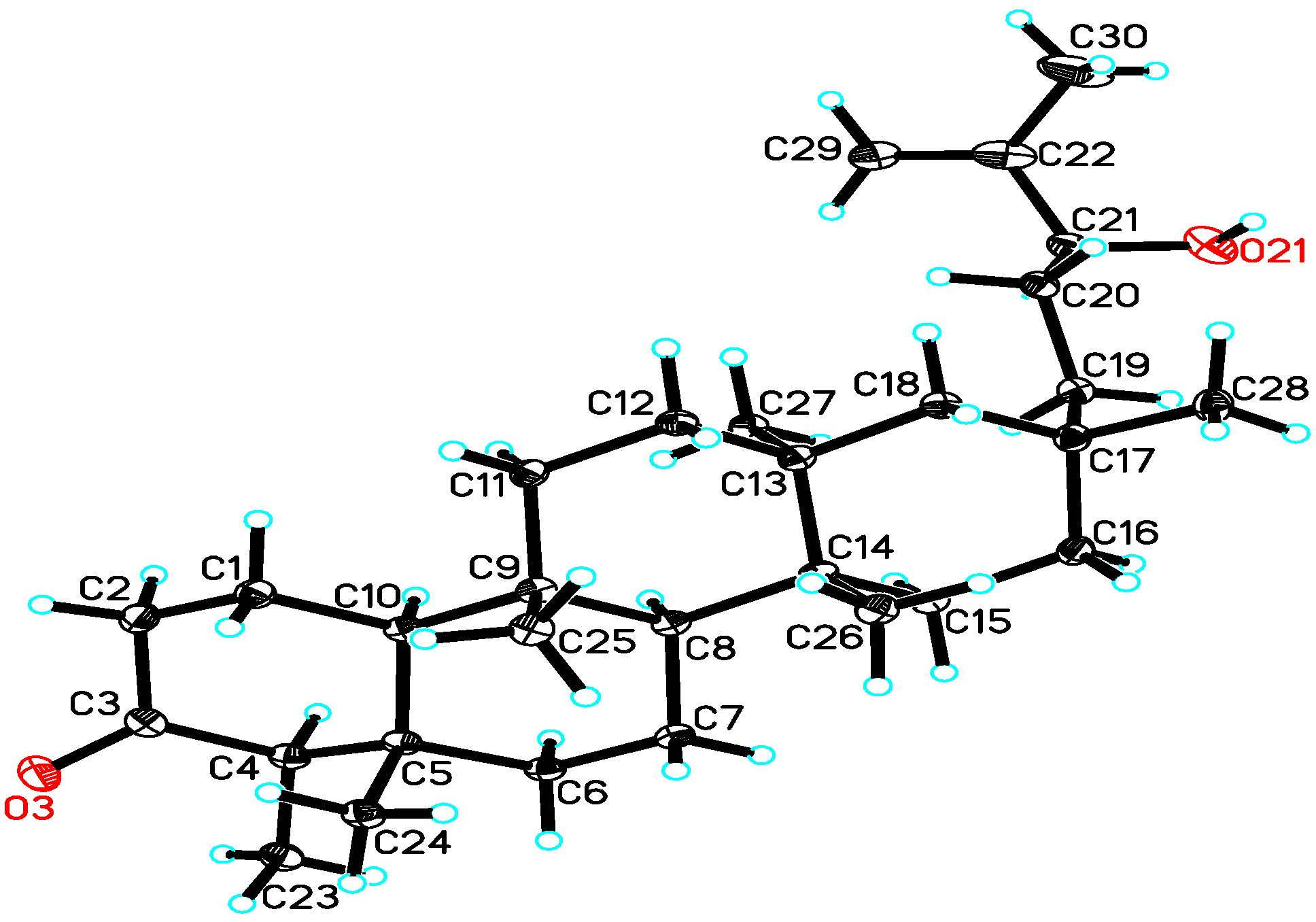
3. Experimental
3.1. General
3.2. Plant Material
3.3. In Vitro Anti-HBV Assay
3.3.1. Cell Line and Cell Culture
3.3.2. Analysis of Secreted HBV Antigens
3.3.3. Assay for HBV DNA Replication
3.3.4. Cytotoxicity Assay
3.4. Extraction and Isolation
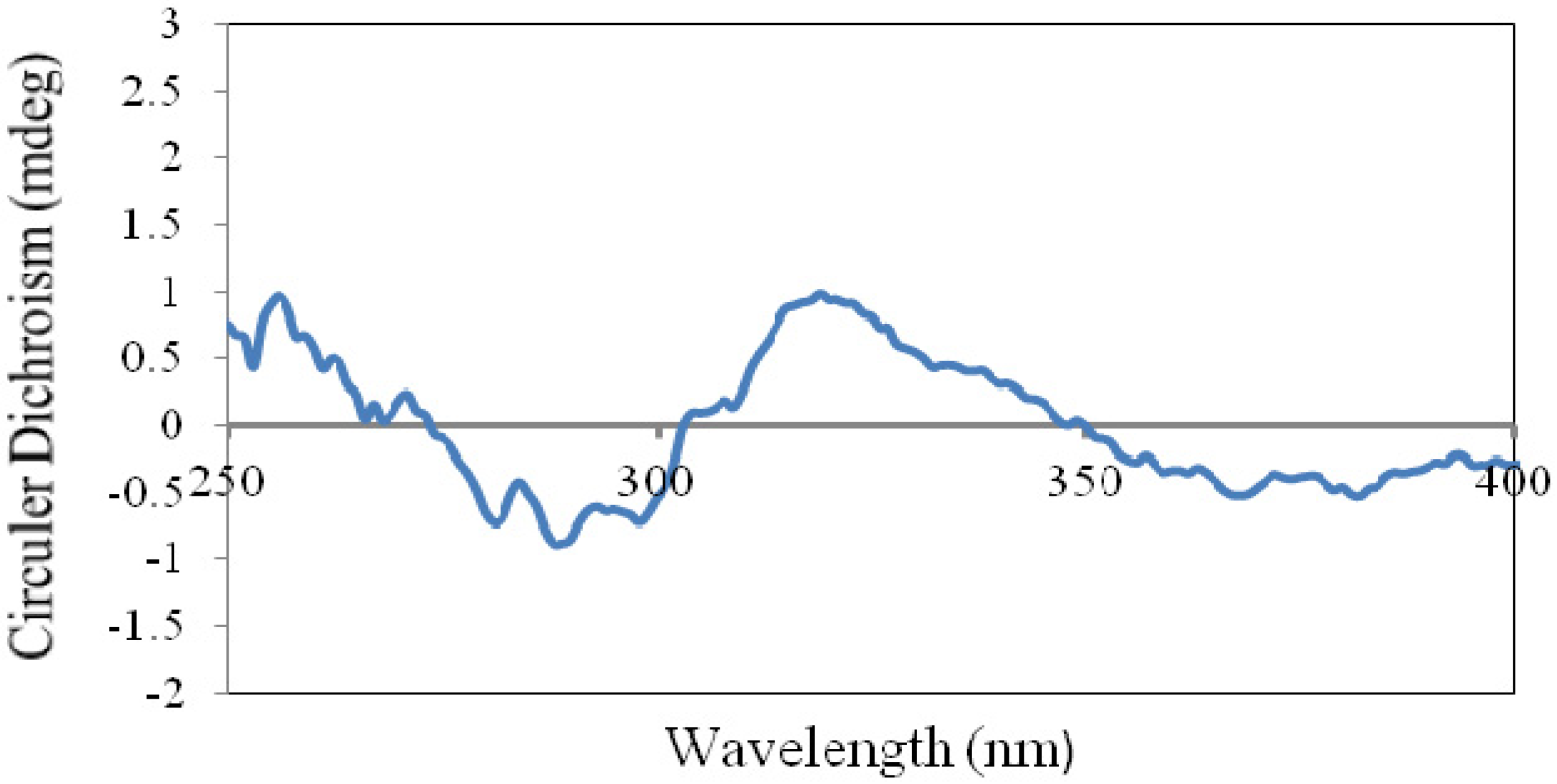
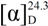 −33.8 (c 1.33, MeOH); UV (MeOH) λmax (logε) 202 (3.12) nm; CD (c 0.64, MeOH) λ (Δε) 290 (−9.5) nm; IR (KBr) νmax 3,498, 2,955, 2,927, 1,697, 1,467, 1,451, 1,390, 1,074, 894 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3808 [M]+ (calcd. for C30H50O2, 442.3811).
−33.8 (c 1.33, MeOH); UV (MeOH) λmax (logε) 202 (3.12) nm; CD (c 0.64, MeOH) λ (Δε) 290 (−9.5) nm; IR (KBr) νmax 3,498, 2,955, 2,927, 1,697, 1,467, 1,451, 1,390, 1,074, 894 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3808 [M]+ (calcd. for C30H50O2, 442.3811).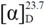 −5.9 (c 0.27, MeOH); UV (MeOH) λmax (log ε) 209 (2.87) nm; CD (c 0.61, MeOH) λ (Δε) 290 (−2.5) nm; IR (KBr) νmax 3,441, 3,425, 2,961, 2,926, 1,704, 1,629, 1,262, 1,097, 1,024, 803 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3804 [M]+ (calcd. for C30H50O2, 442.3811).
−5.9 (c 0.27, MeOH); UV (MeOH) λmax (log ε) 209 (2.87) nm; CD (c 0.61, MeOH) λ (Δε) 290 (−2.5) nm; IR (KBr) νmax 3,441, 3,425, 2,961, 2,926, 1,704, 1,629, 1,262, 1,097, 1,024, 803 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3804 [M]+ (calcd. for C30H50O2, 442.3811).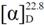 −18.6 (c 0.80, MeOH); UV (MeOH) λmax (logε) 202 (3.18) nm; CD (c 0.64, MeOH) λ (Δε) 290 (−7.8) nm; IR (KBr) νmax 3,533, 3,448, 2,933, 2,876, 1,711, 1,466, 1,387, 1,187, 972 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3824 [M]+ (calcd. for C30H50O2, 442.3811).
−18.6 (c 0.80, MeOH); UV (MeOH) λmax (logε) 202 (3.18) nm; CD (c 0.64, MeOH) λ (Δε) 290 (−7.8) nm; IR (KBr) νmax 3,533, 3,448, 2,933, 2,876, 1,711, 1,466, 1,387, 1,187, 972 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 442.3824 [M]+ (calcd. for C30H50O2, 442.3811).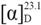 −98.1 (c 0.21, MeOH); UV (MeOH) λmax (logε) 204 (3.41) nm; CD (c 0.60, DMSO) λ (Δε) 290 (−5.8), 300 (−5.7) nm; IR (KBr) νmax 3,475, 3,443, 2,934, 1,701, 1,466, 1,388, 1,072 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 460.3922 [M]+ (calcd. for C30H52O3, 460.3916).
−98.1 (c 0.21, MeOH); UV (MeOH) λmax (logε) 204 (3.41) nm; CD (c 0.60, DMSO) λ (Δε) 290 (−5.8), 300 (−5.7) nm; IR (KBr) νmax 3,475, 3,443, 2,934, 1,701, 1,466, 1,388, 1,072 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 460.3922 [M]+ (calcd. for C30H52O3, 460.3916).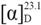 −19.0 (c 0.25, MeOH); UV (MeOH) λmax (logε) 193 (2.56), 202 (2.99) nm; CD (c 0.63, MeOH) λ (Δε) 290 (−0.8) nm; IR (KBr) νmax 3,439, 2,941, 2,925, 1,631, 1,463, 1,376 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 444.3973 [M]+ (calcd. for C30H52O2, 444.3967).
−19.0 (c 0.25, MeOH); UV (MeOH) λmax (logε) 193 (2.56), 202 (2.99) nm; CD (c 0.63, MeOH) λ (Δε) 290 (−0.8) nm; IR (KBr) νmax 3,439, 2,941, 2,925, 1,631, 1,463, 1,376 cm–1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3), see Table 1 and Table 2; HREIMS m/z 444.3973 [M]+ (calcd. for C30H52O2, 444.3967).3.5. X-ray Crystallographic Analysis
3.6. Determination of Absolute Configuration of the 21,22-Diol Group in 4
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Sawai, S.; Uchiyama, H.; Mizuno, S.; Aoki, T.; Akashi, T.; Ayabe, S.-I.; Takahashi, T. Molecular characterization of an oxidosqualene cyclase that yields shionone, a unique tetracyclic triterpene ketone of Aster tataricus. FEBS Lett. 2011, 585, 1031–1036. [Google Scholar] [CrossRef]
- Patil, F.; Ourisson, G.; Tanahashi, Y.; Takahashi, T. Shionone structural study. II. Bull. Soc. Chim. Fr. 1964, 6, 1422–1423. [Google Scholar]
- Takahashi, T.; Moriyama, Y.; Tanahashi, Y.; Ourisson, G. The structure of shionone. Tetrahedron Lett. 1967, 8, 2991–2996. [Google Scholar] [CrossRef]
- Ireland, R.E.; Kowalski, C.J.; Tilly, J.W.; Walba, D.M. Total synthesis of terpenes. XX. Total synthesis of (±)-shionone, a tetracyclic triterpene. J. Org. Chem. 1975, 40, 990–1000. [Google Scholar] [CrossRef]
- Ireland, R.E.; Lipinski, C.A.; Kowaiski, C.J.; Tilley, J.W.; Walba, D.M. Total synthesis of dl-shionone, a tetracyclic triterpene. J. Am. Chem. Soc. 1974, 96, 3333. [Google Scholar] [CrossRef]
- Zhao, S.M.; Kuang, B.; Peng, W.W.; He, W.J.; Xu, H.M.; Ji, C.J.; Han, J.; Zheng, Y.Q.; Song, W.W.; Tan, N.H. Chemical progress in cyclopeptide-containing traditional medicines cited in Chinese pharmacopoeia. Chin. J. Chem. 2012, 30, 1213–1215. [Google Scholar] [CrossRef]
- Lai, G.F.; Chen, J.J.; Luo, S.D. Advances in the studies of chemical constituents and pharmacological activities of Aster L. Nat. Prod. Res. Dev. 2002, 14, 65–70. [Google Scholar]
- Lu, Y.H.; Dai, Y.; Wang, Z.T. Polyphenolic compounds from Aster tataricus. Chin. Tradit. Herbal Drugs 1999, 30, 360–362. [Google Scholar]
- Zhou, W.B.; Tao, J.Y.; Xu, H.M.; Chen, K.L.; Zeng, G.Z.; Ji, C.J.; Zhang, Y.M.; Tan, N.H. Three new antiviral triterpenes from Aster tataricus. Z. Naturforsch. 2010, 65b, 1393–1396. [Google Scholar]
- Tan, N.H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef]
- Xu, H.M.; Yi, H.; Zhou, W.B.; He, W.J.; Zeng, G.Z.; Xu, W.Y.; Tan, N.H. Tataricins A and B, two novel cyclotetrapeptides from Aster tataricus, and their absolute configuration assignment. Tetrahedron Lett. 2013, 54, 1380–1383. [Google Scholar] [CrossRef]
- Xu, H.M.; Zeng, G.Z.; Zhou, W.B.; He, W.J.; Tan, N.H. Astins K–P, six new chlorinated cyclopentapeptides from Aster tataricus. Tetrahedron 2013, 69, 7964–7969. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, Q.; Xu, H.M.; Gong, F.Y.; Zhou, X.B.; Sun, Y.; Wu, X.F.; Liu, W.; Zeng, G.Z.; Tan, N.H.; et al. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclopeptide, for preventing murine experimental colitis. Biochem. Pharmacol. 2011, 82, 260–268. [Google Scholar] [CrossRef]
- Yan, F.L.; Yao, S.M.; Zhou, Y. Two new tetracyclic triterpenoids and other constituents from Aster ageratoides var. oophyllus. J. Chin. Chem. Soc. 2007, 54, 1321–1324. [Google Scholar]
- Toshihiro, A.; Yumiko, K.; Kazuo, K.; Takaaki, T.; Ken, Y.; Koichi, A.S.; Tamotsu, N.Y. Astertarone A: A triterpenoid ketone isolated from the roots of Aster tataricus L. Chem. Pharm. Bull. 1998, 46, 1824–1826. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Moriyama, Y.; Takahashi, T.; Patil, F.; Biellmann, J.-F.; Ourisson, G. Structure of shionone. III. Side chain. Bull. Soc. Chim. Fr. 1966, 5, 1670–1677. [Google Scholar]
- CCDC 926578 (1) contains the supplementary crystallographic data. Available online: www.ccdc.cam.ac.uk/cgi-bin/catreq.cgi?access=referee/ (accessed on 20 November 2013).
- Politi, M.; de Tommasi, N.; Pescitelli, G.; di Bari, L.; Morelli, L.; Brace, A. Structure and absolute configuration of new diterpenes from Lavandula multifida. J. Nat. Prod. 2002, 65, 1742–1745. [Google Scholar] [CrossRef]
- Snatzke, G. Circular dichroism and absolute conformation: Application of qualitative MO theory to chiroptical phenomena. Angew. Chem. Int. Ed. Engl. 1979, 18, 363–377. [Google Scholar] [CrossRef]
- Kim, T.G.; Kang, S.Y.; Jung, K.K.; Kang, J.H.; Lee, E.; Han, H.M.; Kim, S.H. Antiviral activities of extracts isolated from Terminalis chebula Retz., Sanguisorba officinalis L., Rubus coreanus Miq. and Rheum palmatum L. against hepatitis B virus. Phytother. Res. 2001, 15, 718–720. [Google Scholar] [CrossRef]
- Li, H.B.; Zhou, C.X.; Zhou, L.F.; Chen, Z.; Yang, L.X.; Bai, H.; Wu, X.M.; Peng, H.; Zhao, Y. In vitro antiviral activity of three enantiomeric sesquiterpene lactones from Senecio species against hepatitis B virus. Antivir. Chem. Chemother. 2005, 16, 277–282. [Google Scholar]
- Han, H.J.; Tan, N.H.; Zeng, G.Z.; Fan, J.T.; Huang, H.Q.; Ji, C.J.; Jia, R.R.; Zhao, Q.S.; Zhang, Y.J.; Hao, X.J.; et al. Natural inhibitors of DNA topoisomerase I with cytotoxicities. Chem. Biodivers. 2008, 5, 1364–1368. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, W.-B.; Zeng, G.-Z.; Xu, H.-M.; He, W.-J.; Tan, N.-H. Astataricusones A–D and Astataricusol A, Five New Anti-HBV Shionane-Type Triterpenes from Aster tataricus L. f. Molecules 2013, 18, 14585-14596. https://doi.org/10.3390/molecules181214585
Zhou W-B, Zeng G-Z, Xu H-M, He W-J, Tan N-H. Astataricusones A–D and Astataricusol A, Five New Anti-HBV Shionane-Type Triterpenes from Aster tataricus L. f. Molecules. 2013; 18(12):14585-14596. https://doi.org/10.3390/molecules181214585
Chicago/Turabian StyleZhou, Wen-Bing, Guang-Zhi Zeng, Hui-Min Xu, Wen-Jun He, and Ning-Hua Tan. 2013. "Astataricusones A–D and Astataricusol A, Five New Anti-HBV Shionane-Type Triterpenes from Aster tataricus L. f." Molecules 18, no. 12: 14585-14596. https://doi.org/10.3390/molecules181214585
APA StyleZhou, W.-B., Zeng, G.-Z., Xu, H.-M., He, W.-J., & Tan, N.-H. (2013). Astataricusones A–D and Astataricusol A, Five New Anti-HBV Shionane-Type Triterpenes from Aster tataricus L. f. Molecules, 18(12), 14585-14596. https://doi.org/10.3390/molecules181214585




