Biological Activity of Oleanane Triterpene Derivatives Obtained by Chemical Derivatization
Abstract
:1. Introduction
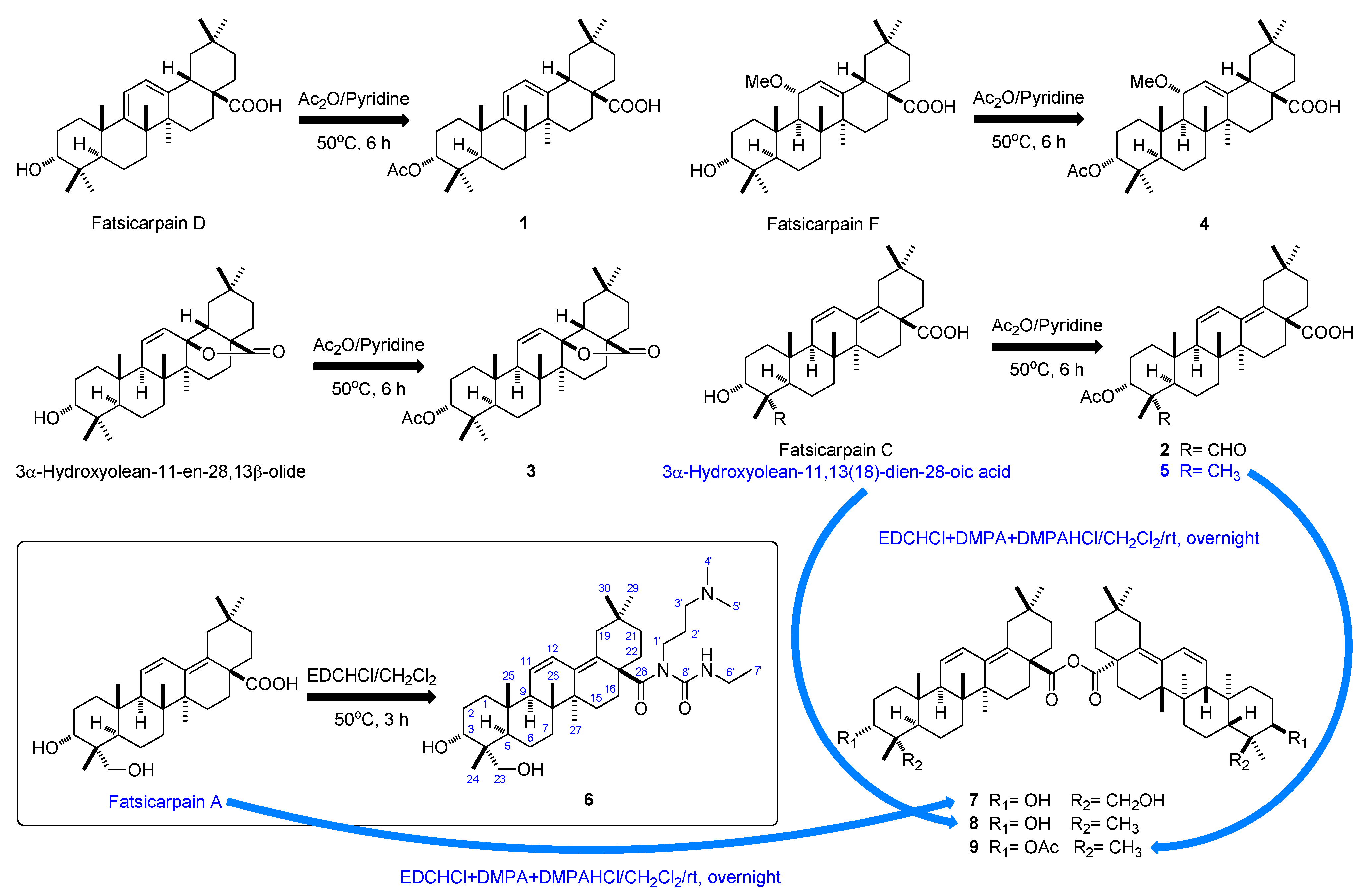
2. Results and Discussion
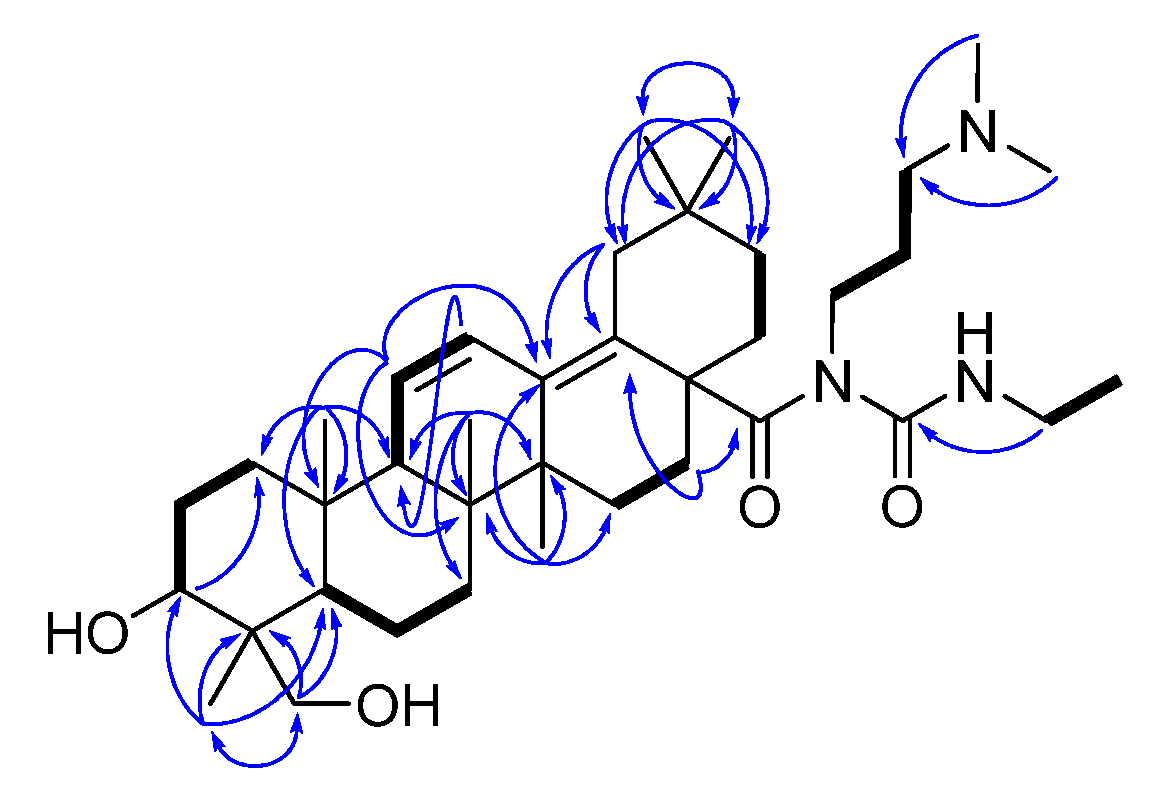
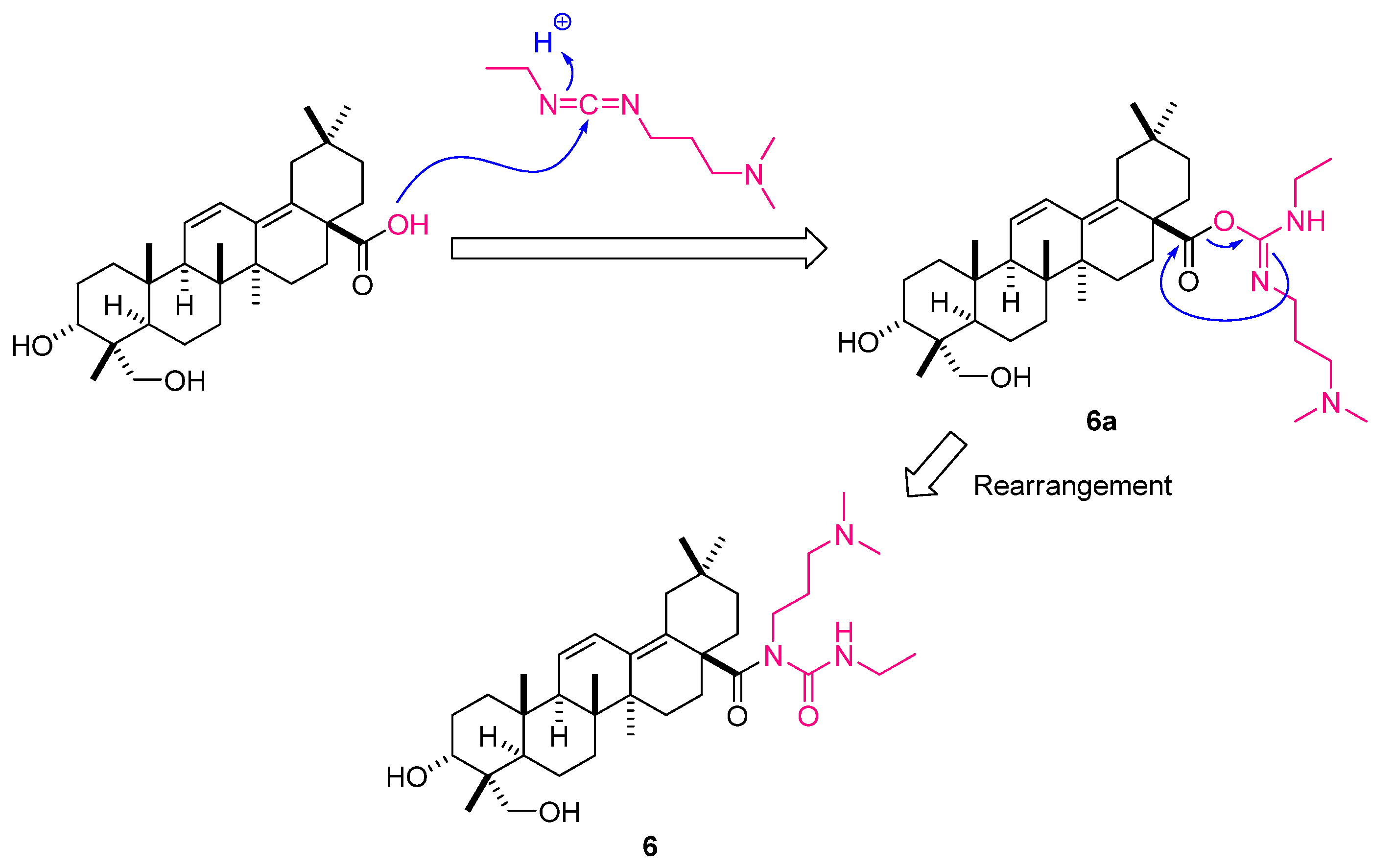
| IC50 (μg/mL) | |||
|---|---|---|---|
| Compounds | HepG2 2.2.15 | HBsAg | HBeAg |
| 1 | 6.5 | >50 | >50 |
| 2 | 17.9 | >50 | >50 |
| 3 | 38.5 | >50 | >50 |
| 4 | 24.1 | >50 | >50 |
| 5 | 9.3 | >50 | >50 |
| 6 | 5.3 | >50 | >50 |
| 7 | >50 | >50 | >50 |
| 8 | >50 | >50 | >50 |
| 9 | >50 | >50 | >50 |
| Fatsicarpain A | 18.9 | >50 | >50 |
| Fatsicarpain C | 16.7 | >50 | >50 |
| Fatsicarpain D | 28.8 | >50 | >50 |
| Fatsicarpain F | 23.9 | >50 | >50 |
| 3 á-Hydroxyolean-11-en-28,13â-olide | >50 | >50 | >50 |
| 3 á-Hydroxyolean-11,13(18)-dien-28-oic acid | >50 | >50 | >50 |
| Minimum Bactericidal Concentrations (MBC) (μg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogens | A | C | D | F | X | Y | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| H. pylori | 64 | 64 | 64 | 64 | >128 | >128 | >128 | 128 | >128 | >128 | >128 | >128 | >128 | >128 | |
| Minimum Inhibitory Concentrations (MIC) (μg/mL) | |||||||||||||||
| S. aureus | >128 | 64 | >128 | >128 | >128 | >128 | >128 | 128 | >128 | >128 | >128 | 16 | >128 | >128 | >128 |
| E. faecalis | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 128 | >128 | >128 | >128 | 32 | 128 | 128 | 128 |
| L. monocytogenes | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 32 | >128 | >128 | >128 |
| B. cereus | 32 | 32 | 8 | 2 | >128 | >128 | >128 | 16 | >128 | >128 | 2 | 8 | >128 | >128 | >128 |
| E. coli | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| S. enterica | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| P. aeruginosa | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |


3. Experimental
3.1. General
3.2. Plant Material
3.3. Acetylation
3.4. Acylation
3.5. Anhydride Coupling Esterification
3.6. Cytotoxicity, Anti-hepatitis B Virus (HBV) Assay and Antibacterial Activity
3.7. Glucose Uptake Assay
3.8. Dual Luciferase Activity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, C.M.; Hsu, Y.M.; Huang, T.J.; Chou, S.C.; Lin, E.H.; Chou, C.H. Oleanane-type triterpenoids from the leaves and twigs of Fatsia polycarpa. J. Nat. Prod. 2011, 74, 1744–1750. [Google Scholar] [CrossRef]
- Sun, H.X.; Zheng, Q.F.; Tu, J. Induction of apoptosis in HeLa cells by 3β-hydroxy-12-oleanen-27-oic acid from the rhizomes of Astilbe chinensis. Bioorg. Med. Chem. 2006, 14, 1189–1198. [Google Scholar] [CrossRef]
- Rojas, R.; Caviedes, L.; Aponte, J.C.; Vaisberg, A.J.; Lewis, W.H.; Lamas, G.; Sarasara, C.; Gilman, R.H.; Hammond, G.B. Aegicerin, the first oleanane triterpene with wide-ranging antimycobacterial activity, isolated from Clavija procera. J. Nat. Prod. 2006, 69, 845–846. [Google Scholar] [CrossRef]
- Chang, C.I.; Kuo, C.C.; Chang, J.Y.; Kuo, Y.H. Three new oleanane-type triterpenes from Ludwigia octovalvis with cytotoxic activity against two human cancer cell lines. J. Nat. Prod. 2004, 67, 91–93. [Google Scholar] [CrossRef]
- Zeng, N.; Shen, Y.; Li, L.Z.; Jiao, W.-H.; Gao, P.Y.; Song, S.J.; Chen, W.S.; Lin, H.W. Anti-inflammatory triterpenes from the leaves of Rosa laevigata. J. Nat. Prod. 2011, 74, 732–738. [Google Scholar] [CrossRef]
- Wang, F.; Hua, H.; Pei, Y.; Chen, D.; Jing, Y. Triterpenoids from the resin of Styrax tonkinensis and their antiproliferative and differentiation effects in human leukemia HL-60 cells. J. Nat. Prod. 2006, 69, 807–810. [Google Scholar] [CrossRef]
- Djoumessi, A.V.B.; Sandjo, L.P.; Liermann, J.C.; Schollmeyer, D.; Kuete, V.; Rincheval, V.; Berhanu, A.M.; Yeboah, S.O.; Wafo, P.; Ngadjui, B.T.; Opatz, T. Donellanic acids A–C: new cyclopropanic oleanane derivatives from Donella ubanguiensis (Sapotaceae). Tetrahedron 2012, 68, 4621–4627. [Google Scholar] [CrossRef]
- Ho, J.C.; Chen, C.M.; Row, L.C. Oleanane-type triterpenes from the flowers, pith, leaves, and fruit of Tetrapanax papyriferus. Phytochemistry 2007, 68, 631–635. [Google Scholar] [CrossRef]
- Cáceres-Castillo, D.; Mena-Rejón, G.J.; Cedillo-Rivera, R.; Quijano, L. 21β-Hydroxy-oleanane-type triterpenes from Hippocratea excelsa. Phytochemistry 2008, 69, 1057–1064. [Google Scholar] [CrossRef]
- Nishino, H.; Nishino, A.; Takayasu, J.; Hasegawa, T.; Iwashima, A.; Hirabayashi, K.; Iwata, S.; Shibata, S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988, 48, 5210–5215. [Google Scholar]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M.; Chen, C.H.; Garrett, P.E.; Lee, K.H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J. Med. Chem. 1996, 39, 1016–1017. [Google Scholar] [CrossRef]
- Qian, K.; Kuo, R.Y.; Chen, C.H.; Huang, L.; Morris-Natschke, S.L.; Lee, K.H. Anti-AIDS agents 81. Design, synthesis, and structure−activity relationship study of betulinic acid and moronic acid derivatives as potent HIV maturation inhibitors. J. Med. Chem. 2010, 53, 3133–3141. [Google Scholar] [CrossRef]
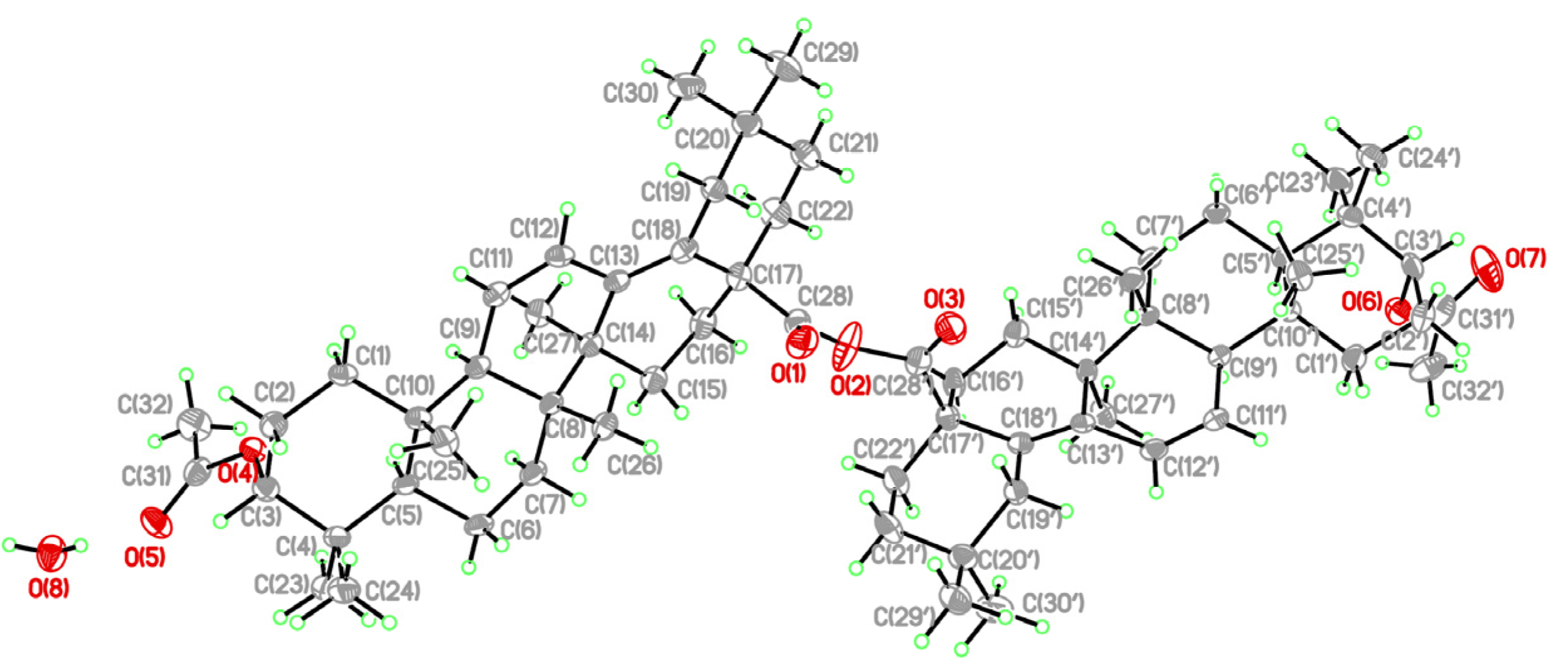
- Crystallographic data for 9 has been deposited with the Cambridge Crystallographic Data Centre (deposition number CCDC 837341). Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: +44-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
- Chen, H.J.; Lin, C.M.; Lin, C.S.; Perez-Olle, R.; Leung, C.L.; Liem, R.K. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 2006, 20, 1933–1945. [Google Scholar] [CrossRef]
- Volonterio, A.; Ramirez de Arellano, C.; Zanda, M. Synthesis of 1,3,5-trisubstituted hydantoins by regiospecific domino condensation/aza-Michael/O→N acyl migration of carbodiimides with activated α,β-unsaturated carboxylic acids. J. Org. Chem. 2005, 70, 2161–2170. [Google Scholar] [CrossRef]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection – natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef]
- Egan, B.J.; Marzio, L.; O'Connor, H.; O'Morain, C. Treatment of Helicobacter pylori infection. Helicobacter 2008, 13, 35–40. [Google Scholar]
- Chang, J.M.; Chen, T.H. Bacterial foodborne outbreaks in central Taiwan, 1991-2000. J. Food Drug Anal. 2003, 11, 53–59. [Google Scholar]
- Tanachatchairtana, T.; Bremner, J.B.; Chokchaisiri, R.; Suksamrarn, A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef]
- Cheng, H.L.; Huang, H.K.; Chang, C.I.; Tsai, C.P.; Chou, C.H. A cell-based screening identifies compounds from the stem of Momordica charantia that overcome insulin resistance and activate AMP-activated protein kinase. J. Agric. Food Chem. 2008, 56, 6835–6843. [Google Scholar] [CrossRef]
- Chang, C.I.; Tseng, H.I.; Liao, Y.W.; Yen, C.H.; Chen, T.M.; Lin, C.C.; Cheng, H.L. In vivo and in vitro studies to identify the hypoglycaemic constituents of Momordica charantia wild variant WB24. Food Chem. 2011, 125, 521–528. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Developmental cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Sen, M.; Lauterbach, K.; El-Gabalawy, H.; Firestein, G.S.; Corr, M.; Carson, D.A. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 2791–2796. [Google Scholar] [CrossRef]
- Sen, M.; Chamorro, M.; Reifert, J.; Corr, M.; Carson, D.A. Blockade of Wnt-5A/Frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001, 44, 772–781. [Google Scholar] [CrossRef]
- Moon, R.T.; Kohn, A.D.; De Ferrari, G.V.; Kaykas, A. WNT and β-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004, 5, 691–701. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Moon, R.T. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene 2006, 25, 7545–7553. [Google Scholar] [CrossRef]
- Johnson, M.L.; Rajamannan, N. Diseases of Wnt signaling. Rev. Endocr. Metab. Disord. 2006, 7, 41–49. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Deng, Z.L.; Luo, X.; Song, W.X.; Sharff, K.A.; Tang, N.; Haydon, R.C.; Luu, H.H.; He, T.C. Wnt signaling and human diseases: what are the therapeutic implications? Lab. Invest. 2007, 87, 97–103. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds may not be available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, S.-Y.; Wang, C.-M.; Cheng, H.-L.; Chen, H.-J.; Hsu, Y.-M.; Lin, Y.-C.; Chou, C.-H. Biological Activity of Oleanane Triterpene Derivatives Obtained by Chemical Derivatization. Molecules 2013, 18, 13003-13019. https://doi.org/10.3390/molecules181013003
Cheng S-Y, Wang C-M, Cheng H-L, Chen H-J, Hsu Y-M, Lin Y-C, Chou C-H. Biological Activity of Oleanane Triterpene Derivatives Obtained by Chemical Derivatization. Molecules. 2013; 18(10):13003-13019. https://doi.org/10.3390/molecules181013003
Chicago/Turabian StyleCheng, Shi-Yie, Chao-Min Wang, Hsueh-Ling Cheng, Hui-Jye Chen, Yuan-Man Hsu, Yu-Chi Lin, and Chang-Hung Chou. 2013. "Biological Activity of Oleanane Triterpene Derivatives Obtained by Chemical Derivatization" Molecules 18, no. 10: 13003-13019. https://doi.org/10.3390/molecules181013003
APA StyleCheng, S.-Y., Wang, C.-M., Cheng, H.-L., Chen, H.-J., Hsu, Y.-M., Lin, Y.-C., & Chou, C.-H. (2013). Biological Activity of Oleanane Triterpene Derivatives Obtained by Chemical Derivatization. Molecules, 18(10), 13003-13019. https://doi.org/10.3390/molecules181013003






