Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments
Abstract
:1. Introduction
2. Suzuki-Miyaura Coupling of Acyl Chlorides and Anhydrides
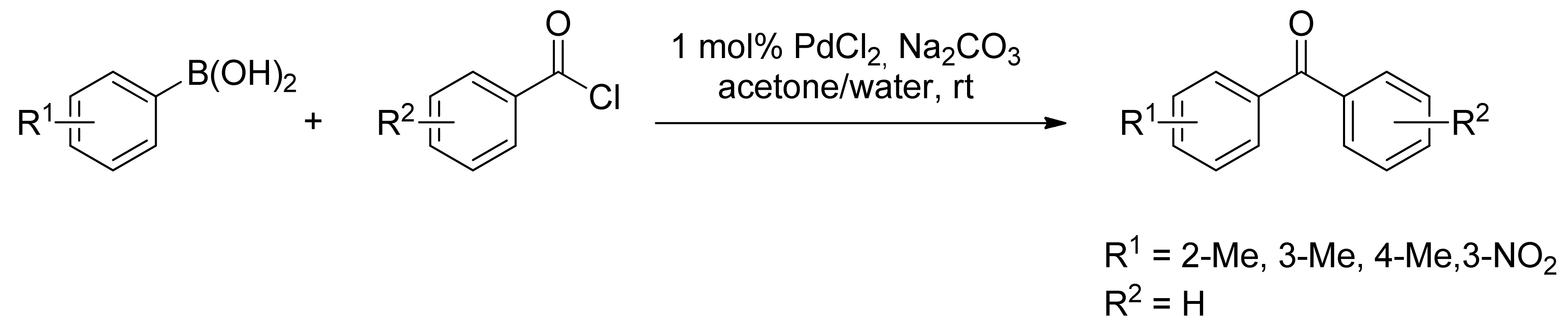
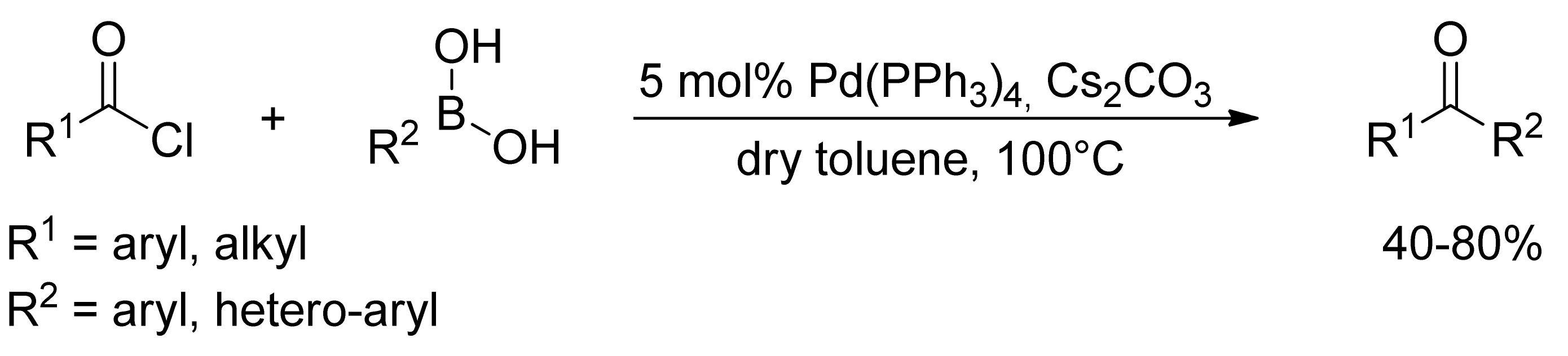
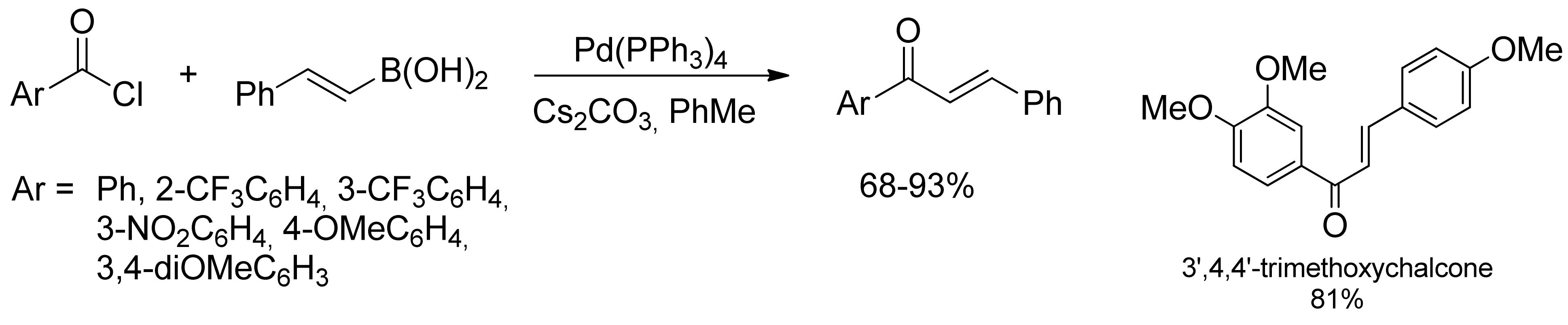
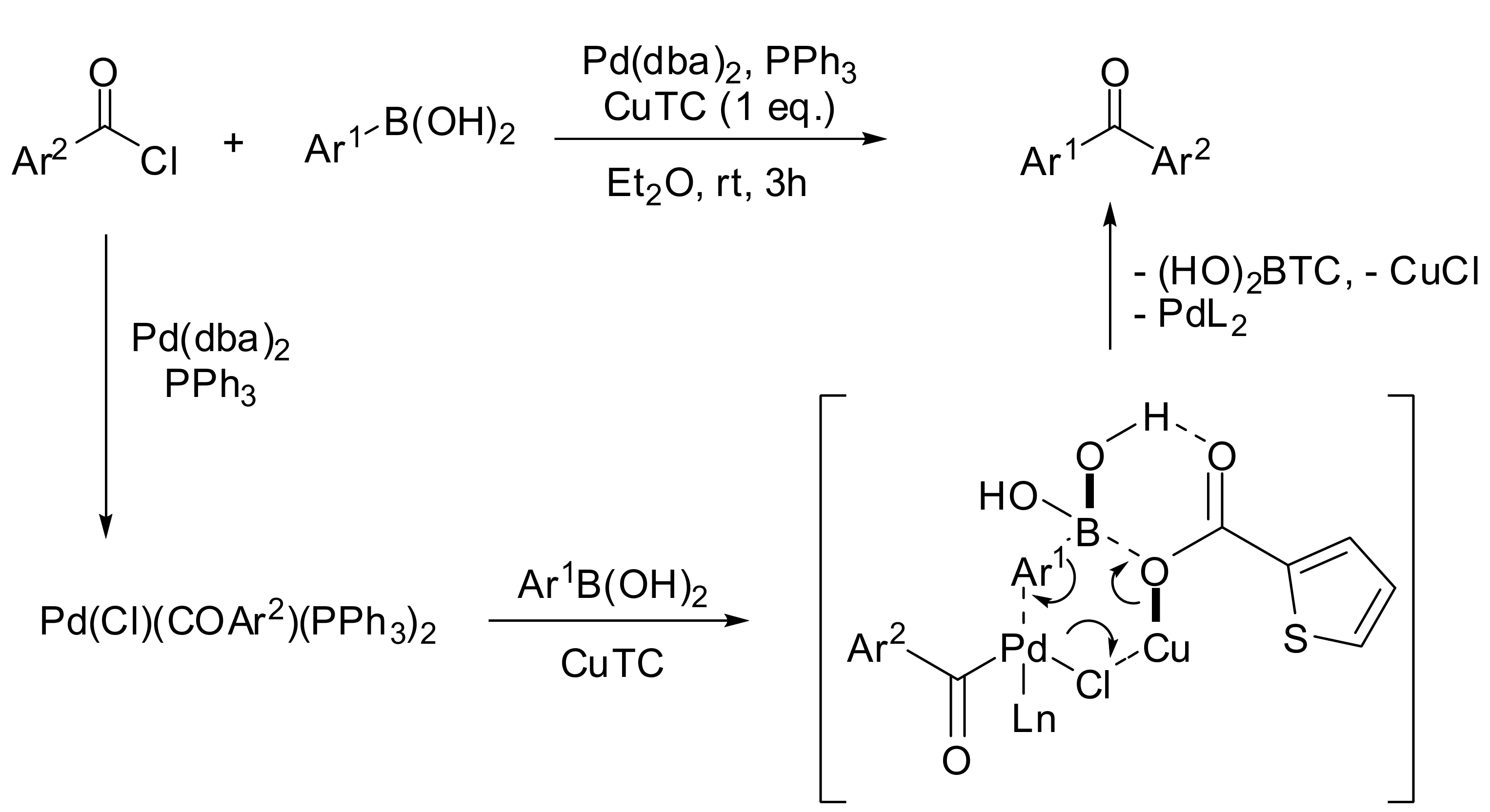
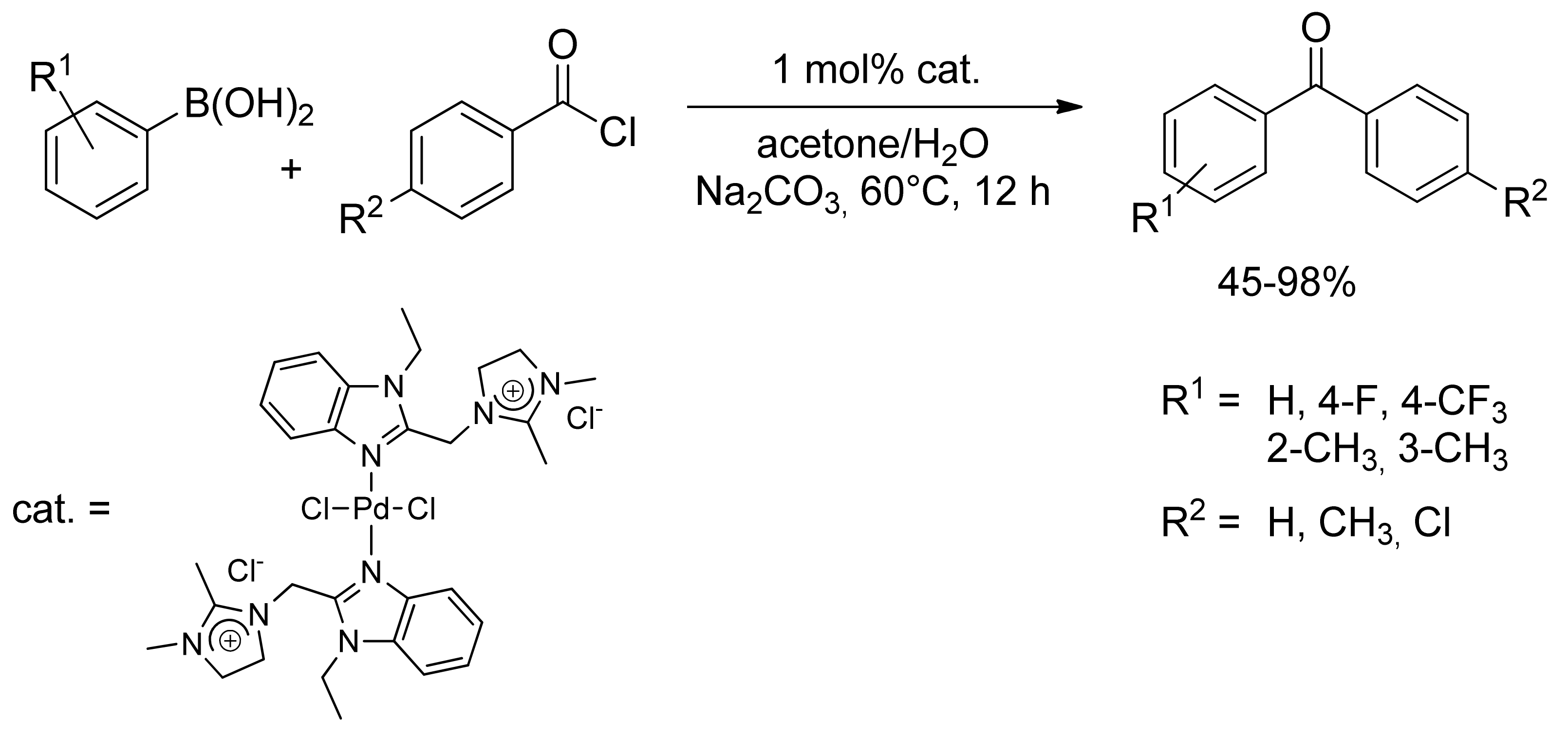
2.1. Coupling of Arylboronic Acid Derivatives with Acyl Chlorides
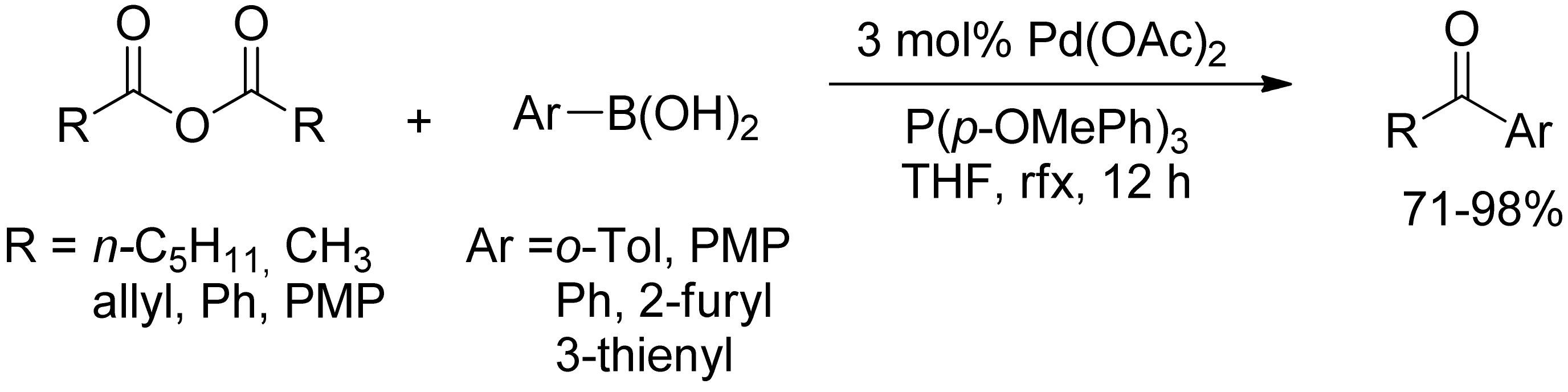
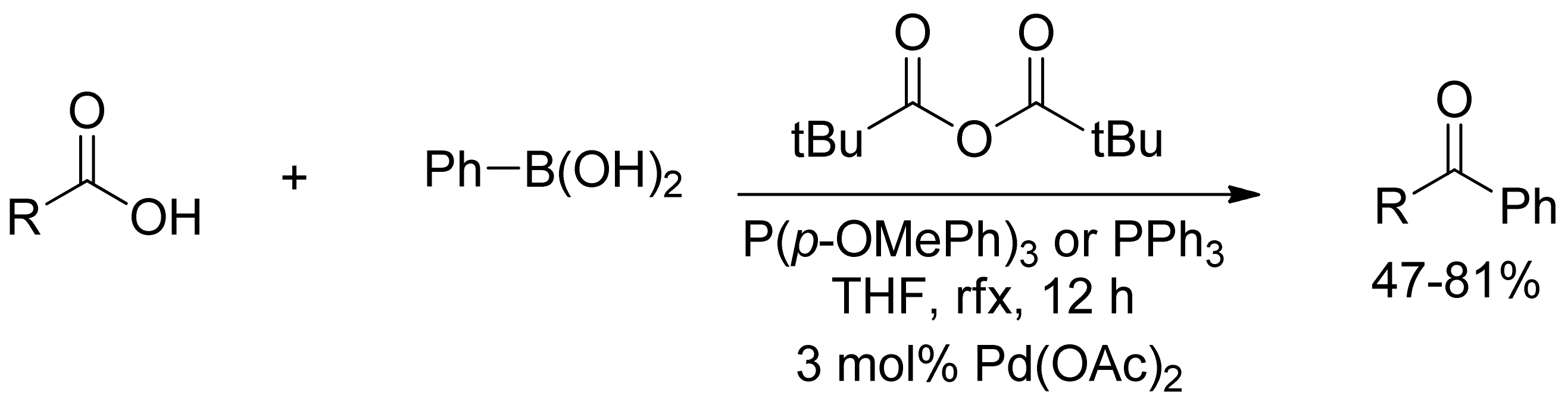
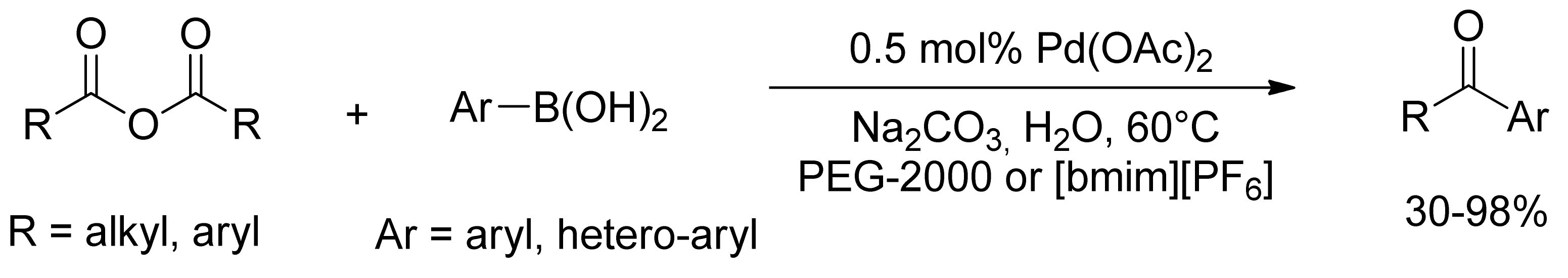
2.2. Coupling of Arylboronic Derivatives with Anhydrides
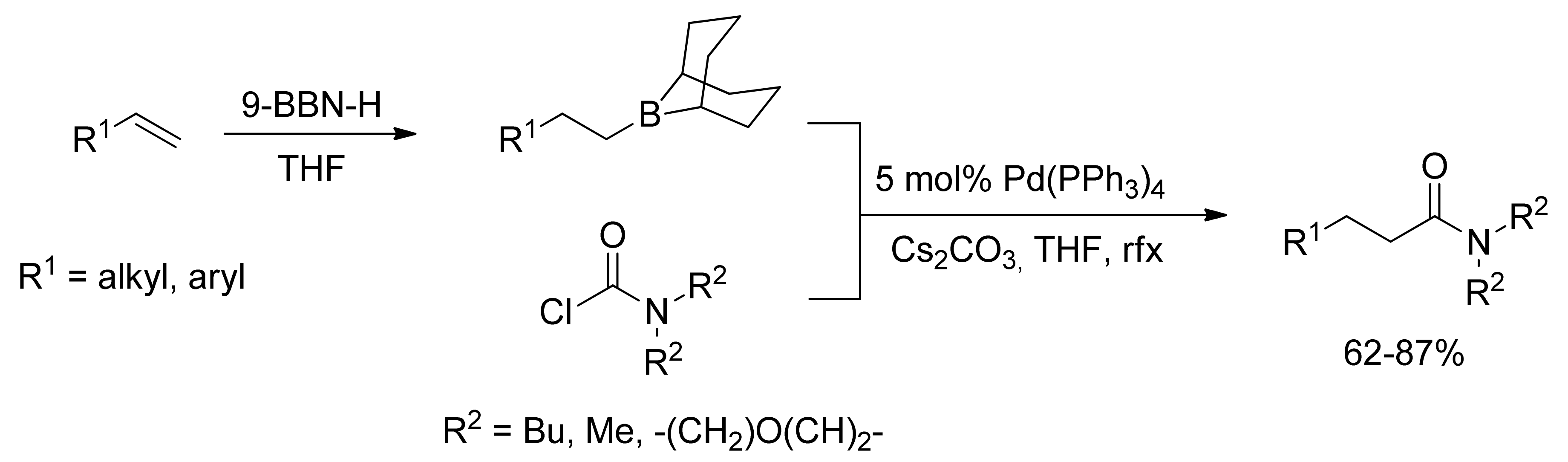
2.3. Coupling with Chloroformates and Carbamoyl Chlorides
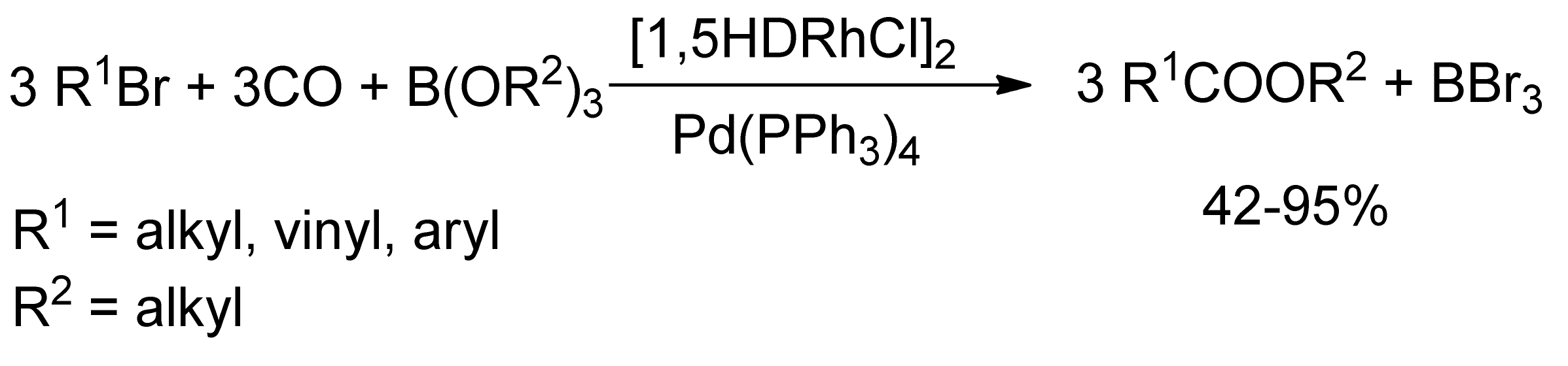
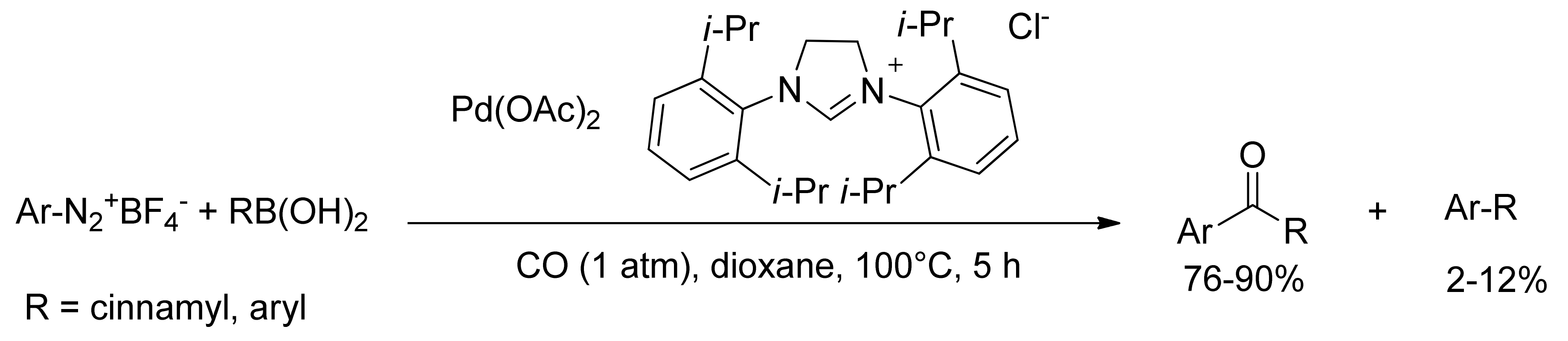
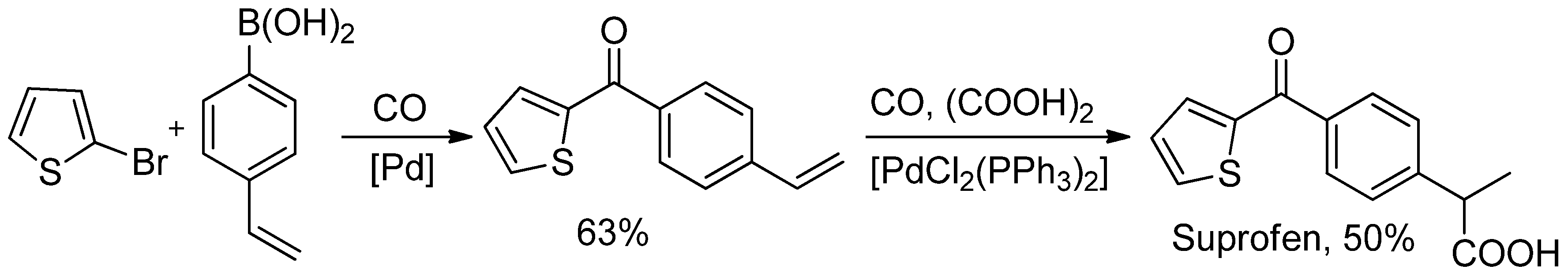
3. Carbonylative SM Reactions
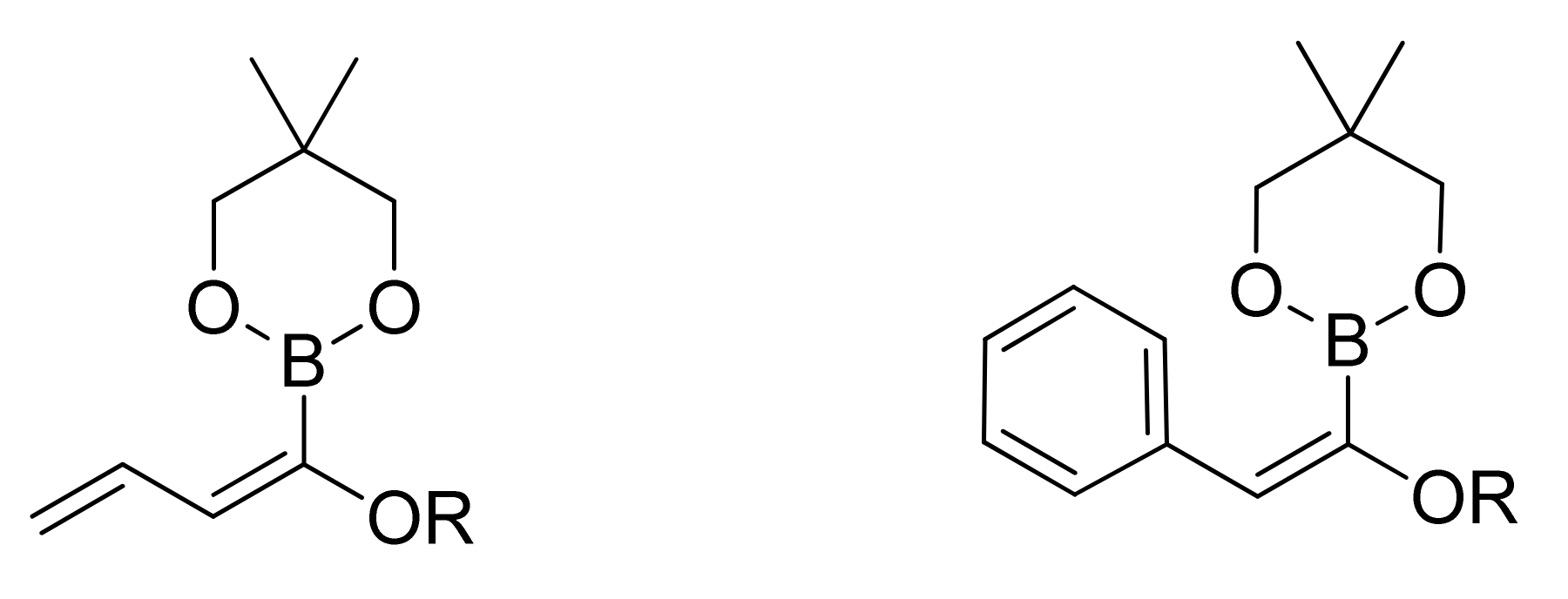
4. SM with Masked Acyl Boronates
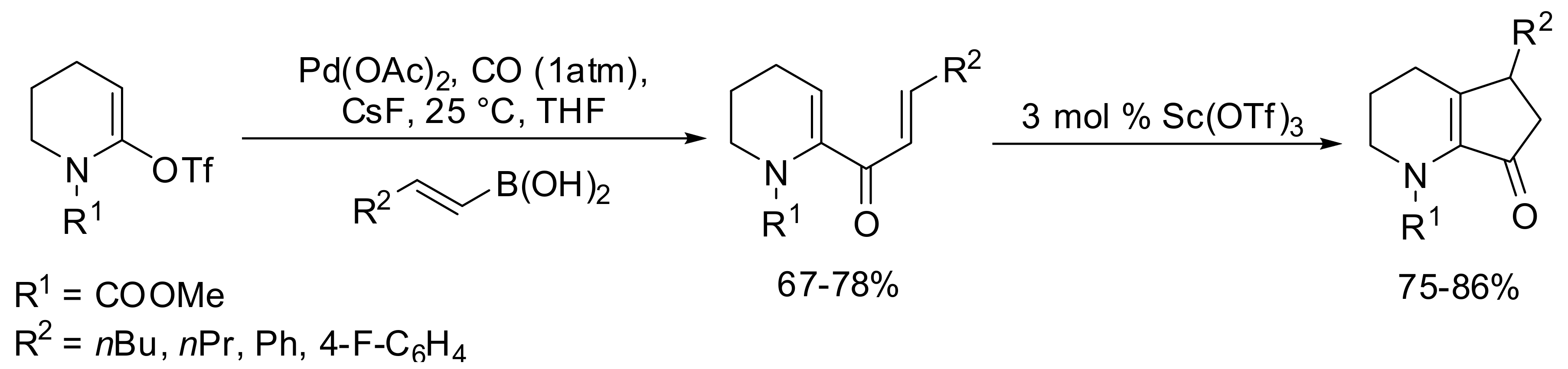
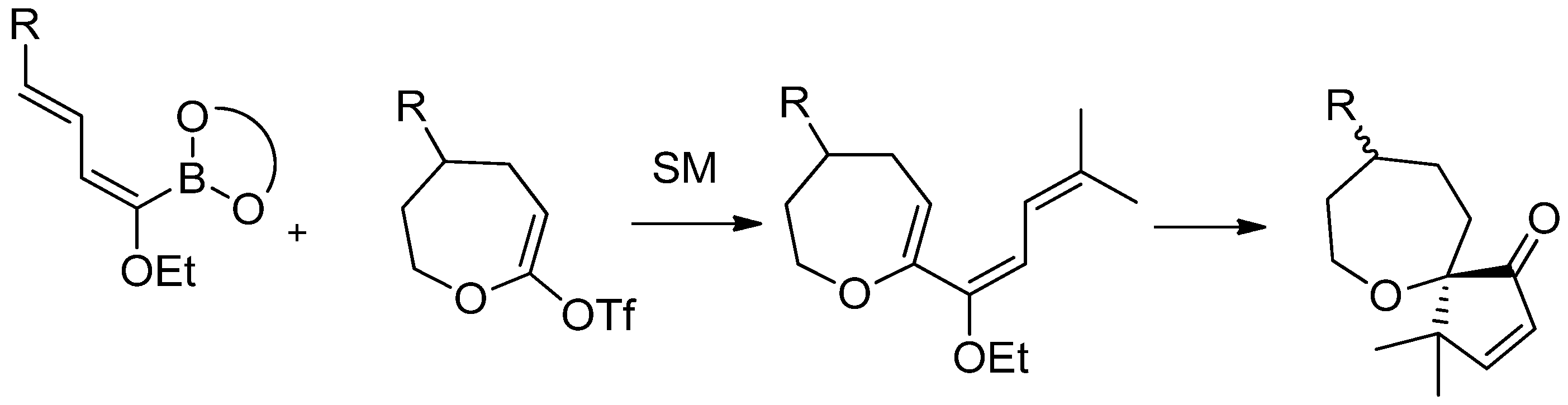
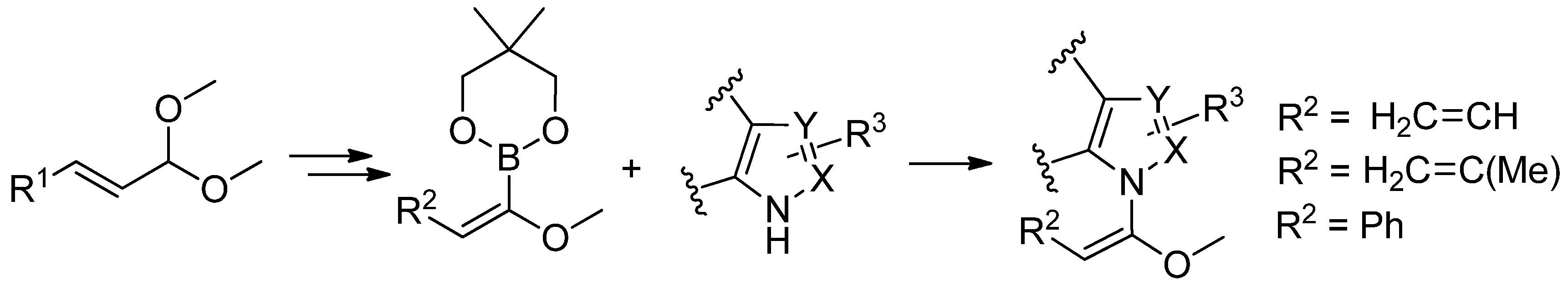
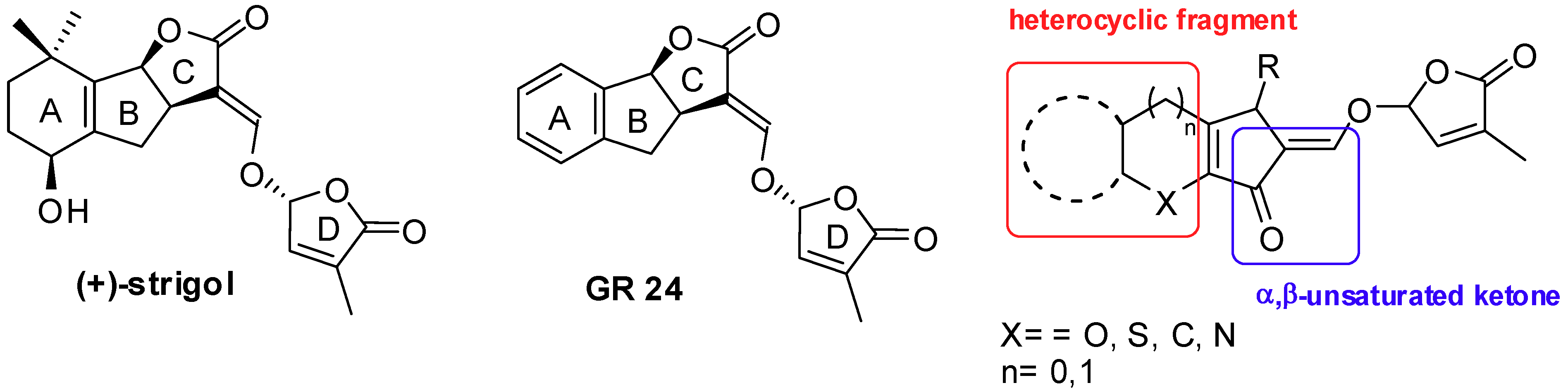
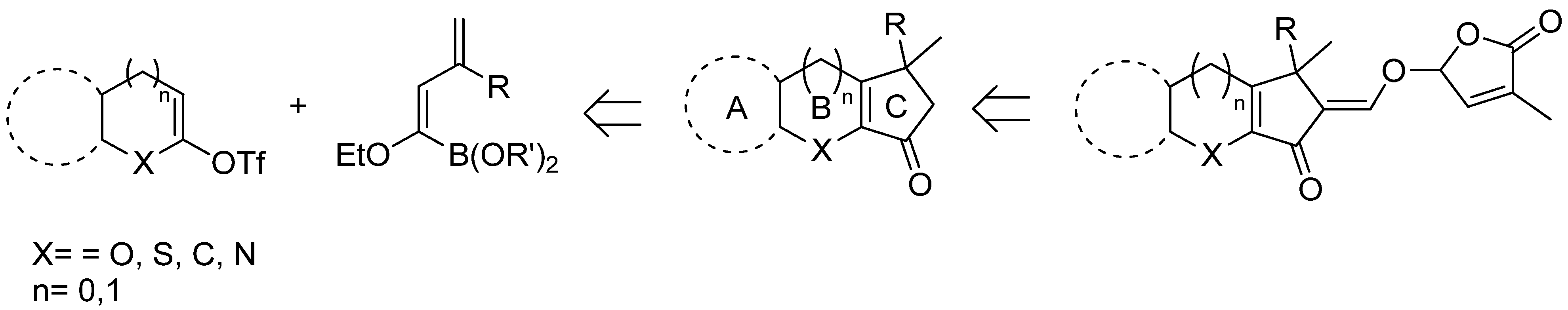
5. Acylation of Heterocycles by SM in the Synthesis of Natural Products
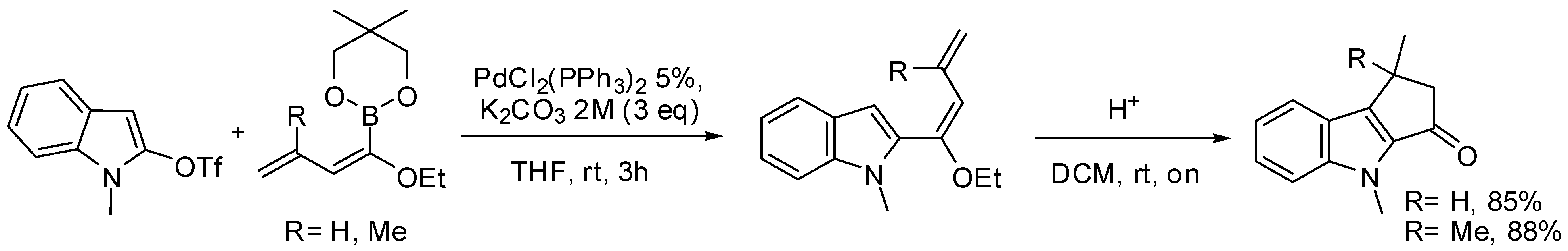
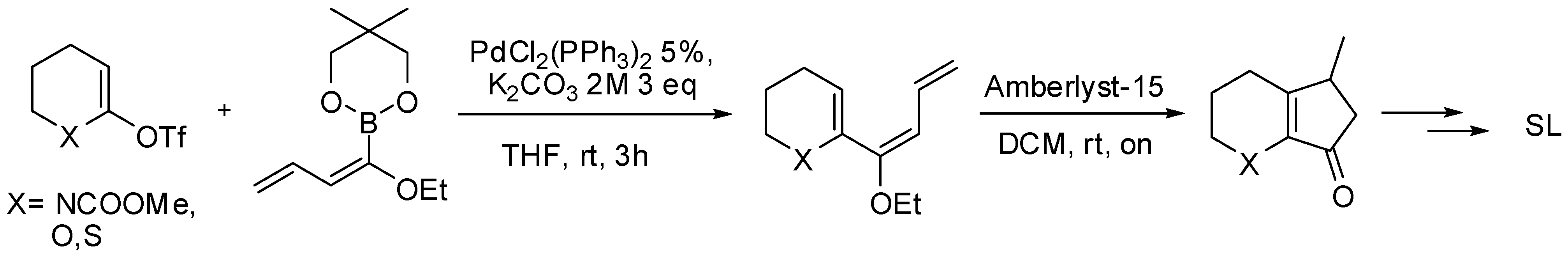
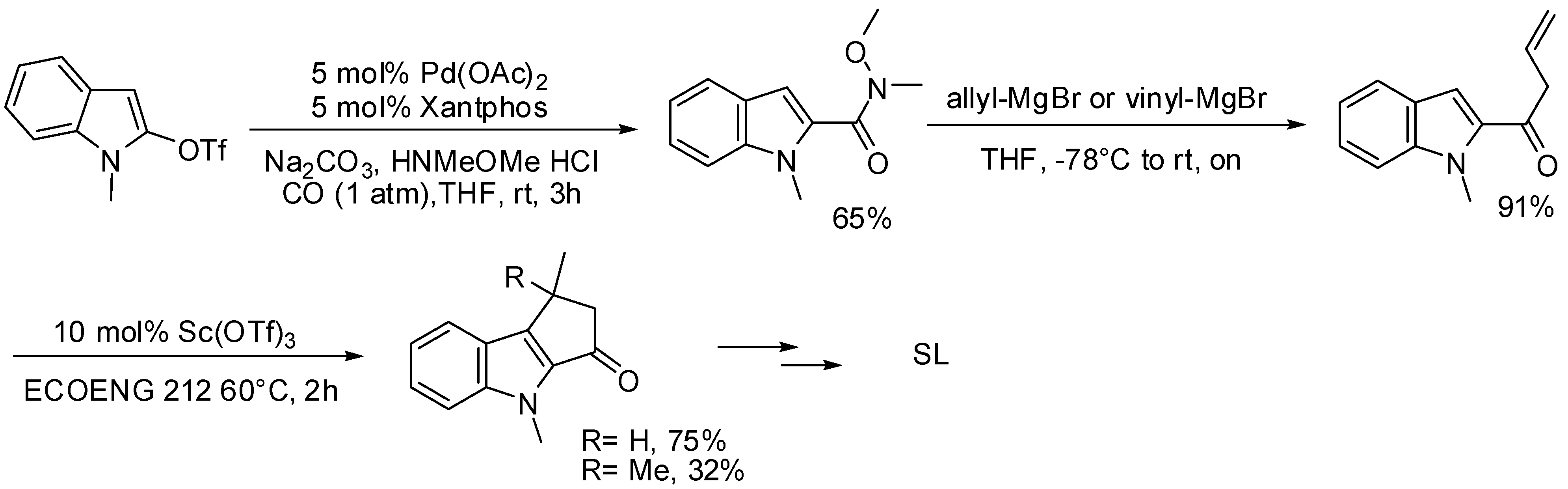
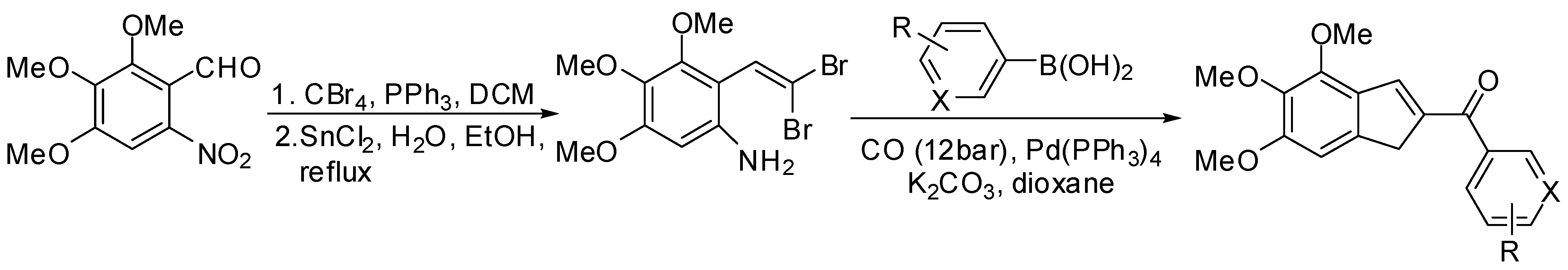
5.1. SM Acylation Reaction for the Synthesis of Strigolactones (SLs)
5.2. SM Coupling Applied to the Synthesis of Other Natural Products
6. Conclusions
Acknowledgments
References
- Dieter, R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Rovis, T. A unique catalyst effects the rapid room-temperature cross-coupling of organozinc reagents with carboxylic acid fluorides, chlorides, anhydrides, and thioesters. J. Am. Chem. Soc. 2004, 126, 15964–15965. [Google Scholar] [CrossRef] [PubMed]
- Furstner, A.; Voigtlander, D.; Schrader, W.; Giebel, D.; Reetz, M.T. A “hard/soft” mismatch enables catalytic Friedel-Crafts acylations. Org. Lett. 2001, 3, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Gmouth, S.; Yang, H.L.; Vaultier, M. Activation of bismuth(III) derivatives in ionic liquids: Novel and recyclable catalytic systems for Friedel-Crafts acylation of aromatic compounds. Org. Lett. 2003, 5, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Fillion, E.; Fishlock, D.; Wilsily, A.; Goll, J.M. Meldrum’s acids as acylating agents in the catalytic intramolecular Friedel-Crafts reaction. J. Org. Chem. 2005, 70, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Xiao, J.L. Friedel-Crafts acylation reactions using metal triflates in ionic liquid. Green Chem. 2002, 4, 129–133. [Google Scholar] [CrossRef]
- Labadie, J.W.; Stille, J.K. Stereochemistry of transmetalation in the palladium-catalyzed coupling of acyl chloride and organostannanes. J. Am. Chem. Soc. 1983, 105, 669–670. [Google Scholar] [CrossRef]
- Reddy, C.K.; Knochel, P. New cobalt- and iron-catalyzed reactions of organozinc compounds. Angew. Chem. Int. Ed. Engl. 1996, 35, 1700–1701. [Google Scholar] [CrossRef]
- Wu, T.C.; Xiong, H.P.; Rieke, R.D. Organocalcium Chemistry: Preparation and Reactions of Highly Reactive Calcium. J. Org. Chem. 1990, 55, 5045–5051. [Google Scholar] [CrossRef]
- Tatamidani, H.; Kakiuchi, F.; Chatani, N. A new ketone synthesis by palladium-catalyzed cross-coupling reactions of esters with organoboron compounds. Org. Lett. 2004, 6, 3597–3599. [Google Scholar] [CrossRef] [PubMed]
- Eberle, M.K.; Kahle, G.G. Preparation of functionalized ketones via low temperature grignard reaction. Tetrahedron Lett. 1980, 21, 2303–2304. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- March, J. Advanced Organic Chemistry, 3rd ed.; Wiley Interscience: New York, NY, USA, 1985; pp. 433–435, 824–827. [Google Scholar]
- Larock, R.C. Comprehensive Organic Transformations: A Guide to Functional Group Preparation; VCH: New York, NY, USA, 1989; pp. 685–702. [Google Scholar]
- O'Nell, B.T. Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991; pp. 397–458. [Google Scholar]
- Olah, G.A. Friedel.–Crafts. and Related Reactions; Interscience: New York, NY, USA, 1963; Volume 1. [Google Scholar]
- Sato, F.; Inoue, M.; Oguro, K.; Sato, M. Preparation of ketones by direct reaction of grignard reagents with acyl chlorides in tetrahydrofuran. Tetrahedron Lett. 1979, 20, 4303–4306. [Google Scholar] [CrossRef]
- Bykov, V.V.; Korolev, D.N.; Bumagin, N.A. Palladium-catalyzed reactions of organoboron compounds with acyl chlorides. Russ. Chem. Bull. 1997, 46, 1631–1632. [Google Scholar] [CrossRef]
- Haddach, M.; McCarthy, J.R. A new method for the synthesis of ketones: The palladium-catalyzed cross-coupling of acyl chlorides with arylboronic acids. Tetrahedron Lett. 1999, 40, 3109–3112. [Google Scholar] [CrossRef]
- Bumagin, N.A.; Korolev, D.N. Synthesis of unsymmetric ketones via ligandless Pd-catalyzed reaction of acyl chlorides with organoboranes. Tetrahedron Lett. 1999, 40, 3057–3060. [Google Scholar] [CrossRef]
- Urawa, Y.; Ogura, K. A convenient method for preparing aromatic ketones from acyl chlorides and arylboronic acids via Suzuki-Miyaura type coupling reaction. Tetrahedron Lett. 2003, 44, 271–273. [Google Scholar] [CrossRef]
- Eddarir, S.; Cotelle, N.; Bakkour, Y.; Rolando, C. An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron Lett. 2003, 44, 5359–5363. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, A.V. A rapid, solvent-free, ligandless and mild method for preparing aromatic ketones from acyl chlorides and arylboronic acids via a Suzuki-Miyaura type of coupling reaction. Tetrahedron Lett. 2005, 46, 7627–7630. [Google Scholar] [CrossRef]
- Nishihara, Y.; Inoue, Y.; Fujisawa, M.; Takagi, K. Room-Temperature Palladium-Catalyzed and Copper(I)-Mediated Coupling Reactions of Acyl Chlorides with Boronic Acids under Neutral Conditions. Synlett 2005, 2309–2312. [Google Scholar] [CrossRef]
- Polàckovà, V.; Toma, S.; Augustìnovà, I. Microwave-promoted cross-coupling of acyl chlorides with arylboronic acids: A convenient method for preparing aromatic ketones. Tetrahedron 2006, 62, 11675–11678. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Xu, H.; Wolf, C. Palladium-phosphinous acid-catalyzed cross-coupling of aliphatic and aromatic acyl chlorides with boronic acids. Tetrahedron Lett. 2008, 49, 5773–5776. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Shi, L.; Xia, C.; Li, F. Ionically tagged benzimidazole palladium(II) complex: Preparation and catalytic application in cross-coupling reactions. Tetrahedron Lett. 2011, 52, 3897–3901. [Google Scholar] [CrossRef]
- Kakino, R.; Shimizu, I.; Yamamoto, A. Synthesis of Trifluoromethyl Ketones by Palladium-Catalyzed Cross-Coupling Reaction of Phenyl Trifluoroacetate with Organoboron Compounds. Bull. Chem. Soc. Jpn. 2001, 74, 371–376. [Google Scholar] [CrossRef]
- Kakino, R.; Yasumi, S.; Shimizu, I.; Yamamoto, A. Synthesis of Unsymmetrical Ketones by Palladium-Catalyzed Cross-Coupling Reaction of Carboxylic Anhydrides with Organoboron Compounds. Bull. Chem. Soc. Jpn. 2002, 75, 137–148. [Google Scholar] [CrossRef]
- Kakino, R.; Narahashi, H.; Shimizu, I.; Yamamoto, A. Palladium-Catalyzed Direct Conversion of Carboxylic Acids into Ketones with Organoboronic Acids Promoted by Anhydride Activators. Bull. Chem. Soc. Jpn. 2002, 75, 1333–1345. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Ghosh, K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Angew. Chem. Int. Ed. 2001, 40, 3458–3460. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Ghosh, K. Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids Activated in situ by Pivalic Anhydride. Eur. J. Org. Chem. 2002, 3254–3267. [Google Scholar]
- Xin, B.; Zhang, Y.; Cheng, K. Phosphine-Free Cross-Coupling Reaction of Arylboronic Acids with Carboxylic Anhydrides or Acyl Chlorides in Aqueous Media. J. Org. Chem. 2006, 71, 5725–5731. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhang, Y.; Cheng, K. The Surfactant-Promoted Cross-Coupling Reactions of Arylboronic Acids with Carboxylic Anhydrides or Acyl Chlorides in Water. Synthesis 2007, 1970–1978. [Google Scholar] [CrossRef]
- Xin, B.-W. Synthesis of Aryl Ketones by Cross-Coupling Reaction of Arylboronic Acids with Carboxylic Anhydrides in Aqueous Phase. Synth. Commun. 2008, 38, 2826–2837. [Google Scholar] [CrossRef]
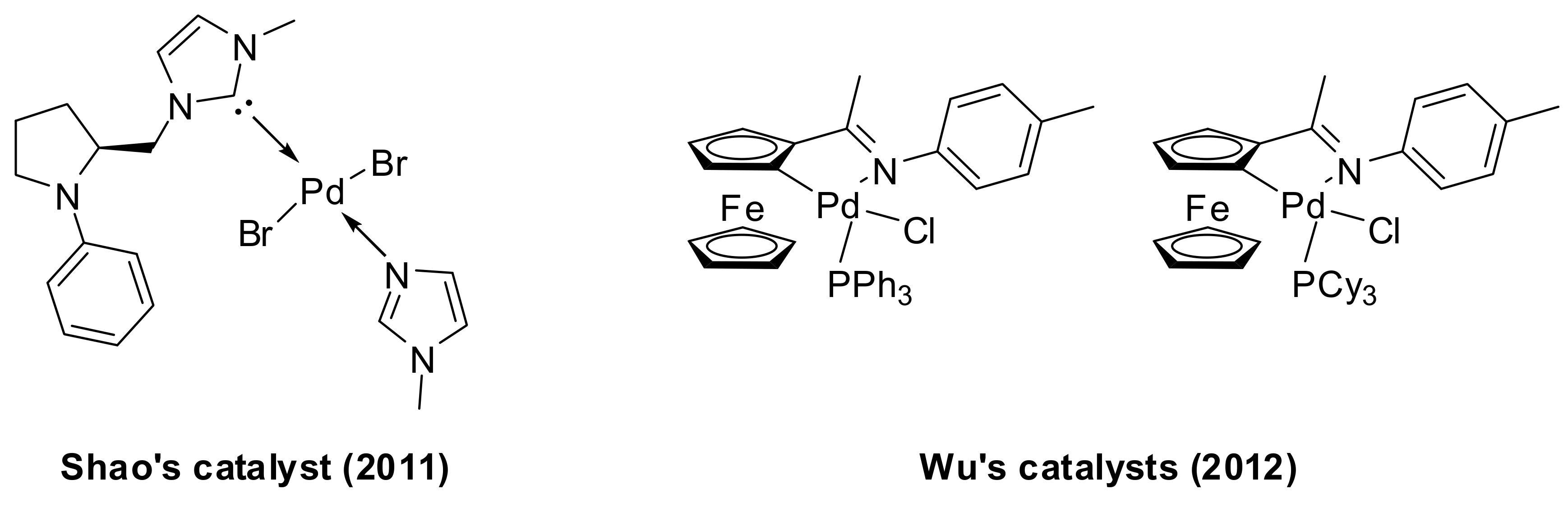
- Shen, X.-B.; Gao, T.-T.; Lu, J.-M.; Shao, L.-X. Imidazole-coordinated monodentate NHC–Pd(II) complex derived from proline and its application to the coupling reaction of arylboronic acids with carboxylic acid anhydrides in water at room temperature. Appl. Organomet. Chem. 2011, 25, 497–501. [Google Scholar] [CrossRef]
- Yu, A.; Shen, L.; Cui, X.; Peng, D.; Wu, Y. Palladacycle-catalyzed cross-coupling reactions of arylboronic acids with carboxylic anhydrides or acyl chlorides. Tetrahedron 2012, 68, 2283–2288. [Google Scholar] [CrossRef]
- Thompson, D.J. Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991; Volume 4, pp. 1028–1035. [Google Scholar]
- Schoenberg, A.; Heck, R.F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J. Org. Chem. 1974, 39, 3327–3331. [Google Scholar] [CrossRef]
- Corey, E.J.; Hegedus, L.S. Base-catalyzed carboxylation of organic halides by nickel carbonyl in protic media. J. Am. Chem. Soc. 1969, 91, 1233–1234. [Google Scholar] [CrossRef]
- Kaiser, N.-F.K.; Hallberg, A.; Larhed, M. In Situ Generation of Carbon Monoxide from Solid Molybdenum Hexacarbonyl. A Convenient and Fast Route to Palladium-Catalyzed Carbonylation Reactions. J. Comb. Chem. 2002, 4, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Collman, J.P.; Winter, S.R.; Komoto, R.G. Selective syntheses of aliphatic carboxylic acids, esters, and amides using sodium tetracarbonylferrate(-II). J. Am. Chem. Soc. 1973, 95, 249–250. [Google Scholar] [CrossRef]
- Blicke, F.F.; Zinnes, H. The Reaction of the Chloromagnesium Derivative of Chloromagnesium Phenylacetate with Isocyanates, Carbamyl Chlorides and Isothiocyanates. J. Am. Chem. Soc. 1955, 77, 4849–4851. [Google Scholar] [CrossRef]
- Mills, R.J.; Taylor, N.J.; Snieckus, V. Directed ortho metalation of N,N-diethylbenzamides. Silicon protection of ortho sites and the o-methyl group. J. Org. Chem. 1989, 54, 4372–4385. [Google Scholar] [CrossRef]
- Nagao, Y.; Miyamoto, S.; Miyamoto, M.; Takeshige, H.; Hayashi, K.; Sano, S.; Shiro, M.; Yamaguchi, K.; Sei, Y. Highly Stereoselective Asymmetric Pummerer Reactions That Incorporate Intermolecular and Intramolecular Nonbonded S···O Interactions. J. Am. Chem. Soc. 2006, 128, 9722–9729. [Google Scholar] [CrossRef] [PubMed]
- Lemoucheux, L.; Seitz, T.; Rouden, J.; Lasne, M.-C. Preparation of Tertiary Amides from Carbamoyl Chlorides and Organocuprates. Org. Lett. 2004, 6, 3703–3706. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-Z.; Deng, M.-Z. Palladium-Catalyzed Cross-Coupling Reaction of Arylboronic Acids with Chloroformate or Carbamoyl Chloride. Synlett 2005, 355–357. [Google Scholar] [CrossRef]
- Yasui, Y.; Tsuchida, S.; Miyabe, H.; Takemoto, Y. One-Pot Amidation of Olefins through Pd-Catalyzed Coupling of Alkylboranes and Carbamoyl Chlorides. J. Org. Chem. 2007, 72, 5898–5900. [Google Scholar] [CrossRef] [PubMed]
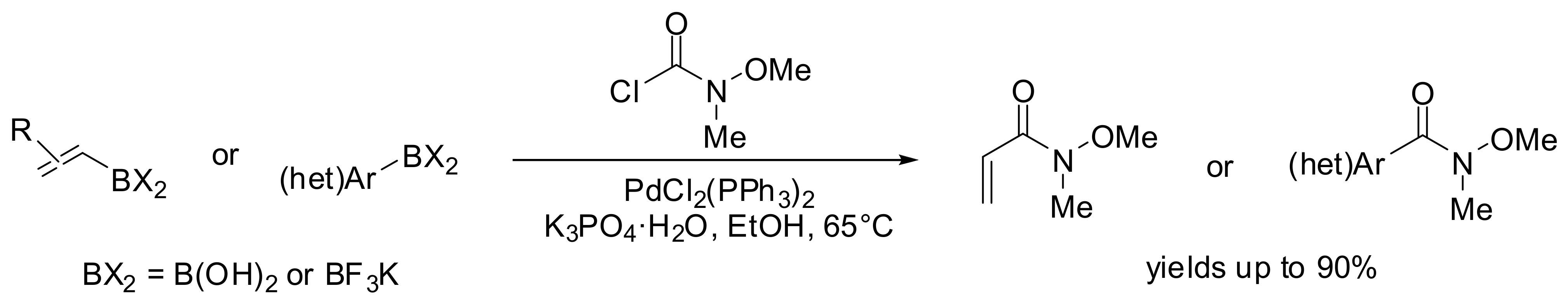
- Krishnamoorthy, R.; Lam, S.Q.; Manley, C.M.; Herr, R.J. Palladium-Catalyzed Preparation of Weinreb Amides from Boronic Acids and N-Methyl-N-methoxycarbamoyl Chloride. J. Org. Chem. 2010, 75, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
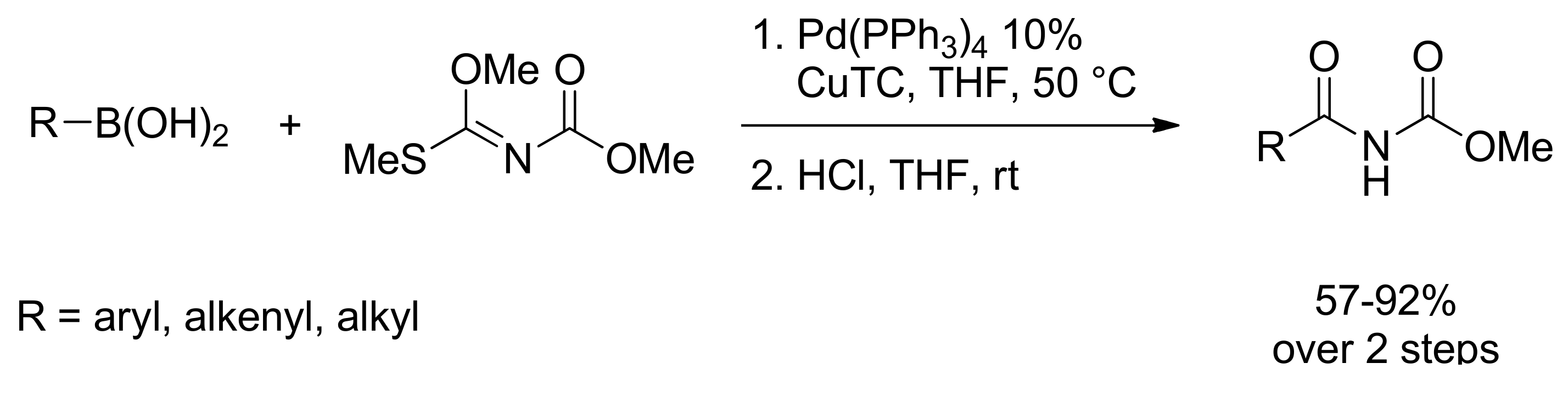
- Tomizawa, T.; Orimoto, K.; Niwa, T.; Nakada, M. Preparation of Imides via the Palladium-Catalyzed Coupling Reaction of Organoborons with Methyl N-[Methoxy(methylthio)methylene]carbamate as a One-Carbon Elongation Reaction. Org. Lett. 2012. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.J.; Chauvin, R. Synthesis of Diarylketones Through Carbonylative Coupling. Chem. Soc. Rev. 1995, 24, 89–95. [Google Scholar] [CrossRef]
- Echavarren, A.M.; Stille, J.K. Palladium-catalyzed carbonylative coupling of aryl triflates with organostannanes. J. Am. Chem. Soc. 1988, 110, 1557–1565. [Google Scholar] [CrossRef]
- Kang, S.-K.; Yamaguchi, T.; Kim, T.-H.; Ho, P.-S. Copper-Catalyzed Cross-Coupling and Carbonylative Cross-Coupling of Organostannanes and Organoboranes with Hypervalent Iodine Compounds. J. Org. Chem. 1996, 61, 9082–9083. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Piarulli, U.; Gennari, C. Effect of Ligands and Additives on the Palladium-Promoted Carbonylative Coupling of Vinyl Stannanes and Electron-Poor Enol Triflates. J. Org. Chem. 2000, 65, 6254–6256. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kohara, T.; Yamamoto, A. Selective Formation of Ketone, Diketone and Aldehyde by the CO Insertion into Nickel-Alkyl Bond of Dialkylnickel Complexes. A Novel Nickel-Catalyzed Syntheses of Ketones and Tertiary Alcohols from Grignard Reagents, Aryl Halides, and Carbon Monoxide. Chem. Lett. 1976, 5, 1217–1220. [Google Scholar] [CrossRef]
- Bumagin, N.A.; Ponomaryov, A.B.; Beletskaya, I.P. Ketone synthesis via palladium-catalyzed carbonylation of organoaluminium compounds. Tetrahedron Lett. 1985, 26, 4819–4822. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Fukushima, S.; Hiyama, T. Carbonylative coupling reaction of organofluorosilanes with organic halides promoted by fluoride ion and palldium catalyst. Tetrahedron 1992, 48, 2113–2126. [Google Scholar] [CrossRef]
- Ishiyama, T.; Kizaki, H.; Miyaura, N.; Suzuki, A. Synthesis of unsymmetrical biaryl ketones via palladium-catalyzed carbonylative cross-coupling reaction of arylboronic acids with iodoarenes. Tetrahedron Lett. 1993, 34, 7595–7598. [Google Scholar]
- Skoda-Foldes, R.; Székvolgi, Z.; Kollàr, L.; Berente, Z.; Horvàth, J.; Tuba, Z. Facile Synthesis of Steroidal Phenyl Ketones via Homogeneous Catalytic Carbonylation. Tetrahedron 2000, 56, 3415–3418. [Google Scholar] [CrossRef]
- Knopff, O.; Alexakis, A. Tandem Asymmetric Conjugate Additional Silylation of Enantiomerically Enriched Zinc Enolates. Synthetic Importance and Mechanistic Implications. Org. Lett. 2002, 4, 3835–3837. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, S.R.; López, F.; Browne, W.R.; Correa, A.; Peña, D.; Badorrey, R.; Meetsma, A.; Minnaard, A.J.; Feringa, B.L. On the Mechanism of the Copper-Catalyzed Enantioselective 1,4-Addition of Grignard Reagents to alfa-beta-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2006, 128, 9103–9118. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Kehrli, S.; d’Augustin, M.; Clavier, H.; Mauduit, M.; Alexakis, A. Copper-Catalyzed Asymmetric Conjugate Addition of Grignard Reagents to Trisubstituted Enones. Construction of All-Carbon Quaternary Chiral Centers. J. Am. Chem. Soc. 2006, 128, 8416–8417. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cavarischia, C.; Guiso, M. The Heck Coupling Reaction Using Aryl Vinyl Ketones: Synthesis of Flavonoids. Eur. J. Org. Chem. 2004, 2894–2898. [Google Scholar] [CrossRef]
- Hinch, M.; Jacques, O.; Drago, C.; Caggiano, L.; Jackson, R.F.W.; Dexter, C.; Anson, M.S.; Macdonald, S.J.F. Effective asymmetric oxidation of enones and alkyl aryl sulfides. J. Mol. Catal. A: Chem. 2006, 251, 123–128. [Google Scholar] [CrossRef]
- Shibuya, M.; Ito, S.; Takahashi, M.; Iwabuchi, Y. Oxidative Rearrangement of Cyclic Tertiary Allylic Alcohols with IBX in DMSO. Org. Lett. 2004, 6, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Ma, Y.; Xu, J.; Zhao, Y. Microwave-Assisted One-Pot Synthesis of 1-Indanones from Arenes and α,β-Unsaturated Acyl Chlorides. J. Org. Chem. 2006, 71, 4312–4315. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S.; Zalatan, D.N.; Lerchner, A.M.; Jacobsen, E.N. Highly Enantioselective Conjugate Additions to alfa,beta-Unsaturated Ketones Catalyzed by a (Salen)Al Complex. J. Am. Chem. Soc. 2005, 127, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
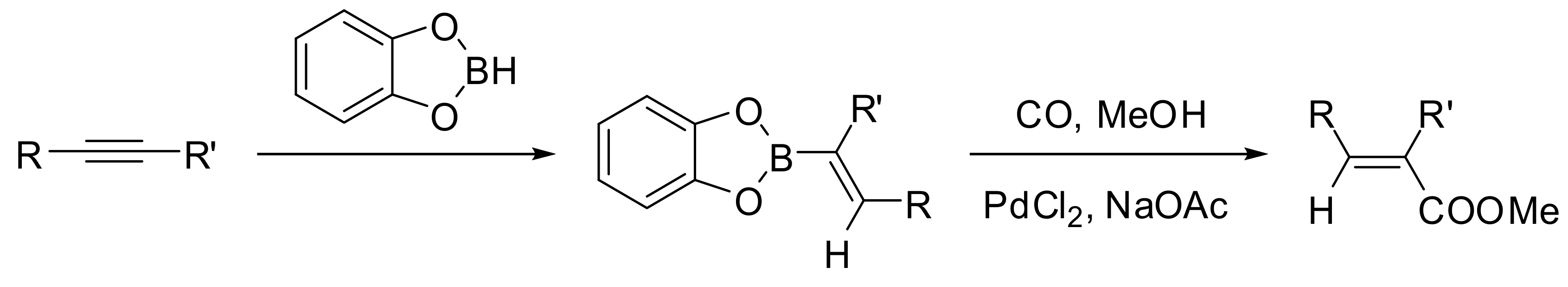
- Miyaura, N.; Suzuki, A. A Convenient Stereospecific Synthesis of Alpha,Beta-Unsaturated Carboxylic Esters via the Palladium-Catalyzed Carbonylation of 1-Alkenylboranes. Chem. Lett. 1981, 7, 879–882. [Google Scholar] [CrossRef]
- Hashem, K.E.; Woell, J.B.; Alper, H. Palladium(0) and rhodium(I) catalysis of the carbonylation of unactivated bromides. Tetrahedron Lett. 1984, 25, 4879–4880. [Google Scholar] [CrossRef]
- Kang, S.K.; Lim, K.H.; Ho, P.S.; Yoon, S.K.; Son, H.J. Palladium-catalyzed carbonylative cross-coupling of organoboranes with hypervalent iodonium salts: Synthesis of aromatic ketones. Synth. Commun. 1998, 28, 1481–1489. [Google Scholar] [CrossRef]
- Andrus, M.B.; Ma, Y.D.; Zang, Y.F.; Song, C. Palladium-imidazolium-catalyzed carbonylative coupling of aryl diazonium ions and aryl boronic acids. Tetrahedron Lett. 2002, 43, 9137–9140. [Google Scholar] [CrossRef]
- Larini, P.; Guarna, A.; Occhiato, E.G. The Lewis acid-catalyzed Nazarov reaction of 2-(N-methoxycarbonylamino)-1,4-pentadien-3-ones. Org. Lett. 2006, 8, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Bartali, L.; Larini, P.; Guarna, A.; Occhiato, E.G. Preparation of cyclopenta-fused N-, O-, and S-heterocycles by Lewis acid catalyzed Nazarov reaction. Synthesis 2007, 1733–1737. [Google Scholar] [CrossRef]
- Bartali, L.; Guarna, A.; Larini, P.; Occhiato, E.G. Carbonylative Suzuki-Miyaura coupling reaction of lactam-, lactone-, and thiolactone-derived enol triflates for the synthesis of unsymmetrical dienones. Eur. J. Org. Chem. 2007, 2152–2163. [Google Scholar] [CrossRef]
- Bartali, L.; Scarpi, D.; Guarna, A.; Prandi, C.; Occhiato, E.G. Chemistry of Lactam-Derived Vinyl Phosphates: Stereoselective Synthesis of (+)-Fagomine. Synlett 2009, 2009, 913–916. [Google Scholar]
- Deagostino, A.; Prandi, C.; Venturello, P. alpha,beta-unsaturated acetals in synthesis. Curr. Org. Chem. 2003, 7, 821–839. [Google Scholar] [CrossRef]
- Occhiato, E.G.; Prandi, C.; Ferrali, A.; Guarna, A.; Deagostino, A.; Venturello, P. Synthesis of alpha-acyl-functionalized azacycles by Pd-catalyzed cross-coupling reactions of alpha-alkoxyboronates with lactam-derived vinyl triflates. J. Org. Chem. 2002, 67, 7144–7146. [Google Scholar] [CrossRef] [PubMed]
- Occhiato, E.G.; Prandi, C.; Ferrali, A.; Guarna, A.; Venturello, P. New synthetic approach to cyclopenta-fused heterocycles based upon a mild Nazarov reaction. J. Org. Chem. 2003, 68, 9728–9741. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Masetti, M.; Recanatini, M.; Prandi, C.; Guarna, A.; Occhiato, E.G. Density functional studies on the Nazarov reaction involving cyclic systems. Chem. Eur. J. 2006, 12, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Pacetti, A.; Recanatini, M.; Prandi, C.; Scarpi, D.; Occhiato, E.G. Predicting Reactivity and Stereoselectivity in the Nazarov Reaction: A Combined Computational and Experimental Study. Chem. Eur. J. 2008, 14, 9292–9304. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Deagostino, A.; Venturello, P.; Occhiato, E.G. Stereoselective synthesis of spirocyclic ketones by Nazarov reaction. Org. Lett. 2005, 7, 4345–4348. [Google Scholar] [CrossRef] [PubMed]
- Deagostino, A.; Prandi, C.; Zavattaro, C.; Venturello, P. N-Functionalization of azoles through coupling reactions with alkoxydienyl and alkoxystyryl boronic esters. Eur. J. Org. Chem. 2007, 1318–1323. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Choi, C.H.; Yang, S. Conjugated Polymers and Aromaticity. Chem. Rev. 2005, 105, 3448–3481. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Fox, J.M.; Hicks, F.A.; Buchwald, S.L. The Use of Catalytic Amounts of CuCl and Other Improvements in the Benzyne Route to Biphenyl-Based Phosphine Ligands. Adv. Synth. Catal. 2001, 343, 789–794. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Bonfante, P.; Deagostino, A.; Kapulnik, Y.; Larini, P.; Occhiato, E.G.; Prandi, C.; Venturello, P. A new class of conjugated strigolactone analogues with fluorescent properties: Synthesis and biological activity. Org. Biomol. Chem. 2009, 7, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Occhiato, E.G.; Tabasso, S.; Bonfante, P.; Novero, M.; Scarpi, D.; Bova, M.E.; Miletto, I. New Potent Fluorescent Analogues of Strigolactones: Synthesis and Biological Activity in Parasitic Weed Germination and Fungal Branching. Eur. J. Org. Chem. 2011, 3781–3793. [Google Scholar] [CrossRef]
- Cohen, M.; Prandi, C.; Occhiato, E.G.; Tabasso, S.; Wininger, S.; Resnick, N.; Steineberger, Y.; Koltai, H.; Kapulnik, Y. Structure-Function Relations of Strigolactone Analogues: Activity as Plant Hormones and Plant Interactions. Mol. Plant 2012. [Google Scholar] [CrossRef]
- Prandi, C.; Rosso, H.N.; Lace, B.; Occhiato, E.G.; Oppedisano, A.; Tabasso, S.; Alberto, G.; Blangetti, M. Strigolactone analogues as molecular probes in chasing the (SLs) receptor/s: Design and synthesis of labeled molecules. Mol. Plant 2012. [Google Scholar] [CrossRef]
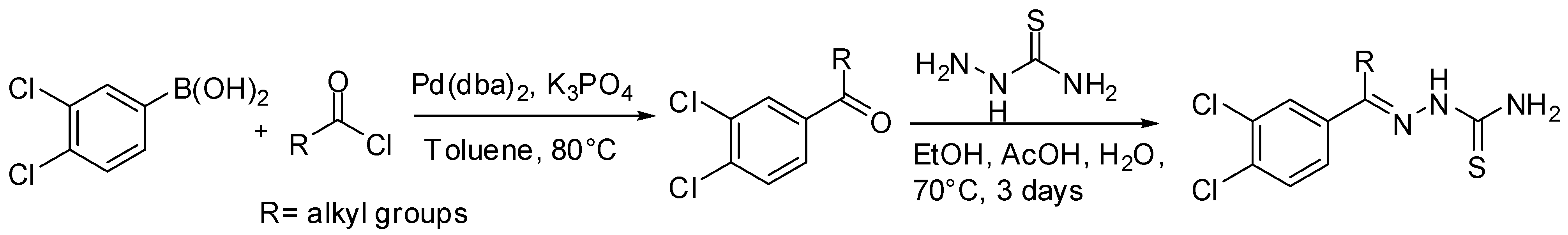
- Fujii, N.; Mallari, J.P.; Hansell, E.J.; Mackey, Z.; Doyle, P.; Zhou, Y.M.; Gut, J.; Rosenthal, P.J.; McKerrow, J.H.; Guy, R.K. Discovery of potent thiosemicarbazone inhibitors of rhodesain and cruzain. Bioorg. Med. Chem. Lett. 2005, 15, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Mallari, J.P.; Shelat, A.; Kosinski, A.; Caffrey, C.R.; Connelly, M.; Zhu, F.; McKerrow, J.H.; Guy, R.K. Discovery of trypanocidal thiosemicarbazone inhibitors of rhodesain and TbcatB. Bioorg. Med. Chem. Lett. 2008, 18, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Kerr, I.D.; Wu, P.; Marion-Tsukamaki, R.; Mackey, Z.B.; Brinen, L.S. Crystal Structures of TbCatB and Rhodesain, Potential Chemotherapeutic Targets and Major Cysteine Proteases of Trypanosoma brucei. PLoS Negl. Trop. Dis. 2010, 4, e701. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.E.; Eakin, A.E.; Engel, J.C.; McKerrow, J.H.; Craik, C.S.; Fletterick, R.J. The Crystal Structure of Cruzain: A Therapeutic Target for Chagas’ Disease. J. Mol. Biol. 1995, 247, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.C.; Mackey, Z.; Hansell, E.; Doyle, P.; Gut, J.; Caffrey, C.R.; Lehrman, J.; Rosenthal, P.J.; McKerrow, J.H.; Chibale, K. Synthesis and Structure-Activity Relationships of Parasiticidal Thiosemicarbazone Cysteine Protease Inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004, 47, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
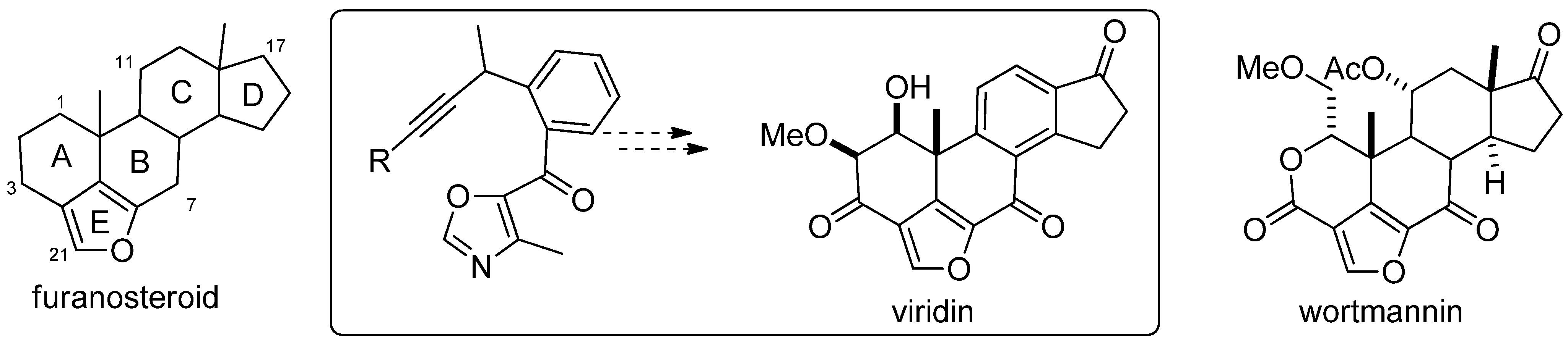
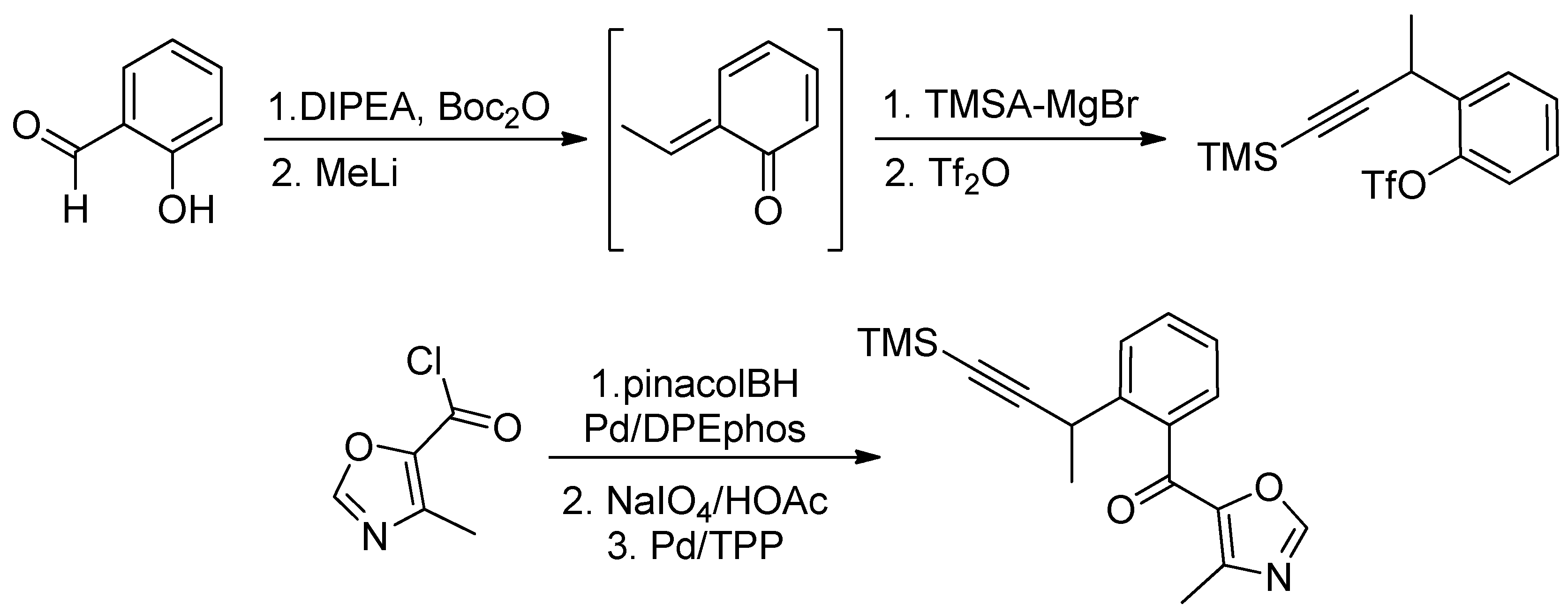
- Sessions, E.H.; Jacobi, P.A. Studies on the Synthesis of Furanosteroids. I. Viridin Models. Org. Lett. 2006, 8, 4125–4128. [Google Scholar] [CrossRef] [PubMed]
- Brian, P.W.; MCGOWAN, J.G. Viridin: A highly fungistatic substance produced by Trichoderma viride. Nature 1945, 156, 144–145. [Google Scholar] [CrossRef]
- Powis, G.; Bonjouklian, R.; Berggren, M.M.; Gallegos, A.; Abraham, R.; Ashendel, C.; Zalkow, L.; Matter, W.F.; Dodge, J.; Grindey, G.; et al. Wortmannin, a Potent and Selective Inhibitor of Phosphatidylinositol-3-kinase. Cancer Res. 1994, 54, 2419–2423. [Google Scholar] [PubMed]
- Ward, S.; Sotsios, Y.; Dowden, J.; Bruce, I.; Finan, P. Therapeutic Potential of Phosphoinositide 3-Kinase Inhibitors. Chem. Biol. 2003, 10, 207–213. [Google Scholar] [CrossRef]
- Neumann, H.; Brennführer, A.; Beller, M. A General Synthesis of Diarylketones by Means of a Three-Component Cross-Coupling of Aryl and Heteroaryl Bromides, Carbon Monoxide, and Boronic acids. Chem. Eur. J. 2008, 14, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, I.; Ruiz, N.; Claver, C.; van der Veen, L.A.; van Leeuwen, P. Hydroxycarbonylation of styrene with palladium catalysts The influence of the mono- and bidentate phosphorus ligand. J. Mol. Catal. A.-Chem. 2000, 161, 39–48. [Google Scholar] [CrossRef]
- Arthuis, M.; Pontikis, R.; Chabot, G.G.; Quentin, L.; Scherman, D.; Florent, J.-C. Domino approach to 2-aroyltrimethoxyindoles as novel heterocyclic combretastatin A4 analogues. Eur. J. Med. Chem. 2011, 46, 95–100. [Google Scholar] [CrossRef] [PubMed]
- West, C.M.L.; Price, P. Combretastatin A4 phosphate. Anti-Cancer Drugs 2004, 15, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin. Investig. Drugs 2009, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Fuchs, P.L. A synthetic method for formyl→ethynyl conversion (RCHO→’RC≡CH or RC≡CR'). Tetrahedron Lett. 1972, 13, 3769–3772. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Lautens, M. A Highly Selective Tandem Cross-Coupling of gem-Dihaloolefins for a Modular, Efficient Synthesis of Highly Functionalized Indoles. J. Org. Chem. 2007, 73, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, M.; Qi, Y.; Xue, J. Ligand-Free Pd-Catalyzed Carbonylative Cross-Coupling Reactions under Atmospheric Pressure of Carbon Monoxide: Synthesis of Aryl Ketones and Heteroaromatic Ketones. Eur. J. Org. Chem. 2011, 2662–2667. [Google Scholar] [CrossRef]
- Qureshi, Z.S.; Deshmukh, K.M.; Tambade, P.J.; Bhanage, B.M. A Simple, Efficient, and Recyclable Phosphine-Free Catalytic System for Carbonylative Suzuki Coupling Reaction of Aryl and Heteroaryl Iodides. Synthesis 2011, 243–250. [Google Scholar] [CrossRef]
- Pickup, P.G. Conjugated metallopolymers. Redox polymers with interacting metal based redox sites. J. Mater. Chem. 1999, 9, 1641–1653. [Google Scholar] [CrossRef]
- Rice, C.R.; Worl, S.; Jeffery, J.C.; Paul, R.L.; Ward, M.D. New multidentate ligands for supramolecular coordination chemistry: Double and triple helical complexes of ligands containing pyridyl and thiazolyl donor units. J. Chem. Soc. Dalton Trans. 2001, 550–559. [Google Scholar] [CrossRef]
- Ishikura, M.; Kamada, M.; Terashima, M. An Efficient Synthesis of 3-Heteroarylpyridines via Diethyl-(3-pyridyl)-borane. Synthesis 1984, 936–938. [Google Scholar] [CrossRef]
- Thompson, W.J.; Jones, J.H.; Lyle, P.A.; Thies, J.E. An efficient synthesis of arylpyrazines and bipyridines. J. Org. Chem. 1988, 53, 2052–2055. [Google Scholar] [CrossRef]
- Mitschke, U.; Bauerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Wang, C.; Jung, G.-Y.; Hua, Y.; Pearson, C.; Bryce, M.R.; Petty, M.C.; Batsanov, A.S.; Goeta, A.E.; Howard, J.A.K. An Efficient Pyridine- and Oxadiazole-Containing Hole-Blocking Material for Organic Light-Emitting Diodes: Synthesis, Crystal Structure, and Device Performance. Chem. Mater. 2001, 13, 1167–1173. [Google Scholar] [CrossRef]
- Chapman, G.M.; Stanforth, S.P.; Tarbit, B.; Watson, M.D. Arylated pyridines: Suzuki reactions of O-substituted 2,6-dihalogenated-3-hydroxypyridines. J. Chem. Soc. Perkin Trans. 1 2002, 581–582. [Google Scholar] [CrossRef]
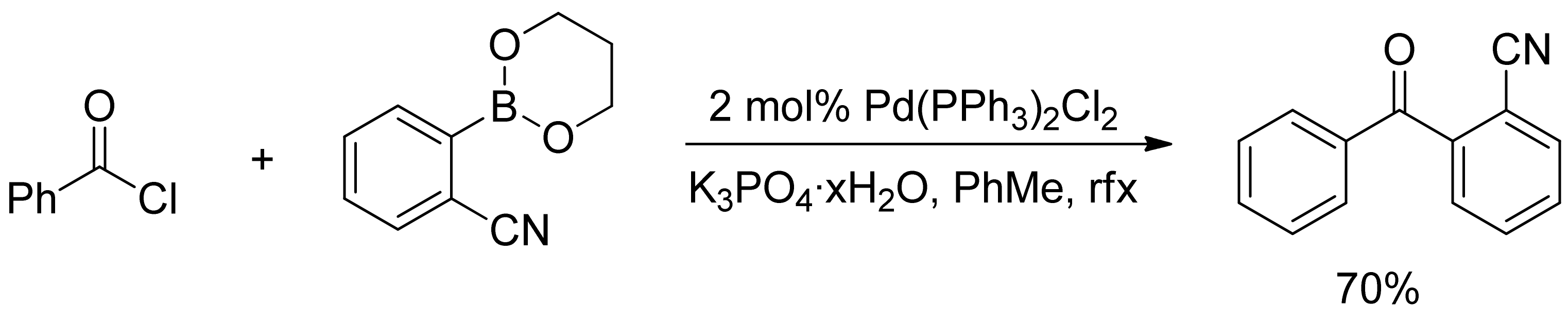
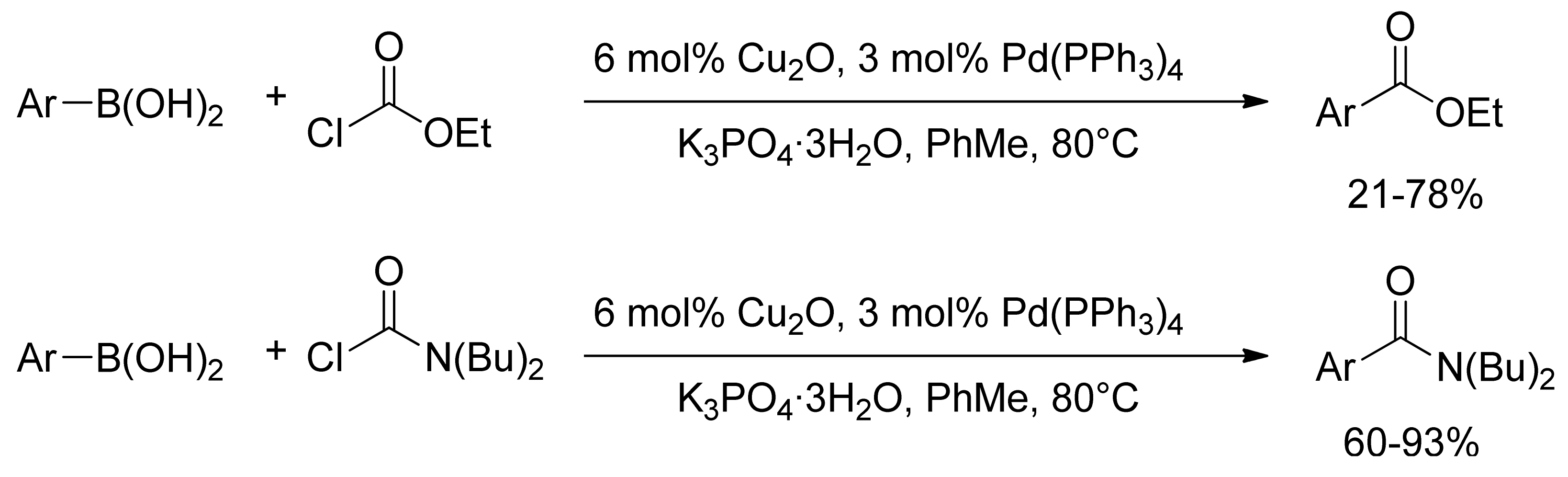
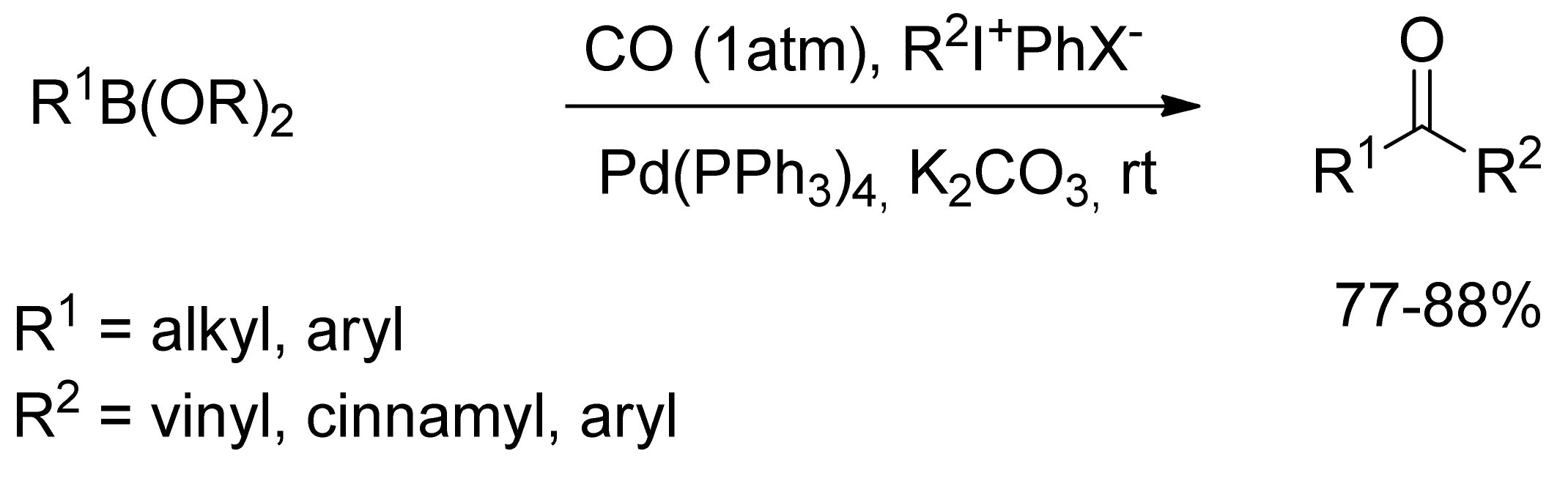
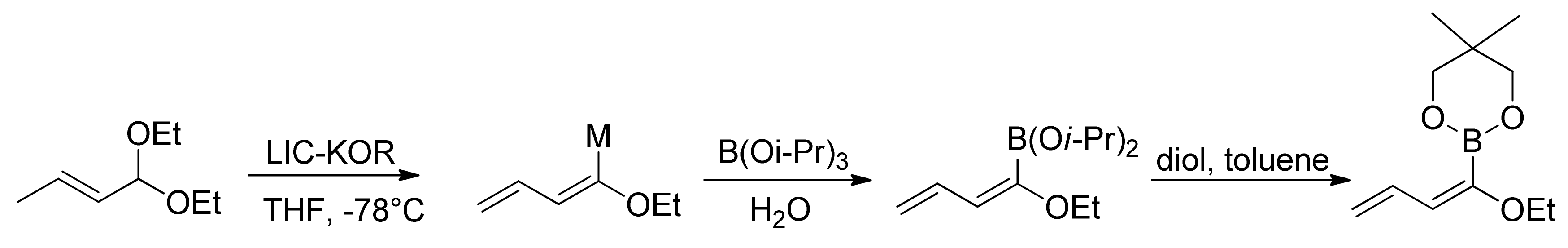
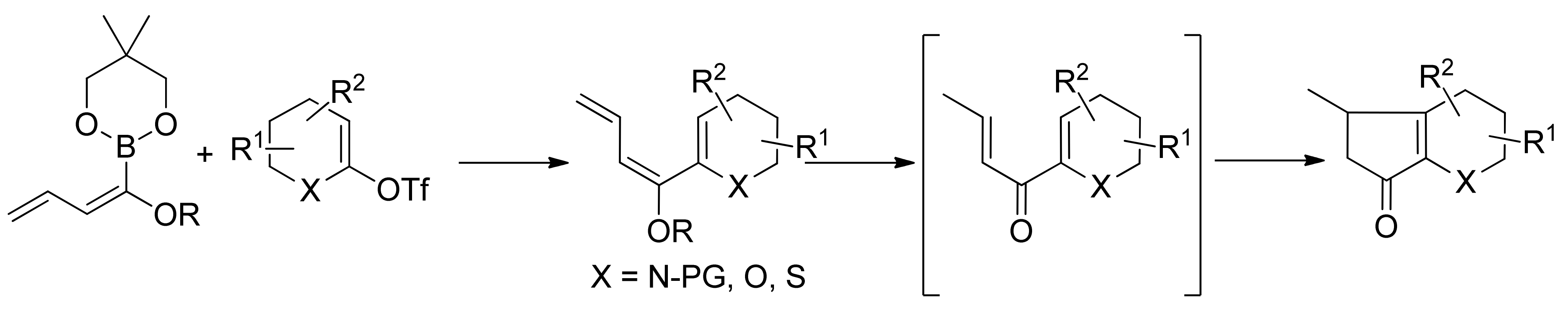
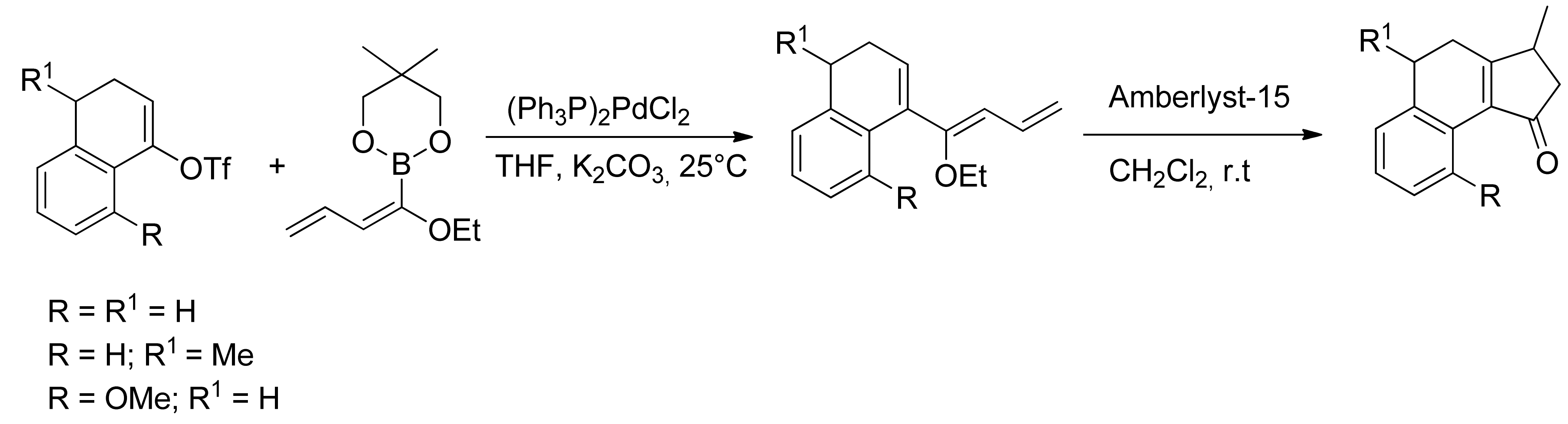
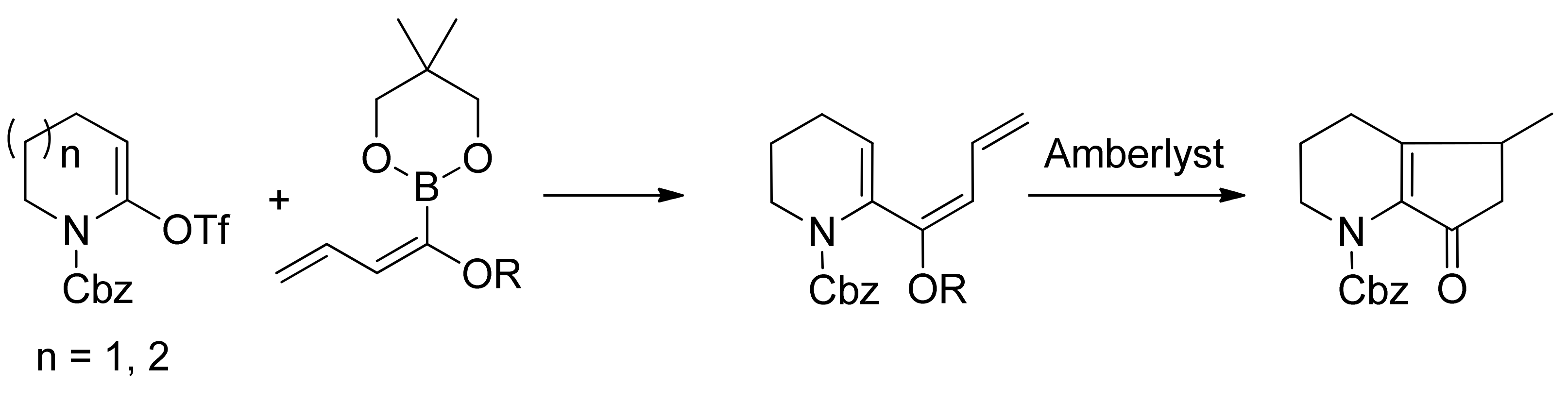


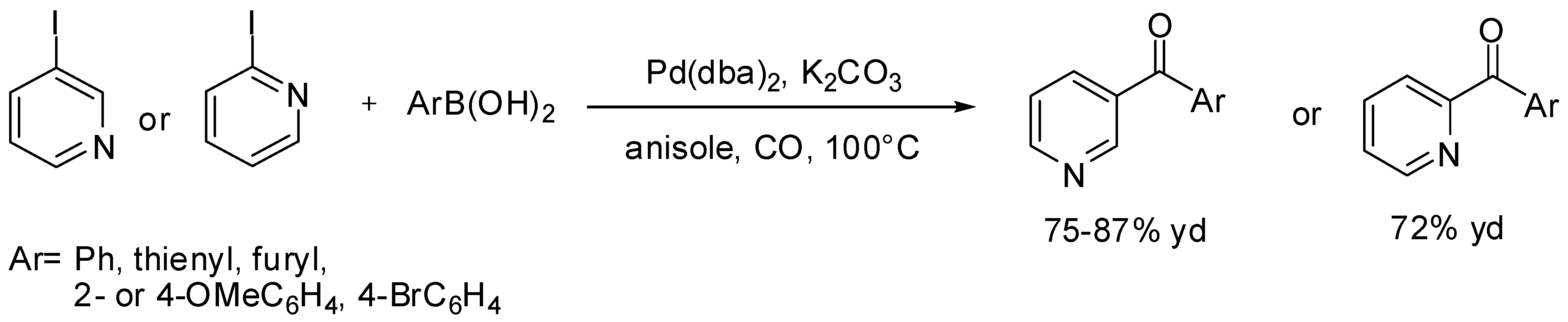
© 2013 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blangetti, M.; Rosso, H.; Prandi, C.; Deagostino, A.; Venturello, P. Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments. Molecules 2013, 18, 1188-1213. https://doi.org/10.3390/molecules18011188
Blangetti M, Rosso H, Prandi C, Deagostino A, Venturello P. Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments. Molecules. 2013; 18(1):1188-1213. https://doi.org/10.3390/molecules18011188
Chicago/Turabian StyleBlangetti, Marco, Heléna Rosso, Cristina Prandi, Annamaria Deagostino, and Paolo Venturello. 2013. "Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments" Molecules 18, no. 1: 1188-1213. https://doi.org/10.3390/molecules18011188
APA StyleBlangetti, M., Rosso, H., Prandi, C., Deagostino, A., & Venturello, P. (2013). Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments. Molecules, 18(1), 1188-1213. https://doi.org/10.3390/molecules18011188





