Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity
Abstract
:1. Introduction
2. Results and Discussion
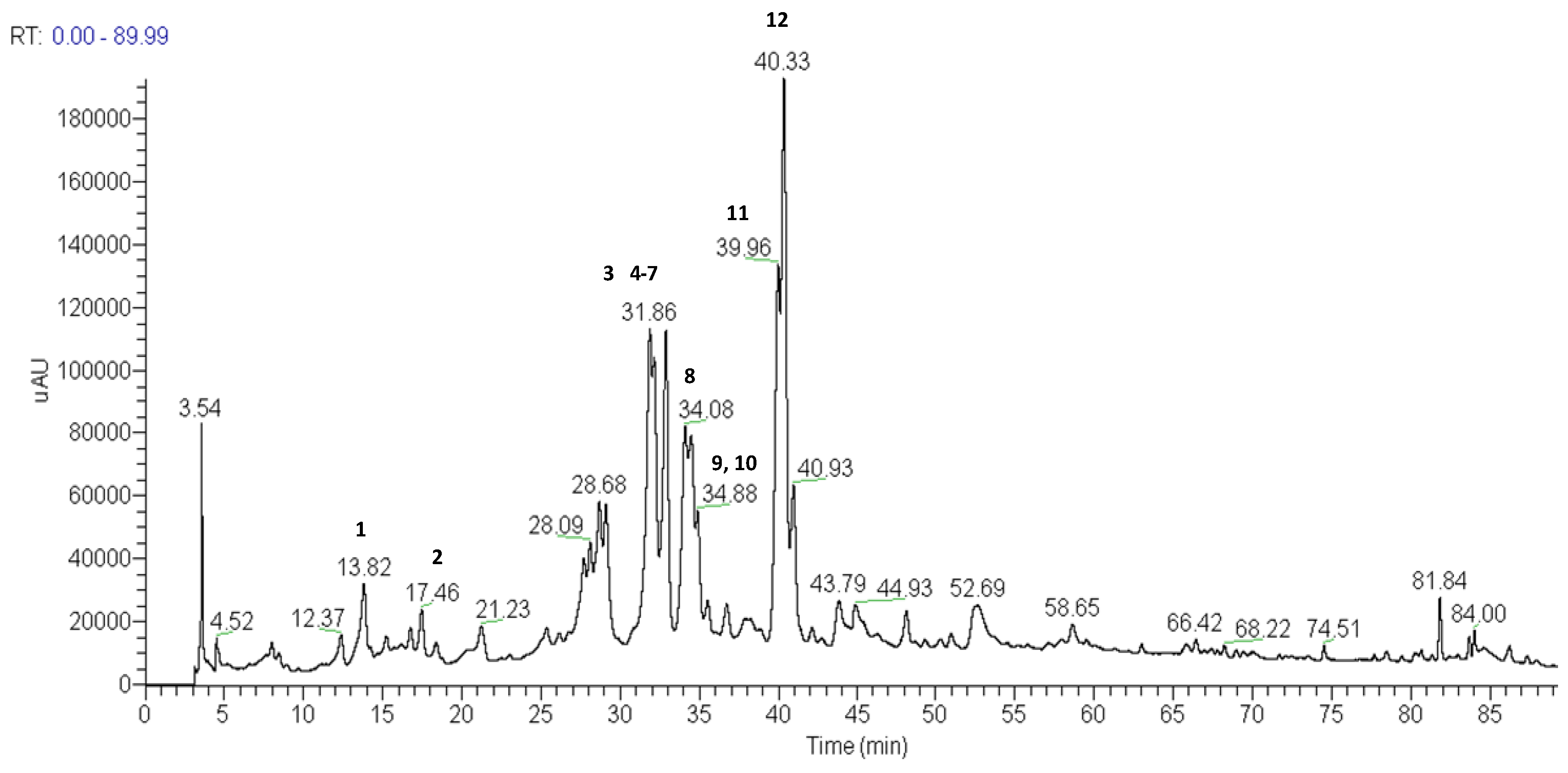
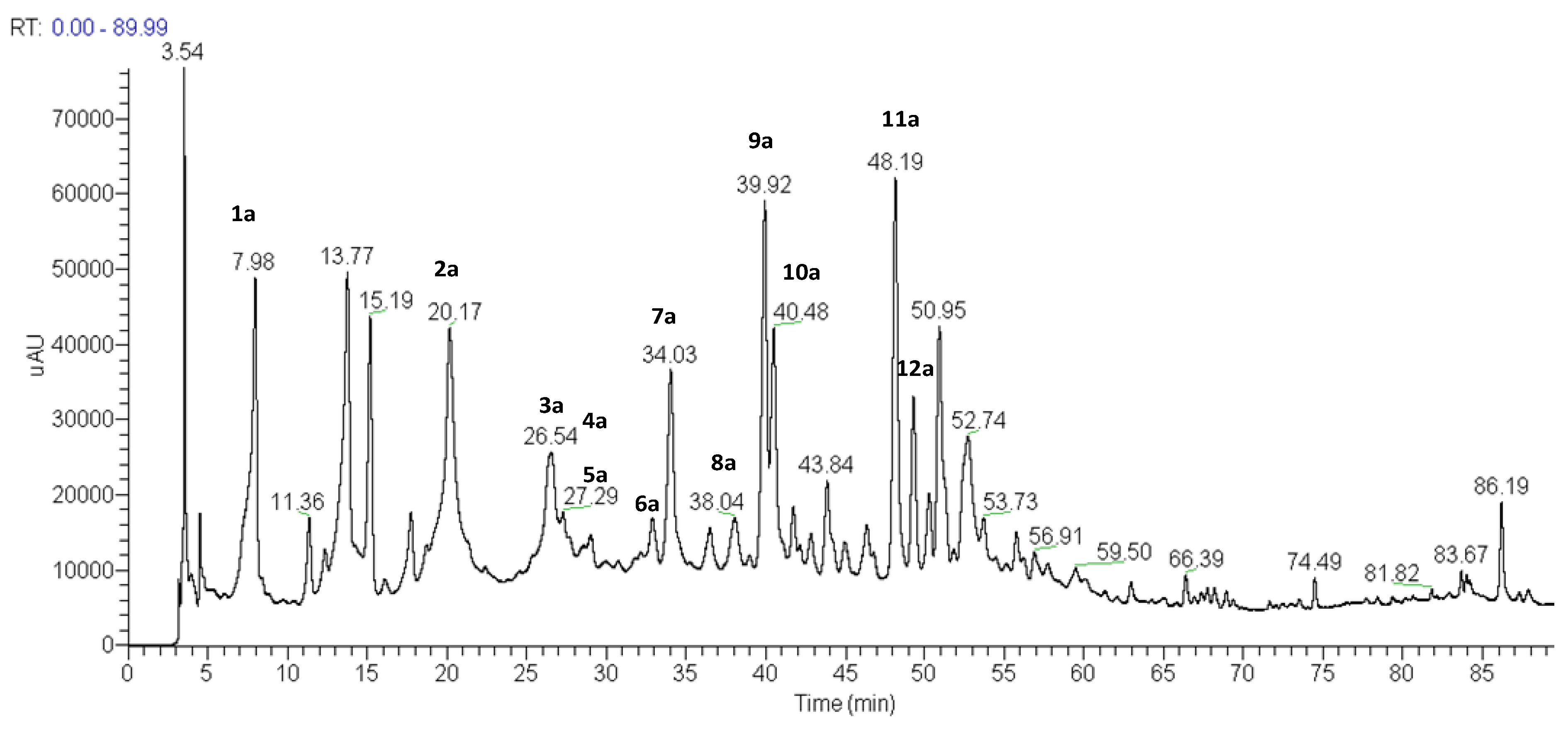
| Substance | Peak | TR | UV spectra λmax (nm) | [M−H]− | Major MS2 and MS3 fragments |
|---|---|---|---|---|---|
| 6-Methoxyquercetin-7-O-β-D-glucopyranosyl-(6→1)-O-β-D-glucopyranoside | 1 | 13.82 | 235, 345 | 655 | 640,493, 331, 316 |
| 6,3'-Dimethoxyquercetin-7-O-β-D-glucopyranosyl-(6→1)-O-β-D-glucopyranoside | 2 | 17.46 | 235, 345 | 669 | 507, 345, 330, 302, 287 |
| 7-Methoxyquercetin-O-hexose | 3 | 31.86 | 268, 342 | 477 | 315, 301, 273 |
| 6-Hydroxy-7,3,4-trimethoxyflavanonol-di-O-hexose | 4 | 32.14 | 259, 349 | 685 | 623, 315 |
| 6-Methoxykaempferol-3-O-β-D-6-(p-coumaroyl)glucopyranoside | 5 | 32.14 | 270, 315 | 623 | 608, 477, 300 |
| 6-Hydroxy-7-4-dimethoxyquercetin-3-O-hexose | 6 | 32.31 | 257, 350 | 507 | 477, 315 |
| 6,3-Dimethoxyquercetin-3-O-β-D-6-(p-coumaroyl)glucopyranoside | 7 | 32.88 | 262, 358 | 653 | 345, 330, 287 |
| 5-10-Ddihydroxy-7-methoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-α-L-rhamnopyranosyl-(1→6)- O-β-D-glucopyranoside | 8 | 34.08 | 271, 280sh, 383 | 595 | 449, 287 |
| 10-Hydroxy-5,7-dimethoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-β-D-allopyranosyl (1→6)-O-β-D-glucopyranoside | 9 | 34.48 | 273, 283sh, 362 | 625 | 593, 463, 301 |
| Quercetin-3-O-di-hexose | 10 | 34.88 | 252 281sh, 362 | 625 | 609, 447, 285 |
| 10-Hydroxy-7-methoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-β-D-glucopyranoside | 11 | 39.96 | 272,280sh381 | 433 | 271, 256 |
| 10-Hydroxy-5-7-dimethoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-β-D-glucopyranoside | 12 | 40.33 | 273,284sh, 384 | 463 | 301, 286, 272, 256 |
| Substance | Peak | TR | UV spectra λmax (nm) | [M−H]− | Major MS2 and MS3 fragments |
|---|---|---|---|---|---|
| 6-Hydroxyquercetin-3-O-di-hexose | 1a | 7.98 | 260,295sh, 340 | 641 | 479, 317 |
| 6-Hydroxyquercetin-3-O-hexose dimer | 2a | 20.17 | 260,274sh, 348 | 958 | 479, 463 |
| 6-Metoxykaempferol-3-O-hexose-O-pentose | 3a | 26.54 | 267, 337 | 577 | 431, 299 |
| 4-Methoxyapigenin-7-O-(3-galloyl)-α-D-arabinopyranosyl-(2→1)-apiofuranosyl-(3→1)- α-D-arabinopyranoside | 4a | 26.94 | 267,337 | 831 | 803, 635, 623, 605, 315, 269 |
| 6-Methoxyquercetin-7-O-glucoside | 5a | 27.29 | 253sh, 345 | 493 | 331, 316 |
| 6,3-Dimethoxyquercetin-3-O-β-D-6-(p-coumaroyl)- glucopyranoside | 6a | 32.89 | 262sh,358 | 653 | 345, 330, 287 |
| 5-10-Dihydroxy-7-methoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-α-L-rhamnopyranosyl-(1→6)- O-β-D-glucopyranosíde | 7a | 34.03 | 268,279sh,349 | 595 | 449, 287 |
| Flavanonol-di-O-hexose | 8a | 38.04 | 242,279sh,325 | 627 | 465, 303 |
| 10-hydroxy-7-methoxy-3-methyl-1H-naphtho[2,3c]pyran-1-one-9-O-β-D-glucopyranoside | 9a | 39.92 | 270,279sh, 387 | 433 | 271, 256 |
| 6-Hydroxy-7-methoxyquercetin-3-O-pentose | 10a | 40.48 | 250,283sh, 326 | 463 | 433, 331 |
| 6-Hydroxy-7,3,4-trimethoxyflavanonol | 11a | 48.19 | 242,261sh, 324 | 361 | 346, 331, 316 |
| 6-Hydroxy-7,4-dimethoxyquercetin-3-O-hexose | 12a | 49.31 | 250,283sh, 326 | 507 | 345, 286 |
| 4a | 7 and 6a | |||
|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 2 | 164.1 | - | 156.4 | |
| 3 | 6.85 s | 103.9 | - | 132.6 |
| 4 | - | 180.0 | - | 177.0 |
| 5 | - | 161.8 | - | 152.5 |
| 6 | 6.42d (2.0) | 99.0 | - | 131.6 |
| 7 | 162.6 | - | 157.4 | |
| 8 | 6.85 d (2.0) | 95.0 | 6.49 s | 94.6 |
| 9 | - | 157.0 | - | 152.2 |
| 10 | - | 105.0 | - | 104.8 |
| 1 | - | 121.0 | - | 121.1 |
| 2 | 7.96 d (8.0) | 129.3 | 7.55 dd (8.5, 2.0) | 116.4 |
| 3 | 6.95 d (8.0) | 116.5 | 6.85d (9.0) | 147.0 |
| 4 | - | 161.0 | - | 149.0 |
| 5 | 6.95 d (8.0) | 116.5 | 6.76 d (8.5) | 115.2 |
| 6 | 7.96 d (8.0) | 129.3 | 7.51 dd (8.5, 2.0) | 121.6 |
| OCH3-4 | 3.74 s | 56.0 | - | - |
| OCH3-3 | - | - | 3.82 | 56.7 |
| OCH3-6 | - | - | 3.70 | 60.0 |
| galloyl | glucose | - | ||
| 1 | 121.2 | 5.43 d (7.5) | 100.8 | |
| 2 | 6.79 s | 112.0 | 3.37 dd (9.0; 7.5) | 73.4 |
| 3 | - | 149.6 | 3.48 dd (9.0; 9.0) | 76.8 |
| 4 | - | 139.0 | 3.27 dd (9.0; 9.0) | 69.8 |
| 5 | - | - | 3.29 m | 75,6 |
| 6 | 6.79 s | - | 4.24 dd (6.0; 10.0)4.02dd (5.0; 12.0) | 62.8 |
| α | - | 147.0 | - | - |
| β | - | 112.5 | - | - |
| (C=O) | - | 165.0 | - | 165.3 |
| arabinopyranosyl | coumaroyl | |||
| 1 | 5.11 d (8.0) | 98.0 | - | 125.0 |
| 2 | 3.82 dd (5.5; 8.5) | 72.8 | 7.36 d (8.5) | 130.0 |
| 3 | 3.70 dd (3.5, 7.5) | 76.7 | 6.78 d (8.5) | 116.2 |
| 4 | 3.64 m | 74,6 | - | 159.4 |
| 5 | 3.38 dd (11.5)3.60 d (11.5) | 60.5 | 6.78 d (8.5) | 116.2 |
| 6′ | - | - | 7.36 d (8.5) | 130.0 |
| α | - | - | 6.14 d (16.0) | 114.5 |
| β | - | - | 7.31 d (16.0) | 144.4 |
| apiofuranosyl | ||||
| 1 | 5.34 d (1.0) | 108.0 | - | -- |
| 2 | 3.74 d (3.0) | 76.7 | - | - |
| 3 | - | 79.0 | - | - |
| 4 | 3.64 d (5.5) | 70.4 | - | - |
| 5 | 3.32 dd | 64.9 | - | - |
| arabinopyranoside | - | |||
| 1 | 5.16d (7.0) | 97.0 | - | - |
| 2 | 3.46 dd (5.5, 7.5) | 72.8 | - | - |
| 3 | 3.50 dd (3.5; 7.5) | 69.4 | - | - |
| 4 | 3.20 m | 71.6 | - | - |
| 5 | 3.38 d (11.5)3.62 d (11.5) | 62.5 | - | - |
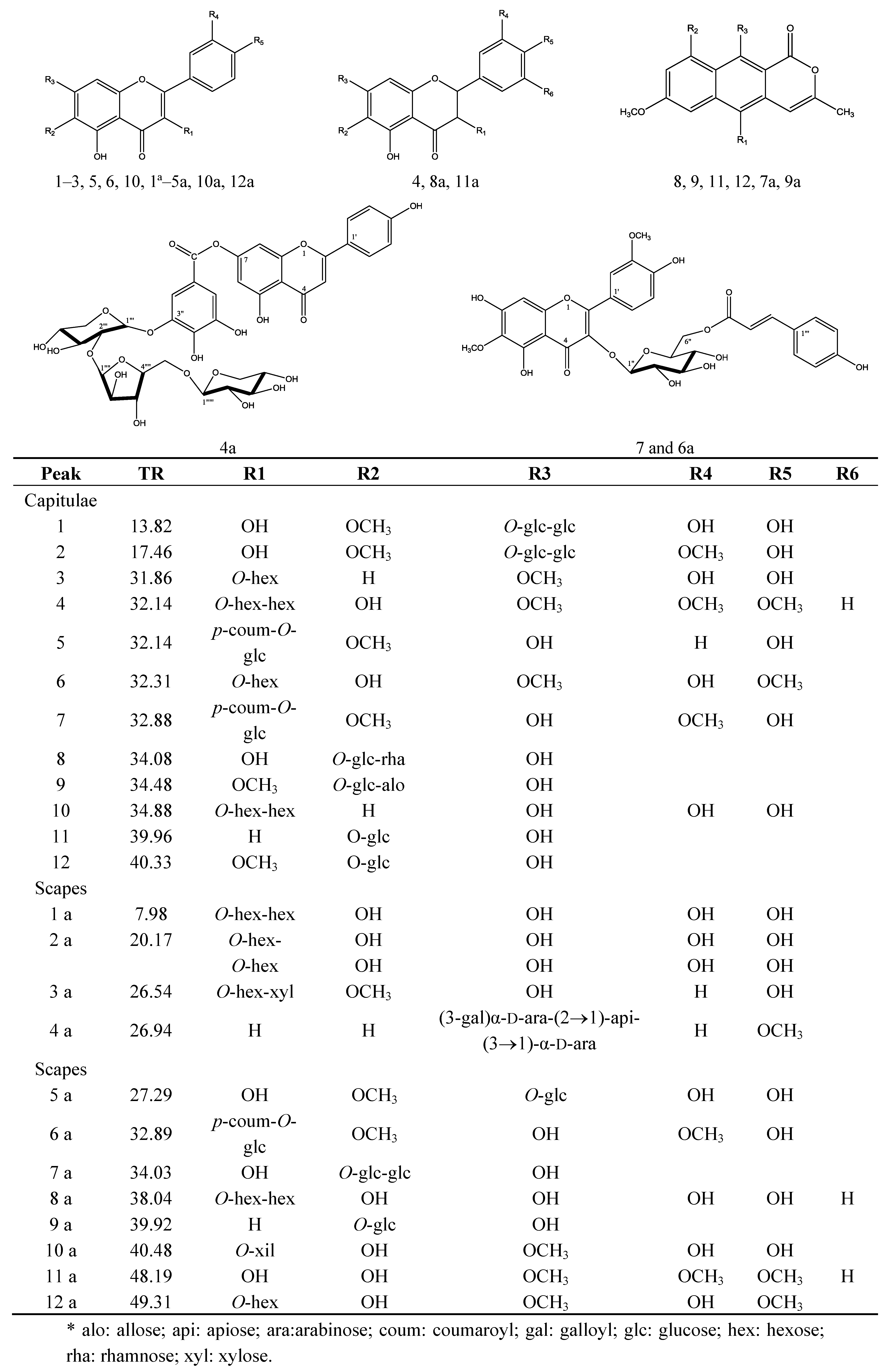
| Treatments mg/plate | Number of revertants/plate in S. typhimurium strains (M ± SD) and (MI) | |||||||
|---|---|---|---|---|---|---|---|---|
| TA98 TA97a TA100 TA102 | ||||||||
| MeOH Ext. Capitulae | −S9 b | +S9 d | −S9 b | +S9 d | −S9 a | +S9 d | −S9 c | +S9 e |
| 0 | 24 ± 1 | 31 ± 2 | 133 ± 17 | 161 ± 24 | 185 ± 11 | 123 ± 9 | 382 ± 30 | 248 ± 3 |
| 0.62 | -- | -- | -- | -- | -- | -- | 245 ± 24 (0.6) | 388 ± 71 (1.5) |
| 1.25 | -- | -- | -- | -- | -- | -- | 277 ± 21 (0.7) | 332 ± 64 (1.3) |
| 1.87 | 34 ± 9 (1.8) | 32 ± 1 (1.5) | 753 ± 127 **(5.6) | 1410 ± 172 **(8.7) | 334 ± 21 (1.7) | 193 ± 12 (1.6) | -- | -- |
| 2.50 | -- | -- | -- | -- | -- | -- | 359 ± 53 (0.9) | 350 ± 7 (1.4) |
| 3.75 | 35 ± 8 (1.1) | 29 ± 5 (1.3) | 419 ± 108 *(3.1) | 1358 ± 76 **(17.9) | 187 ± 12 (0.9) | 148 ± 7 (1.2) | 421 ± 133 1.1) | 341 ± 104(1.4) |
| 7.50 | 57 ± 11 (1.8) | 33 ± 4 (1.5) | 698 ± 259 *(5.2) | 1493 ± 62 **(24.0) | 151 ± 24 (0.7) | 172 ± 11 (1.4) | -- | -- |
| 11.25 | 47 ± 8 (1.4) | 39 ± 2 (1.8) | 231 ± 68 (1.7) | 1348 ± 127 **(10.5) | 118 ± 13 (0.6) | 156 ± 12 (1.3) | -- | -- |
| Control + | 1944 ± 120 | 2877 ± 749 | 1197 ± 57 | 3605 ± 34 | 2033 ± 236 | 1700 ± 311 | 1836 ± 117 | 671 ± 25 |
| MeOH Ext. Scapes | −S9 b | +S9 d | −S9 b | +S9 d | −S9 a | +S9 d | −S9 c | +S9 e |
| 0 | 24 ± 2 | 31 ± 3 | 134 ± 2 | 235 ± 10 | 185 ± 11 | 105 ± 11 | 382 ± 30 | 248 ± 3 |
| 0.62 | -- | -- | -- | -- | -- | -- | 468 ± 10 (1.2) | 370 ± 26 (1.5) |
| 1.25 | -- | -- | -- | -- | -- | -- | 427 ± 33 (1.1) | 320 ± 25 (1.3) |
| 1.87 | 47 ± 2 (1.5) | 32 ± 2 (1.5) | 384 ± 65 (2.3) | 837 ± 77 (2.6) | 382 ± 21 (1.6) | 180 ± 6.1 (1.7) | -- | -- |
| 2.50 | -- | -- | -- | -- | -- | -- | 492 ± 138 1.3) | 377 ± 13 (1.5) |
| 3.75 | 41 ± 1 (1.2) | 34 ± 6 (1.6) | 235 ± 132 (1.4) | 909 ± 180 (2.9) | 187 ± 12 (0.9) | 196 ± 10 (1.9) | 493 ± 21 (1.3) | 321 ± 61 (1.3) |
| 7.50 | 61 ± 4 (1.9) | 37 ± 4 (1.7) | 172 ± 46 (1.0) | 708 ± 73 (2.2) | 151 ± 24 (0.7) | 194 ± 7 (1.9) | -- | -- |
| 11.25 | 41 ± 6 (1.3) | 41 ± 2 (1.9) | 30 ± 19 (0.2) | 580 ± 89 (1.8) | 21 ± 2 (0.1) | 192 ± 28 (1.8) | -- | -- |
| Control + | 1944 ± 120 | 2877 ± 749 | 1465 ± 57 | 2010 ± 536 | 2033 ± 236 | 3221 ± 117 | 1836 ± 117 | 671 ± 25 |
3. Experimental
3.1. Chemicals
3.2. Plant Material
3.3. Extraction
3.4. Sample Preparation
3.5. Isolation of Compounds and Characterization
3.6. Standard Solutions
3.7. HPLC-ESI-IT-MSn Analyses
3.8. ESI-MSn Analysis
3.9. Salmonella Mutagenicity Assay (Ames Test)
3.10. Experimental Procedure
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References
- Giulietti, A.M.; Hensold, N.; Parra, L.R.; Andrade, M.J.G.; Van Den Berg, C.; Harley, R.M. The synonymization of Philodice with Syngonanthus (Eriocaulaceae). Phytotaxa 2012, 60, 50–56. [Google Scholar]
- Dokkedal, A.L.; Santos, L.C.; Sano, P.T.; Vilegas, W. Chemistry in Eriocaulaceae. Z. Naturforsch. C. 2008, 63, 169–175. [Google Scholar]
- Santos, L.C.; Piacente, S.; Montoro, P.; Pizza, C.; Vilegas, W. Atividade antioxidante de xantonas isoladas de espécies de Leiothix (Eriocaulaceae). Rev. Bras. Farmacogn. 2003, 13, 67–74. [Google Scholar]
- Devienne, K.F.; Calgaro-Helena, A.F.; Dorta, D.J.; Prado, I.M.R.; Raddi, M.S.G.; Vilegas, W.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant activity of isocoumarins isolated from Paepalanthus bromelioides on mitochondria. Phytochemistry 2007, 68, 1075–1080. [Google Scholar]
- Varanda, E.A.; Devienne, K.F.; Raddi, M.S.G.; Furuya, E.M.; Vilegas, W. Mutagenicity of paepalantine dimer and glycoside derivatives from Paepalanthus bromelioides. Toxicol. In Vitro 2004, 18, 109–114. [Google Scholar]
- Varanda, E.A.; Raddi, M.S.G.; Dias, F.L.P.; Araujo, M.C.S.; Gibran, S.C.A.; Takahashi, C.S.; Vilegas, W. Mutagenic and cytotoxic activity of an isocoumarin (paepalantine) isolated from Paepalanthus vellozioides. Teratog. Carcinog. Mutagen. 1997, 17, 85–95. [Google Scholar] [CrossRef]
- Silva, M.A.; Oliveira, A.P.S.; Sannomiya, M.; Sano, P.T.; Varanda, E.A.; Vilegas, W.; Santos, L.C. Flavonoids and naphthopyranone from Eriocaulon ligulatum and their mutagenic activity. Chem. Pharm. Bull. 2007, 55, 1635–1639. [Google Scholar] [CrossRef]
- Coelho, R.G.; Batista, L.M.; Santos, L.C.; Brito, A.R.M.S.; Vilegas, W. Phytochemical study and antiulcerogenic activity of Syngonanthus basiculatus (Eriocaulaceae). Rev. Bras. Cienc. Farm. 2006, 42, 413–417. [Google Scholar]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982; p. 113. [Google Scholar]
- Vilegas, W.; Santos, L.C.; Alecio, A.C.; Pizza, C.; Piacente, S.; Pauw, E.; Sano, P.T. Naphthopyranone glycosides from Paepalanthus bromelioides. Phytochemistry 1998, 49, 207–210. [Google Scholar]
- Vilegas, W.; Nehme, C.J.; Dokkedal, A.L.; Piacente, S.; Rastrelli, L.; Pizza, C. Quercetagetin 7-methyl ether glycosides from Paepalanthus velloziodes and Paepalanthus latipes. Phytochemistry 1999, 51, 403–409. [Google Scholar]
- Andrade, F.D.P.; Rastrelli, L.; Pizza, C.; Sano, P.T.; Vilegas, W. Flavonol glycosides and a naphthopyranone glycoside from Paepalanthus macropodus (Eriocaulaceae). Biochem. Syst. Ecol. 2002, 30, 275–277. [Google Scholar] [CrossRef]
- Santos, L.C.; Sannomiya, M.; Piacente, S.; Pizza, C.; Sano, P.T.; Vilegas, W. Chemical profile of the polar extract of Paepalanthus microphyllus (Guill.) Kunth (Eriocaulaceae). Rev. Bras. Cienc. Farm. 2004, 40, 433–436. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Camuesco, D.; Nieto, A.; Vilegas, W.; Zarzuelo, A.; Gálvez, J. Intestinal anti-inflammatory activity of Paepalantine, an isocoumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzenesulphonic acid model of rat colitis. Planta Med. 2004, 70, 315–320. [Google Scholar] [CrossRef]
- Devienne, K.F.; Raddi, M.S.G.; Varanda, E.A.; Vilegas, W. In vitro cytotoxic of some natural and semi-synthetic isocoumarins from Paepalanthus bromelioides. Z. Naturforsch. 2002, 57, 85–88. [Google Scholar]
- Piacente, S.; Santos, L.C.; Mahmood, N.; Zampelli, A.; Pizza, C.; Vilegas, W. Naphthopyranone glycosides from Paepalanthus microphyllus. J. Nat. Prod. 2001, 64, 680–682. [Google Scholar] [CrossRef]
- Trovó, M.; Sano, P.T. Taxonomic survey of Paepalanthus section Diphyomene (Eriocaulaceae). Phytotaxa 2010, 14, 49–55. [Google Scholar]
- Jin, J.; Liu, B.; Zhang, H.; Tian, X.; Cai, Y.; Gao, P. Mutagenicity of Chinese traditional medicine Semen Armeniacae amarum by two modified Ames tests. BMC Complement. Altern. Med. 2009, 15, 43–50. [Google Scholar]
- Gulluce, M.; Agar, G.; Baris, O.; Karadayi, M.; Orhan, F.; Sahin, F. Mutagenic and antimutagenic effects of hexane extract of some Astragalus species grown in the eastern Anatolia region of Turkey. Phytother. Res. 2010, 24, 1014–1018. [Google Scholar]
- Resende, F.A.; Vilegas, W.; Santos, L.C.; Varanda, E.A. Mutagenicity of flavonoids assayed by bacterial reverse mutation (Ames) test. Molecules 2012, 17, 5255–5268. [Google Scholar]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Santos, L.C.; Piacente, S.; Pizza, C.; Albert, K.; Dachtler, M.; Vilegas, W. Planifolin, a New Naphthopyranone Dimer and Flavonoids from Paepalanthus planifolius. J. Nat. Prod. 2001, 64, 122–124. [Google Scholar]
- Amaral, F.P.; Napolitano, A.; Masullo, M.; Santos, L.C.; Pizza, C.; Vilegas, W.; Piacente, S. HPLC-ESIMSn Profiling, Isolation, Structural Elucidation, and Evaluation of the Antioxidant Potential of Phenolics from Paepalanthus geniculatus. J. Nat. Prod. 2012, 75, 547–556. [Google Scholar]
- Santos, L.C.; Silva, M.A.; Rodrigues, C.M.; Carbone, V.; Napolitano, A.; Bassarello, C.; Mari, A.; Piacente, S.; Pizza, C.; Vilegas, W. Characterization of Flavonoid and Naphthopyranone Derivatives from Eriocaulon ligulatum using Liquid Chromatography Tandem Mass Spectrometry. Nat. Prod. Commun. 2009, 4, 1651–1656. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer-Verlag: New York, NY, USA, 1970. [Google Scholar]
- Dokkedal, A.L.; Lavarda, F.; Santos, L.C.; Vilegas, W. Xeractinol-A new flavanonol C-glucoside from Paepalanthus argenteus var. argenteus (Bongard) Hensold (Eriocaulaceae). J. Braz. Chem. Soc. 2007, 18, 437–439. [Google Scholar] [CrossRef]
- Santos, L.C.; Piacente, S.; Pizza, C.; Toro, R.; Sano, P.T.; Vilegas, W. 6-Methoxyquercetin-3-O-(6E-feruloyl)-β-D-glucopyranoside from Paepalanthus polyanthus (Eriocaulaceae). Biochem. Syst. Ecol. 2002, 30, 451–456. [Google Scholar]
- Park, E.J.; Kim, Y.; Kim, J. Acylated Flavonol Glycosides from the Flower of Inula britannica. J. Nat. Prod. 2000, 63, 34–36. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon 13H-NMR of Flavonoids; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Andrade, F.D.P.; Santos, L.C.; Dokkedal, A.L.; Vilegas, W. Acyl glucosylated flavonols from Paepalanthus species. Phytochemistry 1999, 51, 411–415. [Google Scholar] [CrossRef]
- Cardoso, C.A.L.; Zanutto, F.V.; Varanda, E.A.; Sano, P.T.; Vilegas, W.; Santos, L.C. Quantification of Flavonoids, Naphthopyranones and Xanthones in Eriocaulaceae Species by LC-PDA. Am. J. Analyt. Chem. 2012, 3, 138–146. [Google Scholar]
- Tavares, D.C.; Varanda, E.A.; Andrade, F.D.P.; Vilegas, W.; Takahashi, C.S. Evaluation of the genotoxic potential of the isocoumarin paepalantine in vivo and in vitro mammalian systems. J. Ethnopharmacol. 1999, 68, 115–120. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Boersma, M.G.; van der Woude, H.; Jeurissen, S.M.F.; Schutte, M.E.; Alink, G.M. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005, 574, 124–138. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Bernstein, L.; Kaldor, J.; Mccann, J.; Pike, M.C. An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test. Mutat. Res. 1982, 97, 267–281. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zanutto, F.V.; Boldrin, P.K.; Varanda, E.A.; Souza, S.F.d.; Sano, P.T.; Vilegas, W.; Santos, L.C.d. Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity. Molecules 2013, 18, 244-262. https://doi.org/10.3390/molecules18010244
Zanutto FV, Boldrin PK, Varanda EA, Souza SFd, Sano PT, Vilegas W, Santos LCd. Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity. Molecules. 2013; 18(1):244-262. https://doi.org/10.3390/molecules18010244
Chicago/Turabian StyleZanutto, Fabiana Volpe, Paula Karina Boldrin, Eliana Aparecida Varanda, Samara Fernandes de Souza, Paulo Takeo Sano, Wagner Vilegas, and Lourdes Campaner dos Santos. 2013. "Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity" Molecules 18, no. 1: 244-262. https://doi.org/10.3390/molecules18010244
APA StyleZanutto, F. V., Boldrin, P. K., Varanda, E. A., Souza, S. F. d., Sano, P. T., Vilegas, W., & Santos, L. C. d. (2013). Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity. Molecules, 18(1), 244-262. https://doi.org/10.3390/molecules18010244





