Diterpenoids with Anti-Inflammatory Activity from the Wood of Cunninghamia konishii
Abstract
:1. Introduction

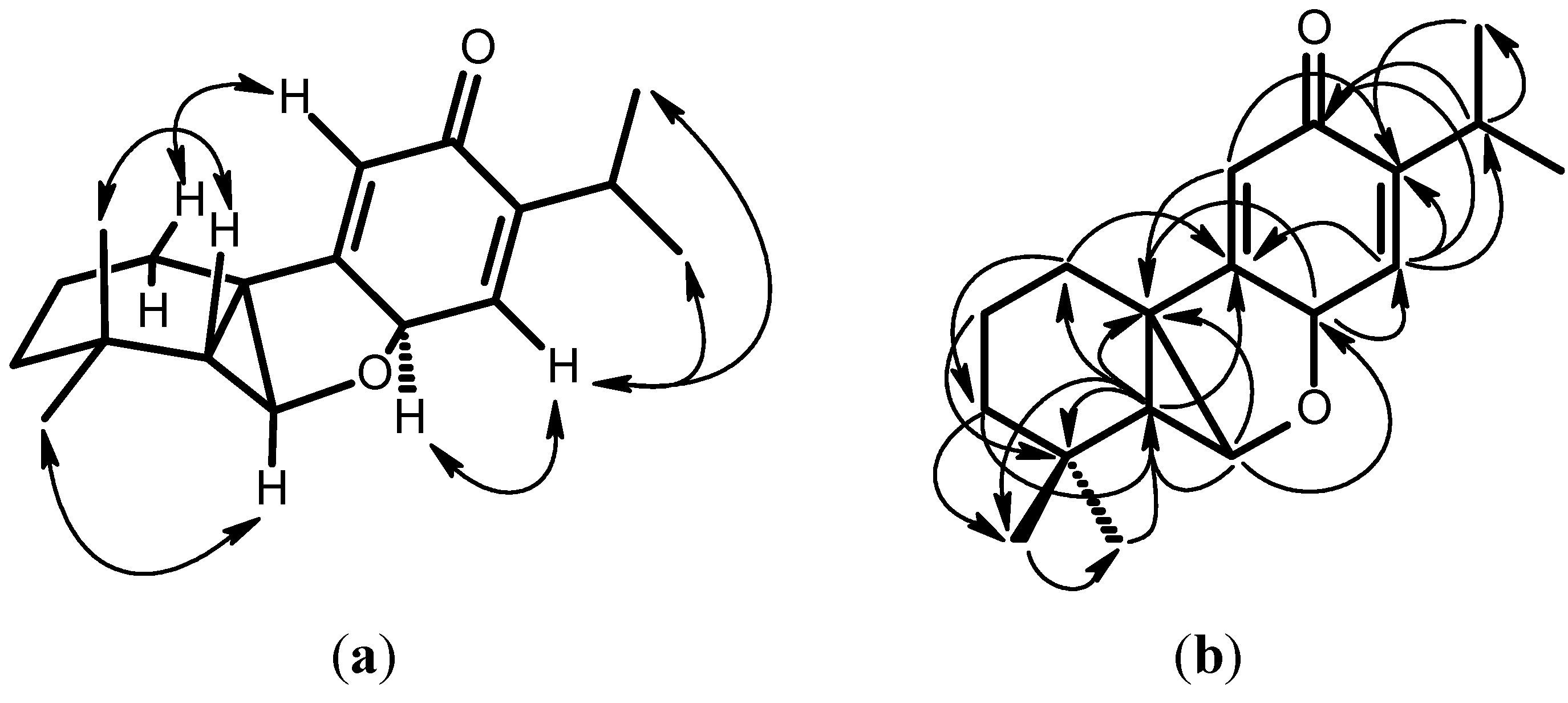
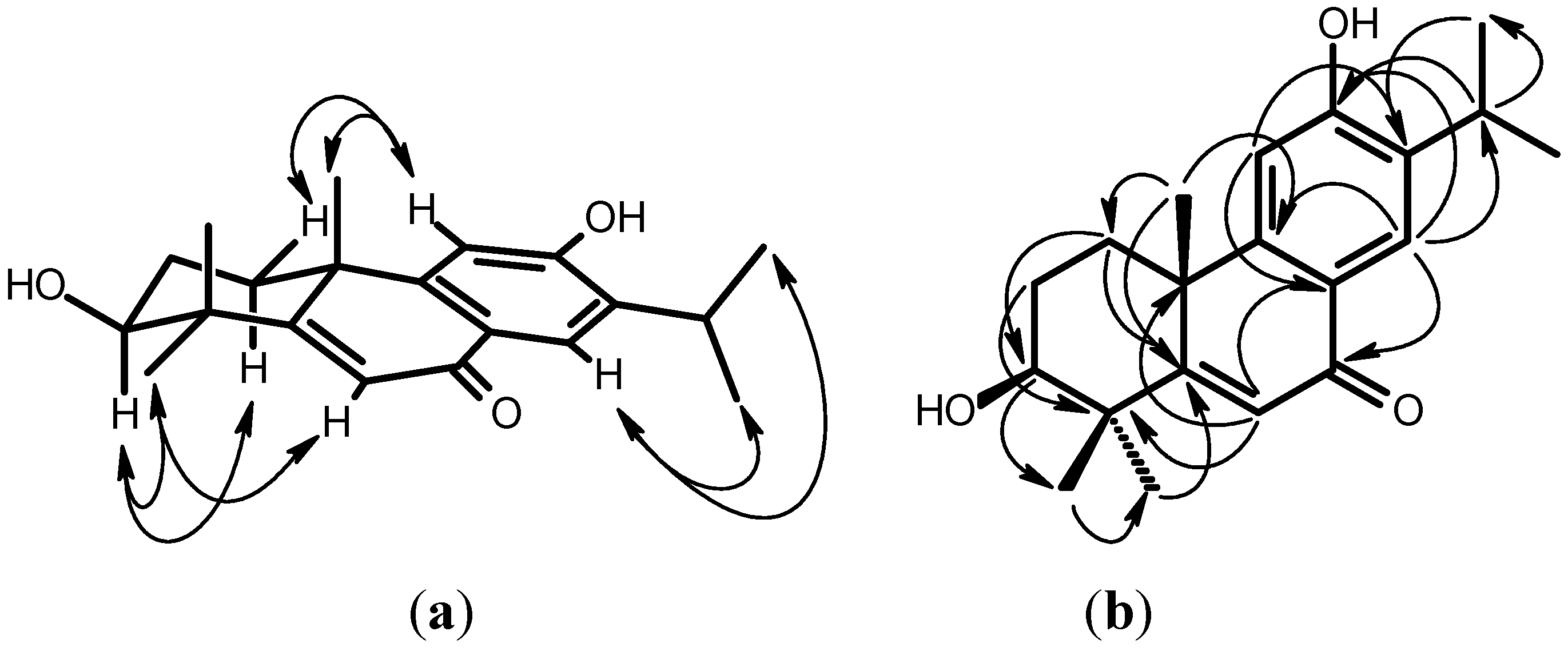
2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 1.42 (m, 1H), 1.72 (m, 1H) | 38.1 | 1.63 (ddd, 13.6, 3.6 , 3.6, 1H) | 34.5 |
| 2.13 (ddd, 13.6, 3.6, 3.6, 1H) | ||||
| 2 | 1.58 (m, 1H), 1.72 (m, 1H) | 18.1 | 1.95 (m, 1H), 2.01 (m, 1H) | 27.2 |
| 3 | 1.20 (m, 1H), 1.41 (m, 1H) | 42.9 | 3.39 (dd, 11.6, 4.8, 1H) | 76.5 |
| 4 | – | 34.5 | – | 43.2 |
| 5 | 1.33 (d,10.3, 1H) | 52.5 | – | 171.0 |
| 6 | 4.61 (d, 10.3, 1H) | 79.7 | 6.51 (s, 1H) | 125.7 |
| 7 | – | – | – | 185.1 |
| 8 | 4.11 (s, 1H) | 67.5 | – | 129.3 |
| 9 | – | 161.9 | – | 153.3 |
| 10 | – | 56.0 | – | 40.6 |
| 11 | 6.31 (s, 1H) | 126.5 | 6.82 (s, 1H) | 111.1 |
| 12 | – | 187.1 | – | 157.5 |
| 13 | – | 149.6 | – | 133.7 |
| 14 | 6.06 (s, 1H) | 138.5 | 7.98 (s, 1H) | 125.0 |
| 15 | 2.96 (sept, 6.0, 1H) | 26.3 | 3.16 (sept, 6.8, 1H) | 26.9 |
| 16 | 1.05 (d, 6.0, 3H) | 21.6 | 1.25 (d, 6.8, 3H) | 22.3 |
| 17 | 1.07 (d, 6.0, 3H) | 22.4 | 1.28 (d, 6.8, 3H) | 22.5 |
| 18 | 1.21 (s, 3H) | 21.3 | 1.33 (s, 3H) | 22.4 |
| 19 | 1.00 (s, 3H) | 34.8 | 1.28 (s, 3H) | 27.4 |
| 20 | – | – | 1.49 (s, 3H) | 32.3 |
| Compound | Dose (μg/mL) | Cell viability (% of control) | NO level | NO inhibition (% of control) | IC50 (μg/mL) |
|---|---|---|---|---|---|
| Control | (−) | 93.0 ± 4.9 | −0.5 ± 0.1 | (−) | |
| LPS | (+) | 98.7 ± 8.0 | 45.4 ± 2.7 ### | (−) | |
| 1 | 2.5 | 103.7 ± 2.0 | 39.0 ± 1.2 | 14.2 ± 2.7 | 9.8 ± 0.7 |
| 5 | 101.1 ± 3.1 | 34.6 ± 1.1 * | 23.9 ± 2.5 | ||
| 10 | 98.9 ± 6.7 | 22.2 ± 1.1 ** | 51.0 ± 2.4 | ||
| 20 | 34.6 ± 9.6 | (−) | (−) | ||
| 3 | 2.5 | 96.2 ± 1.5 | 39.3 ± 3.7 | 13.5 ± 8.2 | 7.9 ± 0.9 |
| 5 | 87.1 ± 1.5 | 32.5 ± 0.6 * | 28.5 ± 1.4 | ||
| 10 | 82.6 ± 1.5 | 16.0 ± 0.7 ** | 64.8 ± 1.5 | ||
| 20 | 75.8 ± 2.5 | (−) | (−) | ||
| 4 | 2.5 | 90.4 ± 1.7 | 44.9 ± 1.9 | 1.2 ± 4.2 | >20 |
| 5 | 83.1 ± 1.8 | 40.5 ± 1.3 | 10.8 ± 2.9 | ||
| 10 | 81.8 ± 1.0 | 28.7 ± 2.1 * | 36.9 ± 4.7 | ||
| 20 | 53.8 ± 1.9 | (–) | (–) | ||
| 5 | 2.5 | 98.0 ± 1.2 | 38.0 ± 1.1 | 16.2 ± 2.4 | 9.3 ± 1.3 |
| 5 | 100.4 ± 4.5 | 33.0 ± 1.3 | 27.4 ± 2.8 | ||
| 10 | 91.1 ± 2.0 | 21.1 ± 2.3 ** | 53.5 ± 5.1 | ||
| 20 | 82.2 ± 1.2 | 11.1 ± 4.4*** | 75.6 ± 9.7 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Konishone (1)
3.5. 3β-Hydroxy-5,6-dehydrosugiol (2)
3.6. Chemicals
3.7. Cell Culture
3.8. Cell Viability
3.9. Measurement of Nitric Oxide/Nitrite
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Li, H.L.; Keng, H. Taxodiaceae. In Flora of Taiwan, 2nd ed; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1994; Volume 1, pp. 582–585. [Google Scholar]
- Ikeda, T.; Fujita, Y. Essential oils of Cunninghamia konishii Hayata. J. Chem. Soc. Jpn. 1929, 50, 32–45. [Google Scholar]
- Ikeda, T.; Fujita, Y. The pinene in Cunninghamia konishii Hayata. J. Chem. Soc. Jpn. 1929, 50, 66–70. [Google Scholar]
- Cheng, Y.S.; Lin, C.S. Study of the extractive constituents from the wood of Cunninghamia konishii Hayata. J. Chin. Chem. Soc. 1979, 26, 169–172. [Google Scholar]
- Chang, S.T.; Yin, H.W. Identificition of the needle crystal appeared on the wood surface of Cunninghamia konishii Hyata. Bull. Taiwan For. Res. Inst. New Ser. 1991, 6, 57–63. [Google Scholar]
- Li, Y.C.; Kuo, Y.H. Five New Diterpenoids from the Wood of Cunninghamia konishii. J. Nat. Prod. 1998, 61, 997–1000. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuo, Y.H. Labdane-type diterpenoids from the wood of Cunninghamia konishii. Chem. Pharm. Bull. 2002, 50, 498–500. [Google Scholar] [CrossRef]
- Cheng, S.S.; Lin, C.Y.; Gu, H.J.; Chang, S.T. Antifungal Activities and Chemical Composition of Wood and Leaf Essential Oils from Cunninghamia konishii. J. Wood Chem. Technol. 2011, 31, 204–217. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chung, M.J.; Lin, C.Y.; Wang, Y.N.; Chang, S.T. Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J. Agric. Food Chem. 2012, 60, 124–128. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Tsai, M.D. Terpenes and sterols of Cunninghamia konishii. Phytochemistry 1972, 11, 2108–2109. [Google Scholar] [CrossRef]
- He, K.; Shi, G.; Zeng, L.; Ye, Q.; McLaughlin, J.L. Konishiol, a new sesquiterpene, and bioactive components from Cunninghamia konishi. Planta Med. 1997, 63, 158–160. [Google Scholar] [CrossRef]
- Yang, Z.; Kitano, Y.; Chiba, K.; Shibata, N.; Kurokawa, H.; Doi, Y.; Arakawa, Y.; Tada, M. Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE. Bioorg. Med. Chem. 2001, 9, 347–356. [Google Scholar] [CrossRef]
- Matsumoto, T.; Usui, S.; Kawashima, H.; Mitsuki, M. Synthesis of (+)-hinokiol, (+)-hinokione, (+)-salviol, and (+)-2-oxoferruginol. Bull. Chem. Soc. Jpn. 1981, 54, 581–584. [Google Scholar] [CrossRef]
- Chang, H.M.; Cheng, K.P.; Choang, T.F.; Chow, H.F.; Chui, K.Y. Structure elucidation and total synthesis of new tanshinones isolated from Salvia miltiorrhiza Bunge (Danshen). J. Org. Chem. 1990, 55, 3537–3543. [Google Scholar] [CrossRef]
- Katoh, T.; Akagi, T.; Noguchi, C.; Kajimoto, T.; Node, M.; Tanaka, R.; Nishizawa, M.; Ohtsu, H.; Suzuki, N.; Saito, K. Synthesis of DL-standishinal and its related compounds for the studies on structure-activity relationship of inhibitory activity against aromatase. Bioorg. Med. Chem. 2007, 15, 2736–2748. [Google Scholar]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-C.; Li, Y.-C.; You, B.-J.; Chang, W.-T.; Chao, L.K.; Lo, L.-C.; Wang, S.-Y.; Huang, G.-J.; Kuo, Y.-H. Diterpenoids with Anti-Inflammatory Activity from the Wood of Cunninghamia konishii. Molecules 2013, 18, 682-689. https://doi.org/10.3390/molecules18010682
Chen Y-C, Li Y-C, You B-J, Chang W-T, Chao LK, Lo L-C, Wang S-Y, Huang G-J, Kuo Y-H. Diterpenoids with Anti-Inflammatory Activity from the Wood of Cunninghamia konishii. Molecules. 2013; 18(1):682-689. https://doi.org/10.3390/molecules18010682
Chicago/Turabian StyleChen, Yu-Chang, Yen-Cheng Li, Bang-Jau You, Wen-Te Chang, Louis Kuoping Chao, Lee-Chiang Lo, Sheng-Yang Wang, Guan-Jhong Huang, and Yueh-Hsiung Kuo. 2013. "Diterpenoids with Anti-Inflammatory Activity from the Wood of Cunninghamia konishii" Molecules 18, no. 1: 682-689. https://doi.org/10.3390/molecules18010682
APA StyleChen, Y.-C., Li, Y.-C., You, B.-J., Chang, W.-T., Chao, L. K., Lo, L.-C., Wang, S.-Y., Huang, G.-J., & Kuo, Y.-H. (2013). Diterpenoids with Anti-Inflammatory Activity from the Wood of Cunninghamia konishii. Molecules, 18(1), 682-689. https://doi.org/10.3390/molecules18010682





