Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of TP-SLN
2.2. Designing the Models
| Variables | Responses | |||||
|---|---|---|---|---|---|---|
| Experiments | X1 | X2 | X3 | Y1 | Y2 | Y3 |
| 1 | 55.77 | 2.85 | 89.43 | 421 | 48.1 | 0.54 |
| 2 | 50.00 | 4.00 | 75.00 | 113.3 | 52.4 | 0.70 |
| 3 | 44.23 | 2.85 | 60.57 | 117.3 | 50.5 | 0.83 |
| 4 | 50.00 | 4.00 | 50.00 | 123.5 | 53.3 | 1.07 |
| 5 | 44.23 | 5.15 | 60.57 | 79.5 | 44.0 | 0.73 |
| 6 | 55.77 | 5.15 | 60.57 | 131.5 | 47.7 | 0.79 |
| 7 | 55.77 | 2.85 | 60.57 | 391.9 | 54.0 | 0.89 |
| 8 | 50.00 | 4.00 | 100.00 | 125.4 | 45.8 | 0.46 |
| 9 | 50.00 | 4.00 | 75.00 | 124.1 | 50.6 | 0.67 |
| 10 | 44.23 | 5.15 | 89.43 | 95.4 | 41.5 | 0.46 |
| 11 | 44.23 | 2.85 | 89.43 | 113.7 | 42.4 | 0.47 |
| 12 | 60.00 | 4.00 | 75.00 | 327.7 | 45.0 | 0.60 |
| 13 | 50.00 | 4.00 | 75.00 | 116.5 | 51.0 | 0.68 |
| 14 | 55.77 | 5.15 | 89.43 | 131.8 | 44.8 | 0.50 |
| 15 | 50.00 | 6.00 | 75.00 | 95.9 | 40.9 | 0.55 |
| 16 | 50.00 | 2.00 | 75.00 | 540.8 | 52.5 | 0.70 |
| 17 | 50.00 | 4.00 | 75.00 | 116.3 | 49.2 | 0.66 |
| 18 | 40.00 | 4.00 | 75.00 | 80.9 | 42.1 | 0.56 |
| 19 | 50.00 | 4.00 | 75.00 | 121.7 | 50.0 | 0.67 |
| 20 | 50.00 | 4.00 | 75.00 | 120.3 | 49.6 | 0.66 |
| Source | Particle size | Encapsulation efficiency | Drug loading | |||
|---|---|---|---|---|---|---|
| coefficient | p-value | coefficient | p-value | coefficient | p-value | |
| Model | <0.0001 | <0.0001 | <0.0001 | |||
| Intercept | 118.70 | 50.47 | 0.67 | |||
| X1 | 78.41 | <0.0001 | 1.52 | 0.0016 | 0.020 | 0.0029 |
| X2 | −98.31 | <0.0001 | −2.65 | <0.0001 | −0.038 | <0.0001 |
| X3 | 3.21 | 0.7522 | −2.31 | <0.0001 | −0.17 | <0.0001 |
| X1 X2 | −61.69 | 0.0008 | −0.27 | 0.5713 | −0.0029 | 0.6796 |
| X1 X3 | 2.15 | 0.8730 | 0.23 | 0.6423 | −0.0023 | 0.7484 |
| X2 X3 | −1.17 | 0.9305 | 1.08 | 0.0452 | 0.021 | 0.0140 |
| X12 | 22.74 | 0.0349 | −2.30 | <0.0001 | −0.031 | <0.0001 |
| X22 | 60.75 | <0.0001 | −1.25 | 0.0039 | −0.017 | 0.0056 |
| X32 | −3.88 | 0.6861 | −0.30 | 0.3902 | 0.029 | 0.0001 |
2.3. Response Surface Analysis
2.3.1. Effects on Particle Size

2.3.2. Effects on Encapsulation Efficiency (EE)
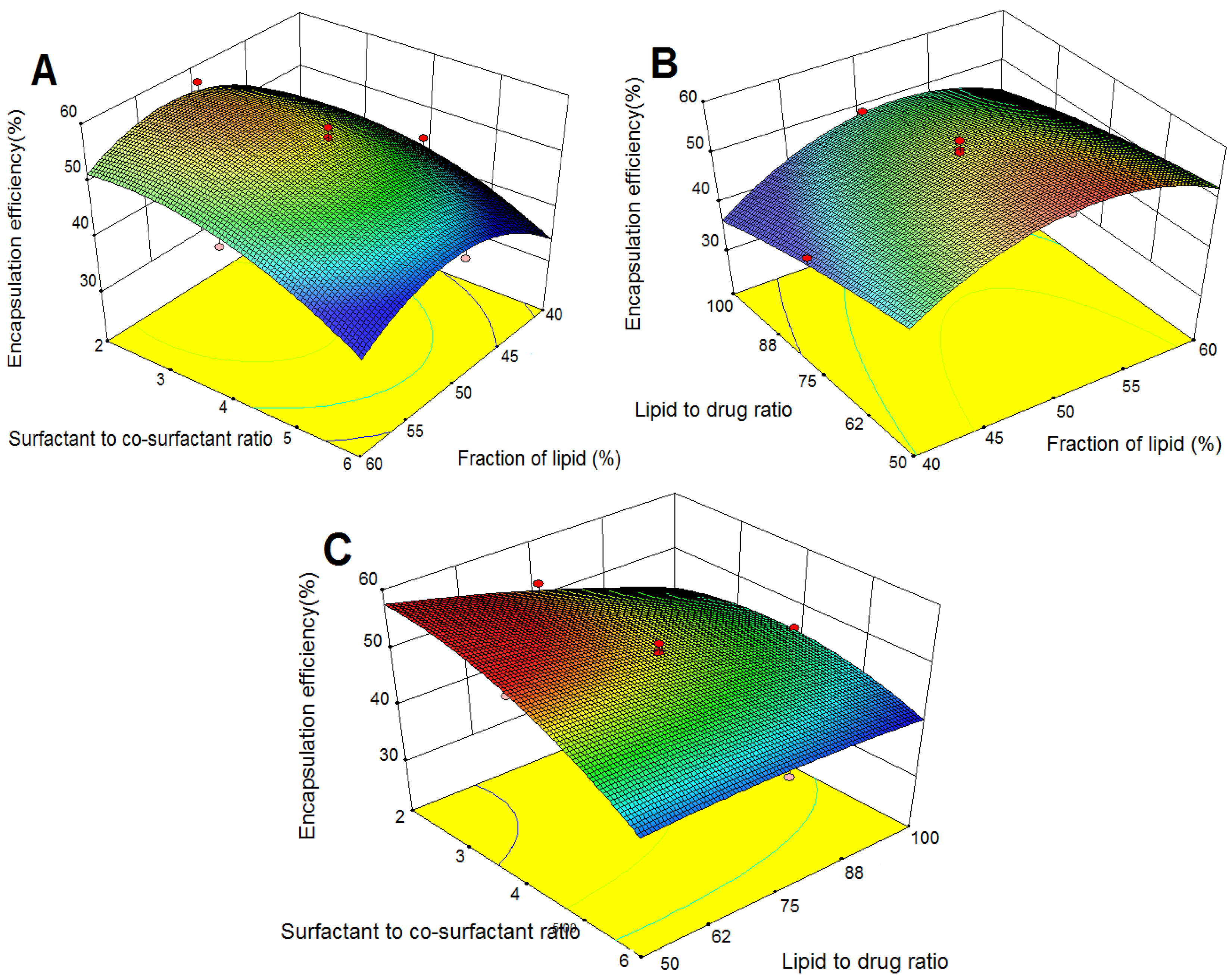
2.3.3. Effects on Drug Loading (DL)

2.4. Optimization and Validation
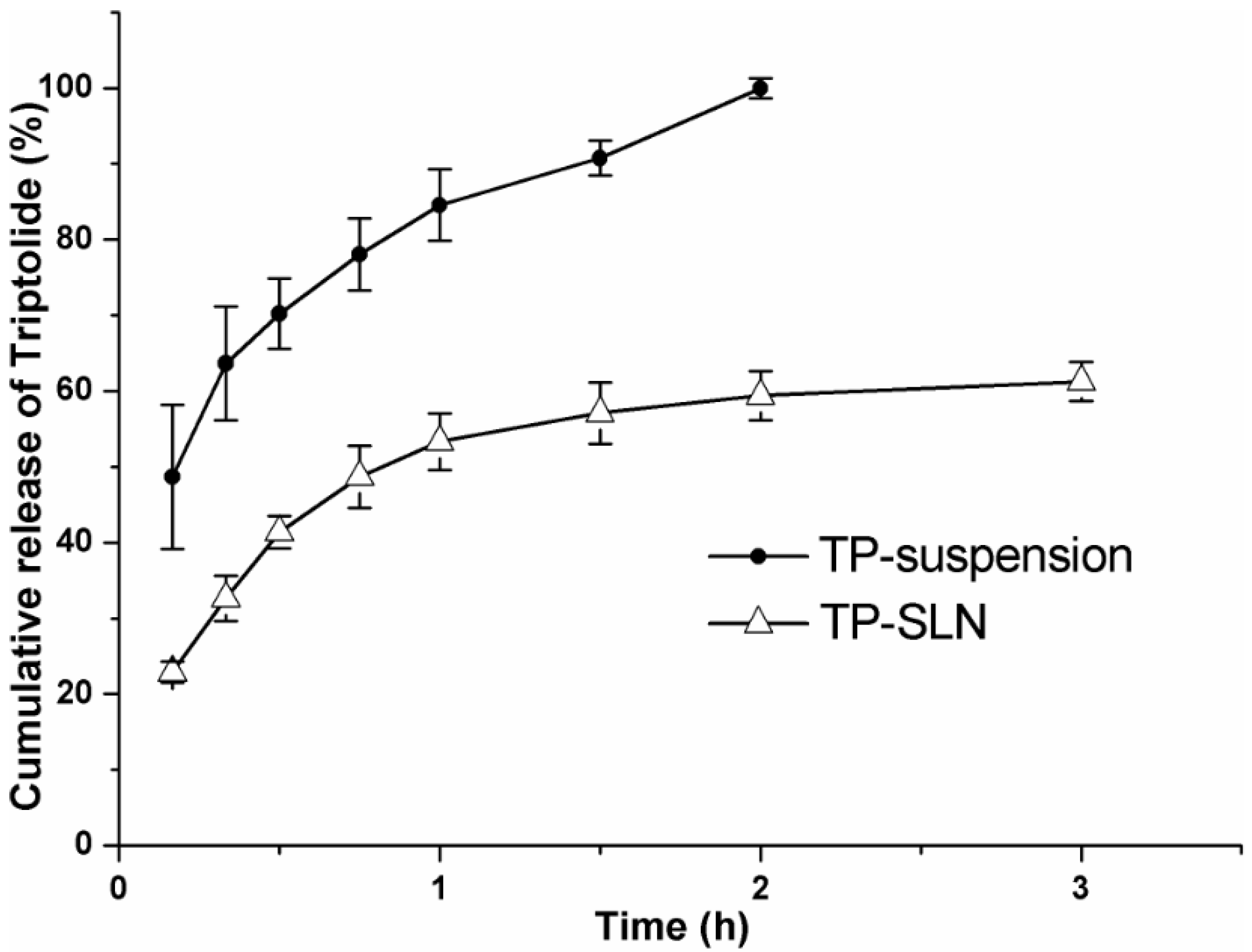
2.5. In Vitro Release
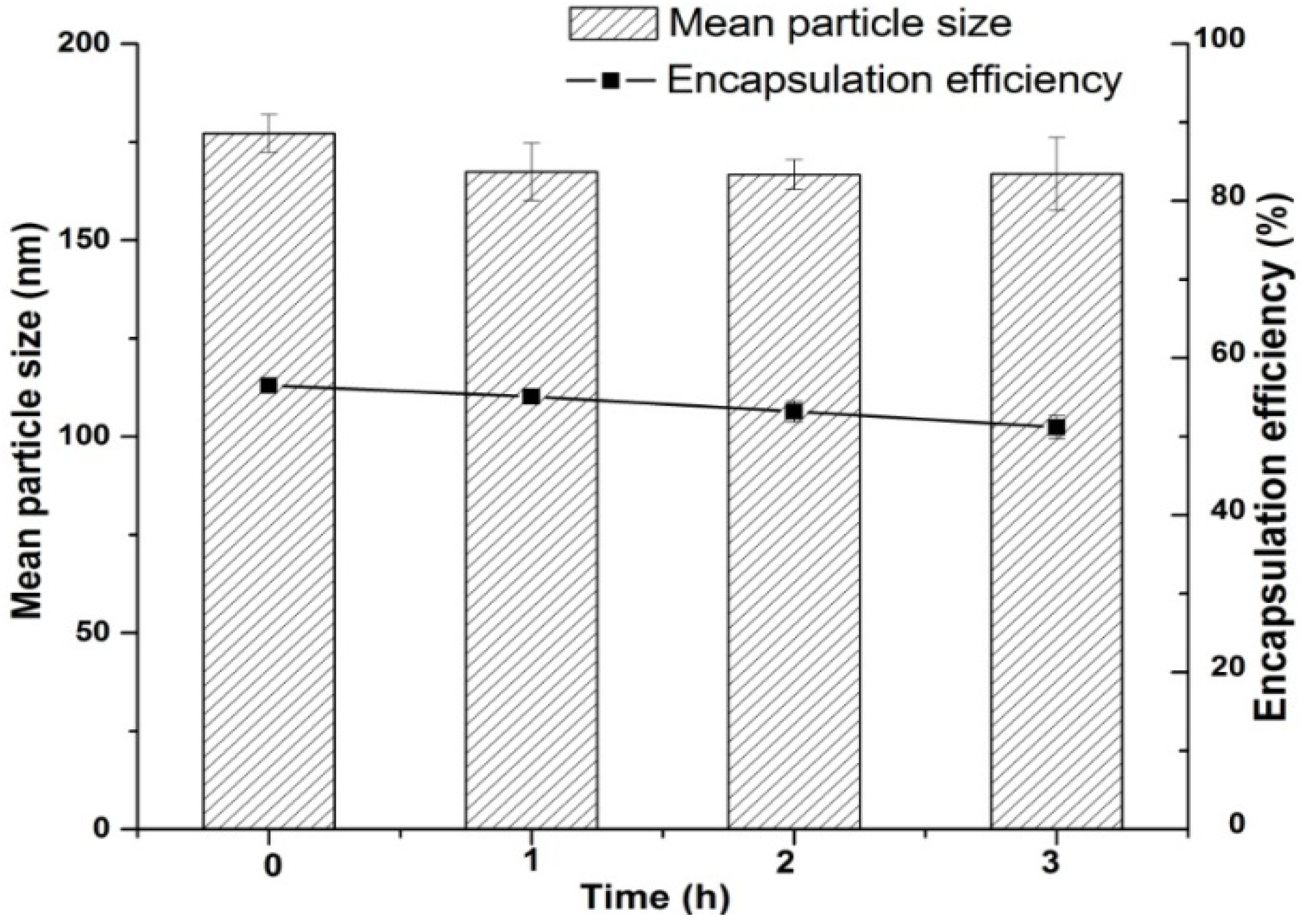
2.6. Stability Study in Simulated Gastric Fluid
2.7. Assessment of Gastric Mucosa Irritation
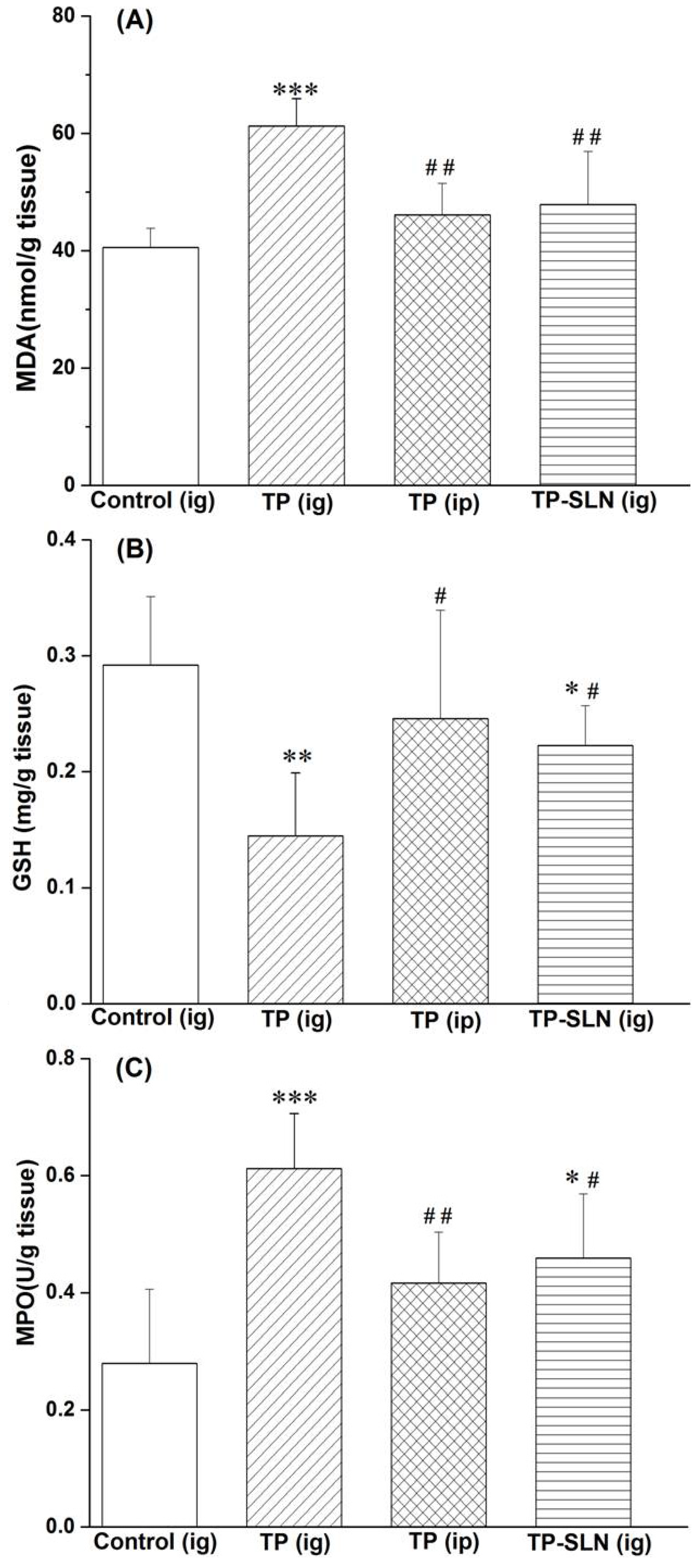
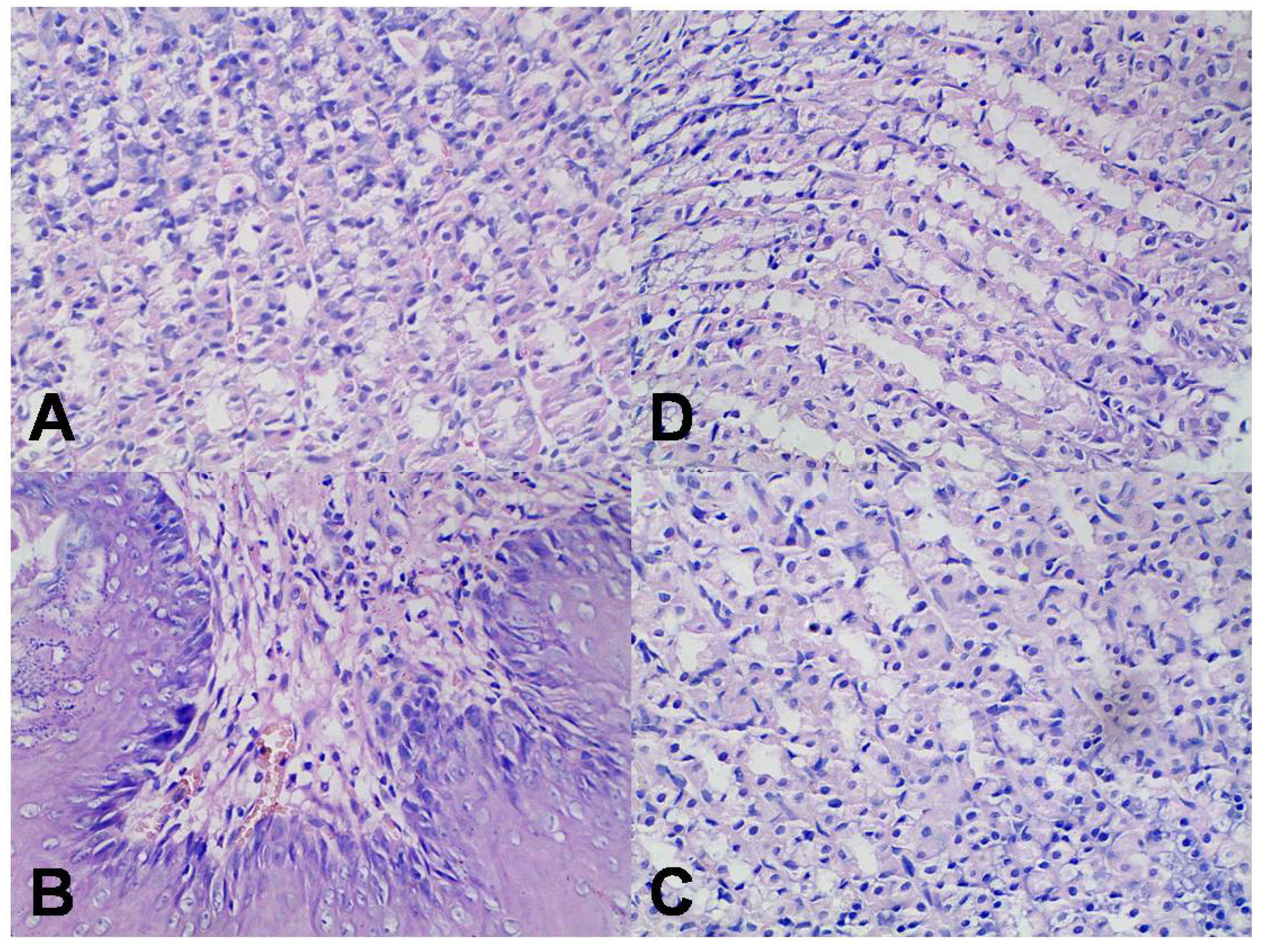
3. Experimental
3.1. Materials
3.2. Animals
3.3. Preparation of TP-SLN Using the Microemulsion Technique
3.4. Central Composite Design (CCD)
| Independent Variables | Coded levels | ||||
|---|---|---|---|---|---|
| −1.732 | −1 | 0 | +1 | +1.732 | |
| X1: fraction of lipid (%, w/w) | 40.00 | 44.23 | 50.00 | 55.77 | 60.00 |
| X2: surfactant to co-surfactant ratio (w/w) | 2.00 | 2.85 | 4.00 | 5.15 | 6.00 |
| X3: lipid to drug ratio (w/w) | 50.00 | 60.57 | 75 | 89.43 | 100.00 |
3.5. Characterization of TP-SLN
3.5.1. Particle Size
3.5.2. Encapsulation Efficiency (EE) and Drug Loading (DL)
3.6. In Vitro Release
3.7. Stability Study in Simulated Gastric Fluid
3.8. Assessment of Gastric Mucosa Irritation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.L.; Yang, Y.X.; Ding, J.; Li, Y.C.; Miao, Z.H. Triptolide: Structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef]
- Li, J.; Jin, J.; Li, M.; Guan, C.; Wang, W.; Zhu, S.; Qiu, Y.; Huang, M.; Huang, Z. Role of Nrf2 in protection against triptolide-induced toxicity in rat kidney cells. Toxicol. Lett. 2012, 213, 194–202. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Zhou, G.B. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules 2011, 16, 5283–5297. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Liu, J.; Huang, X.; Wang, T.; Zhang, Y.; Zhou, Z.; Guo, J.; Yang, L.; Chen, Y.; et al. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology 2010, 271, 57–63. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Z.; Zhang, L. Advances in studies on toxicity and attenuation of triptolide. Drug Eval. Res. 2012, 35, 211–215. [Google Scholar]
- Zhao, P.; Jiang, H.; Jiang, T.; Zhi, Z.; Wu, C.; Sun, C.; Zhang, J.; Wang, S. Inclusion of celecoxib into fibrous ordered mesoporous carbon for enhanced oral bioavailability and reduced gastric irritancy. Eur. J. Pharm. Sci. 2012, 45, 639–647. [Google Scholar] [CrossRef]
- Desai, P.P.; Date, A.A.; Patravale, V.B. Overcoming poor oral bioavailability using nanoparticle formulations—Opportunities and limitations. Drug Discov. Today Technol. 2012, 9, e87–e95. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Aji Alex, M.R.; Chacko, A.J.; Jose, S.; Souto, E.B. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur. J. Pharm. Sci. 2011, 42, 11–18. [Google Scholar] [CrossRef]
- Müller, R.H.; Runge, S.; Ravelli, V.; Mehnert, W.; Thunemann, A.F.; Souto, E.B. Oral bioavailability of cyclosporine: Solid lipid nanoparticles (SLN) versus drug nanocrystals. Int. J. Pharm. 2006, 317, 82–89. [Google Scholar] [CrossRef]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 14–155. [Google Scholar]
- Müller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; Garcia, M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z.; Yang, Y.; Xu, H.; Yang, X. A study of microemulsion systems for transdermal delivery of triptolide. J. Control. Release 2004, 98, 427–436. [Google Scholar] [CrossRef]
- Xue, J.; Jia, X.B.; Tan, X.B.; Jia, D.S.; Jiang, J.; Zhang, L.Y. Determination of apparent oil/water partition coefficient and absorption prediction of triptolide. Chin. Pharm. J. 2009, 44, 1560–1563. [Google Scholar]
- Muchow, M.; Maincent, P.; Müller, R.H. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev. Ind. Pharm. 2008, 34, 1394–1405. [Google Scholar] [CrossRef]
- Souza, L.G.; Silva, E.J.; Martins, A.L.; Mota, M.F.; Braga, R.C.; Lima, E.M.; Valadares, M.C.; Taveira, S.F.; Marreto, R.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196. [Google Scholar] [CrossRef]
- Ghadiri, M.; Fatemi, S.; Vatanara, A.; Doroud, D.; Najafabadi, A.R.; Darabi, M.; Rahimi, A.A. Loading hydrophilic drug in solid lipid media as nanoparticles: Statistical modeling of entrapment efficiency and particle size. Int. J. Pharm. 2012, 424, 128–137. [Google Scholar] [CrossRef]
- Bhandari, R.; Kaur, I.P. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int. J. Pharm. 2013, 441, 202–212. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 167–175. [Google Scholar]
- Hao, J.; Wang, F.; Wang, X.; Zhang, D.; Bi, Y.; Gao, Y.; Zhao, X.; Zhang, Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharm. Sci. 2012, 47, 497–505. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef]
- Jain, S.; Valvi, P.U.; Swarnakar, N.K.; Thanki, K. Gelatin coated hybrid lipid nanoparticles for oral delivery of amphotericin B. Mol. Pharm. 2012, 9, 2542–2553. [Google Scholar] [CrossRef]
- Negi, L.M.; Jaggi, M.; Talegaonkar, S. A logical approach to optimize the nanostructured lipid carrier system of irinotecan: Efficient hybrid design methodology. Nanotechnology 2013, 24, 015104. [Google Scholar] [CrossRef]
- Varshosaz, J.; Tabbakhian, M.; Mohammadi, M.Y. Formulation and optimization of solid lipid nanoparticles of buspirone HCl for enhancement of its oral bioavailability. J. Liposome Res. 2010, 20, 286–296. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Smith, E. Experimental design for the optimization of lipid nanoparticles. J. Pharm. Sci. 2009, 98, 1813–1819. [Google Scholar] [CrossRef]
- Lv, Q.; Yu, A.; Xi, Y.; Li, H.; Song, Z.; Cui, J.; Cao, F.; Zhai, G. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int. J. Pharm. 2009, 372, 191–198. [Google Scholar] [CrossRef]
- Chen, C.C.; Tsai, T.H.; Huang, Z.R.; Fang, J.Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010, 74, 474–482. [Google Scholar] [CrossRef]
- Abdelbary, G.; Fahmy, R.H. Diazepam-loaded solid lipid nanoparticles: Design and characterization. AAPS Pharm. Sci. Tech. 2009, 10, 211–219. [Google Scholar] [CrossRef]
- Chalikwar, S.S.; Belgamwar, V.S.; Talele, V.R.; Surana, S.J.; Patil, M.U. Formulation and evaluation of Nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf. B 2012, 97, 109–116. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Benoit, J.P. The gastrointestinal stability of lipid nanocapsules. Int. J. Pharm. 2009, 379, 260–265. [Google Scholar] [CrossRef]
- Rawat, M.K.; Jain, A.; Singh, S. Studies on binary lipid matrix based solid lipid nanoparticles of repaglinide: In vitro and in vivo evaluation. J. Pharm. Sci. 2011, 100, 2366–2378. [Google Scholar] [CrossRef]
- Menozzi, A.; Pozzoli, C.; Poli, E.; Passeri, B.; Gianelli, P.; Bertini, S. Diazoxide attenuates indomethacin-induced small intestinal damage in the rat. Eur. J. Pharmacol. 2011, 650, 378–383. [Google Scholar] [CrossRef]
- Morsy, M.A.; Heeba, G.H.; Abdelwahab, S.A.; Rofaeil, R.R. Protective effects of nebivolol against cold restraint stress-induced gastric ulcer in rats: Role of NO, HO-1, and COX-1,2. Nitric Oxide 2012, 27, 117–122. [Google Scholar] [CrossRef]
- Al-Abbasi, F.A. Acrylonitrile-induced gastric toxicity in rats: The role of xanthine oxidase. Med. Sci. Monit. 2012, 18, BR208–BR214. [Google Scholar]
- Odabasoglu, F.; Halici, Z.; Cakir, A.; Halici, M.; Aygun, H.; Suleyman, H.; Cadirci, E.; Atalay, F. Beneficial effects of vegetable oils (corn, olive and sunflower oils) and alpha-tocopherol on anti-inflammatory and gastrointestinal profiles of indomethacin in rats. Eur. J. Pharmacol. 2008, 591, 300–306. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yasuhiro, T.; Asada, Y.; Sugawa, Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion 1998, 59, 298–307. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- Murakami, K.; Okajima, K.; Harada, N.; Isobe, H.; Liu, W.; Johno, M.; Okabe, H. Plaunotol prevents indomethacin-induced gastric mucosal injury in rats by inhibiting neutrophil activation. Aliment. Pharmacol. Ther. 1999, 13, 521–530. [Google Scholar] [CrossRef]
- Castro, G.A.; Coelho, A.L.; Oliveira, C.A.; Mahecha, G.A.; Orefice, R.L.; Ferreira, L.A. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int. J. Pharm. 2009, 381, 77–83. [Google Scholar] [CrossRef]
- Potta, S.G.; Minemi, S.; Nukala, R.K.; Peinado, C.; Lamprou, D.A.; Urquhart, A.; Douroumis, D. Preparation and characterization of ibuprofen solid lipid nanoparticles with enhanced solubility. J. Microencapsul. 2011, 28, 74–81. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.J.; Peng, D.Y.; Li, Q.L.; Wang, X.S.; Wang, D.L.; Chen, W.D. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf. B 2013, 102, 391–397. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, D.; Ren, L.; Zhao, X.; Qin, J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release 2006, 114, 53–59. [Google Scholar] [CrossRef]
- Hejri, A.; Khosravi, A.; Gharanjig, K.; Hejazi, M. Optimisation of the formulation of beta-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013, 141, 117–123. [Google Scholar] [CrossRef]
- Zhuang, C.Y.; Li, N.; Wang, M.; Zhang, X.N.; Pan, W.S.; Peng, J.J.; Pan, Y.S.; Tang, X. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010, 394, 179–185. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Xu, H.; Yang, X. Anti-tumour and immuno-modulation effects of triptolide-loaded polymeric micelles. Eur. J. Pharm. Biopharm. 2008, 70, 741–748. [Google Scholar] [CrossRef]
- Han, H.K.; Shin, H.J.; Ha, D.H. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur. J. Pharm. Sci. 2012, 46, 50–507. [Google Scholar]
- Alarcon de la Lastra, C.; Nieto, A.; Martin, M.J.; Cabre, F.; Herrerias, J.M.; Motilva, V. Gastric toxicity of racemic ketoprofen and its enantiomers in rat: Oxygen radical generation and COX-expression. Inflamm. Res. 2002, 51, 51–57. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, C.; Gu, C.; Peng, F.; Liu, W.; Wan, J.; Xu, H.; Lam, C.W.; Yang, X. Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation. Molecules 2013, 18, 13340-13356. https://doi.org/10.3390/molecules181113340
Zhang C, Gu C, Peng F, Liu W, Wan J, Xu H, Lam CW, Yang X. Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation. Molecules. 2013; 18(11):13340-13356. https://doi.org/10.3390/molecules181113340
Chicago/Turabian StyleZhang, Cong, Conghui Gu, Fan Peng, Wei Liu, Jiangling Wan, Huibi Xu, Christopher Waikei Lam, and Xiangliang Yang. 2013. "Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation" Molecules 18, no. 11: 13340-13356. https://doi.org/10.3390/molecules181113340
APA StyleZhang, C., Gu, C., Peng, F., Liu, W., Wan, J., Xu, H., Lam, C. W., & Yang, X. (2013). Preparation and Optimization of Triptolide-Loaded Solid Lipid Nanoparticles for Oral Delivery with Reduced Gastric Irritation. Molecules, 18(11), 13340-13356. https://doi.org/10.3390/molecules181113340