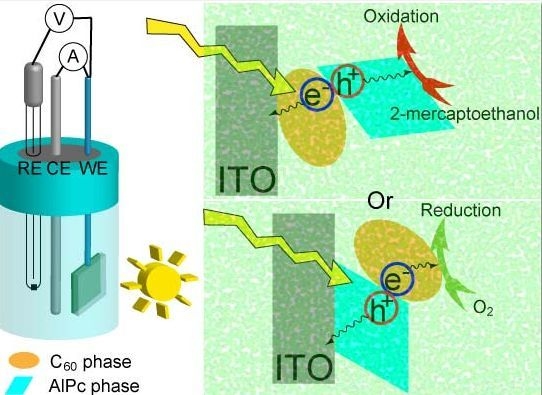
Photoelectrode Characteristics of Partially Hydrolyzed Aluminum Phthalocyanine Chloride/Fullerene C60 Composite Nanoparticles Working in a Water Phase
Abstract
:1. Introduction
2. Results and Discussion
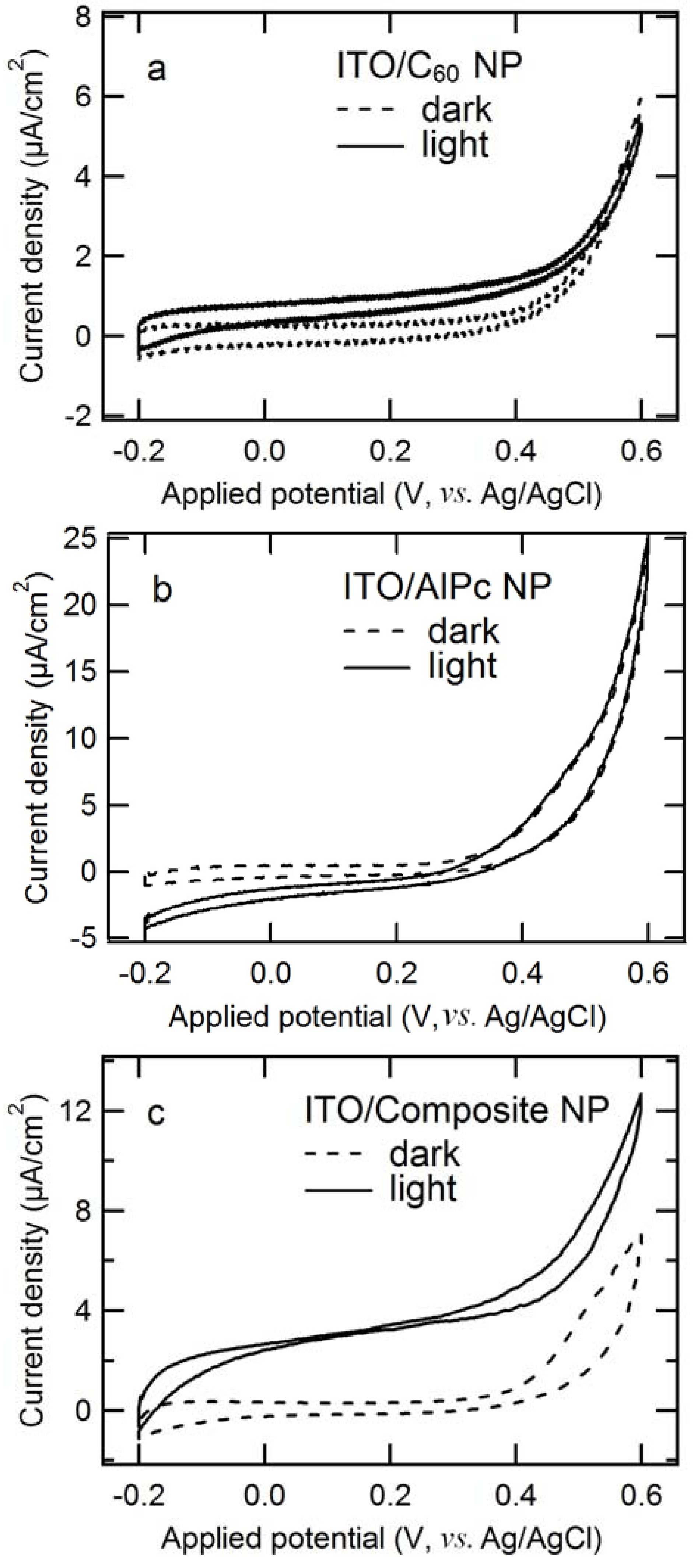
2.1. Comparison of Photoanodic Characteristics for Electrodes Composed of Different Nanoparticles
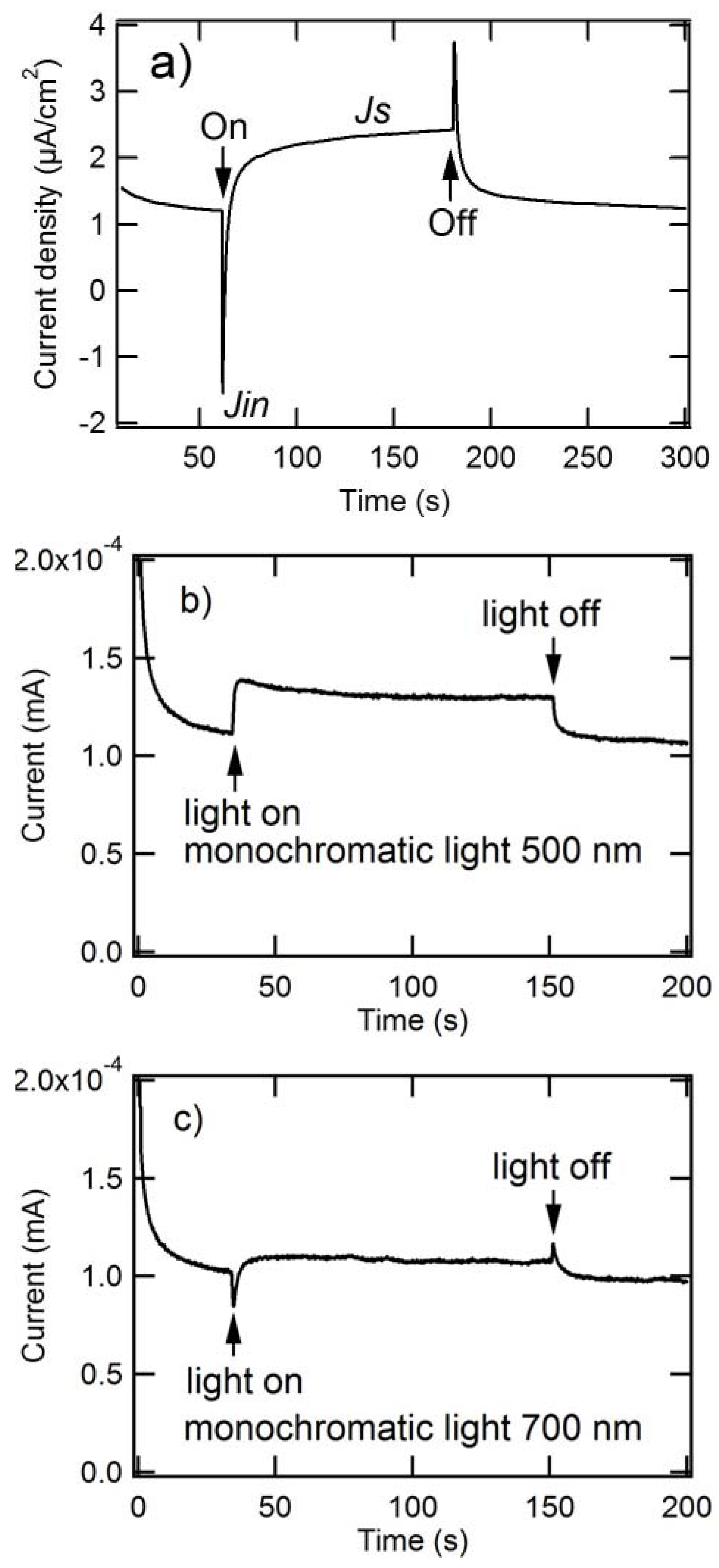
2.2. Kinetic Characteristics of Photoanode Composed of Composite Nanoparticles
2.2.1. Transient Photoanodic Current Generated at ITO/Composite Nanaoparticles
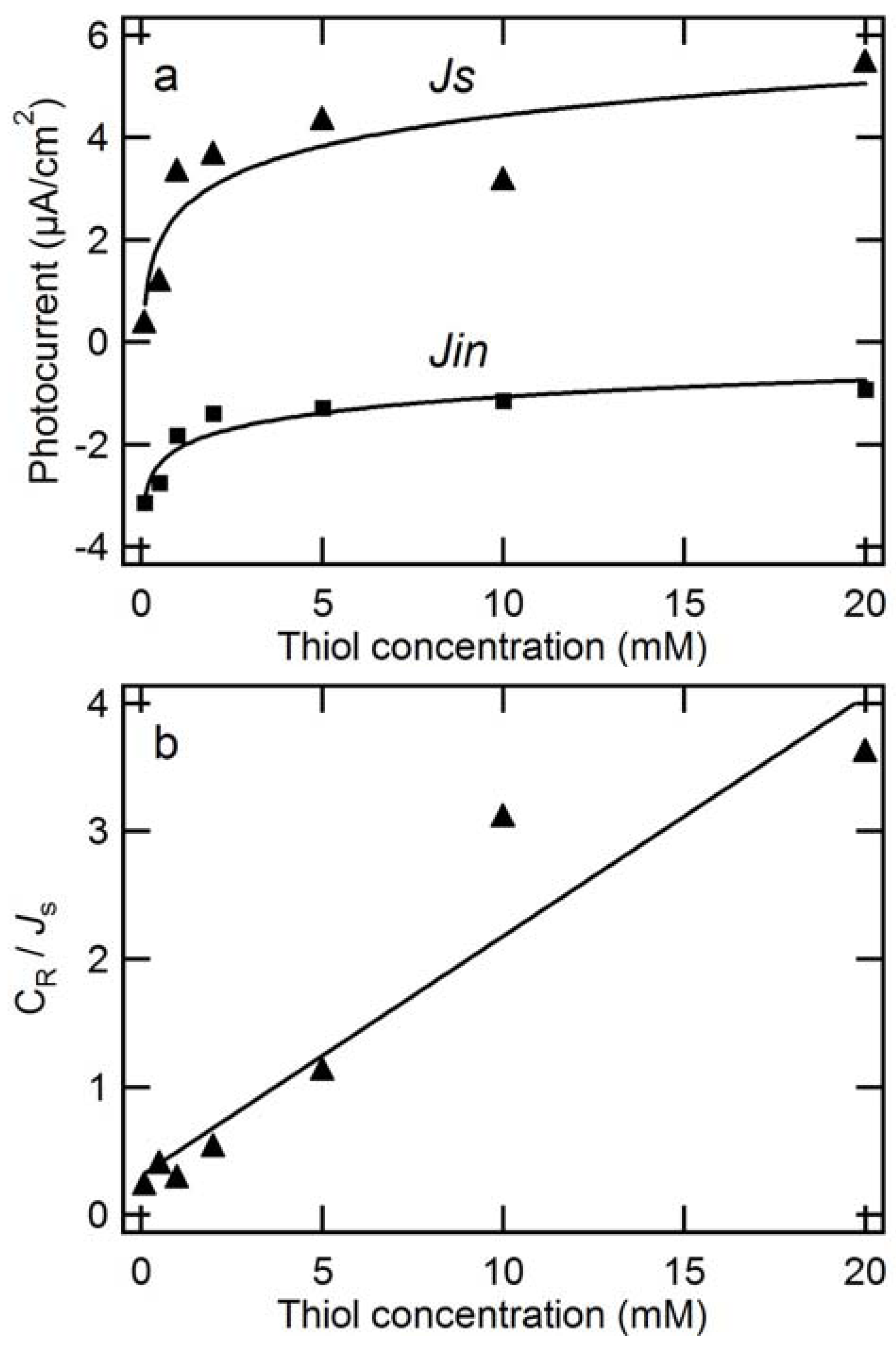
2.2.2. Dependence of Photocurrent on ME Concentration
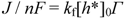

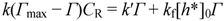
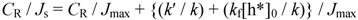
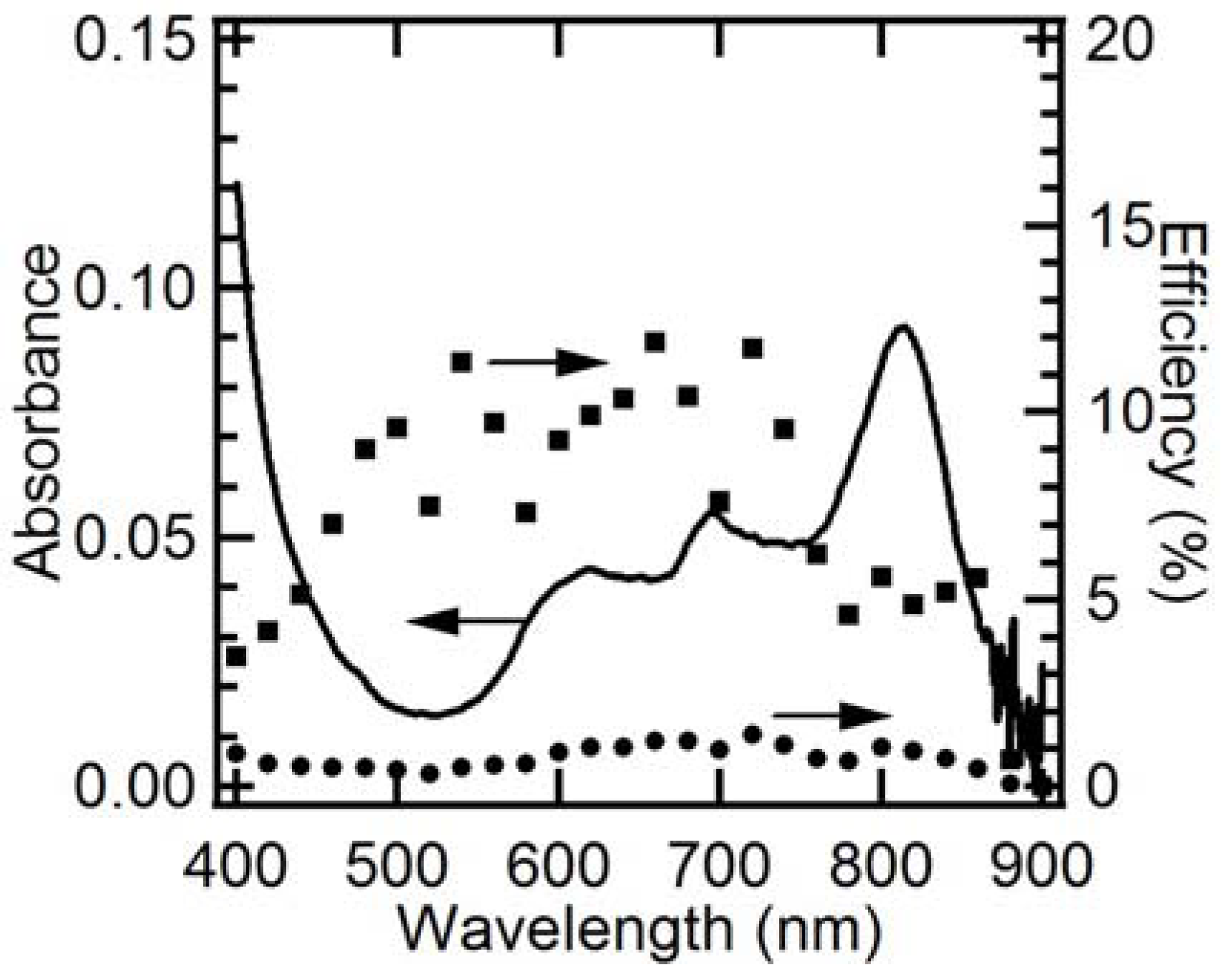
2.3. EQE and IQE Spectra for Steady-State Photoanodic Current
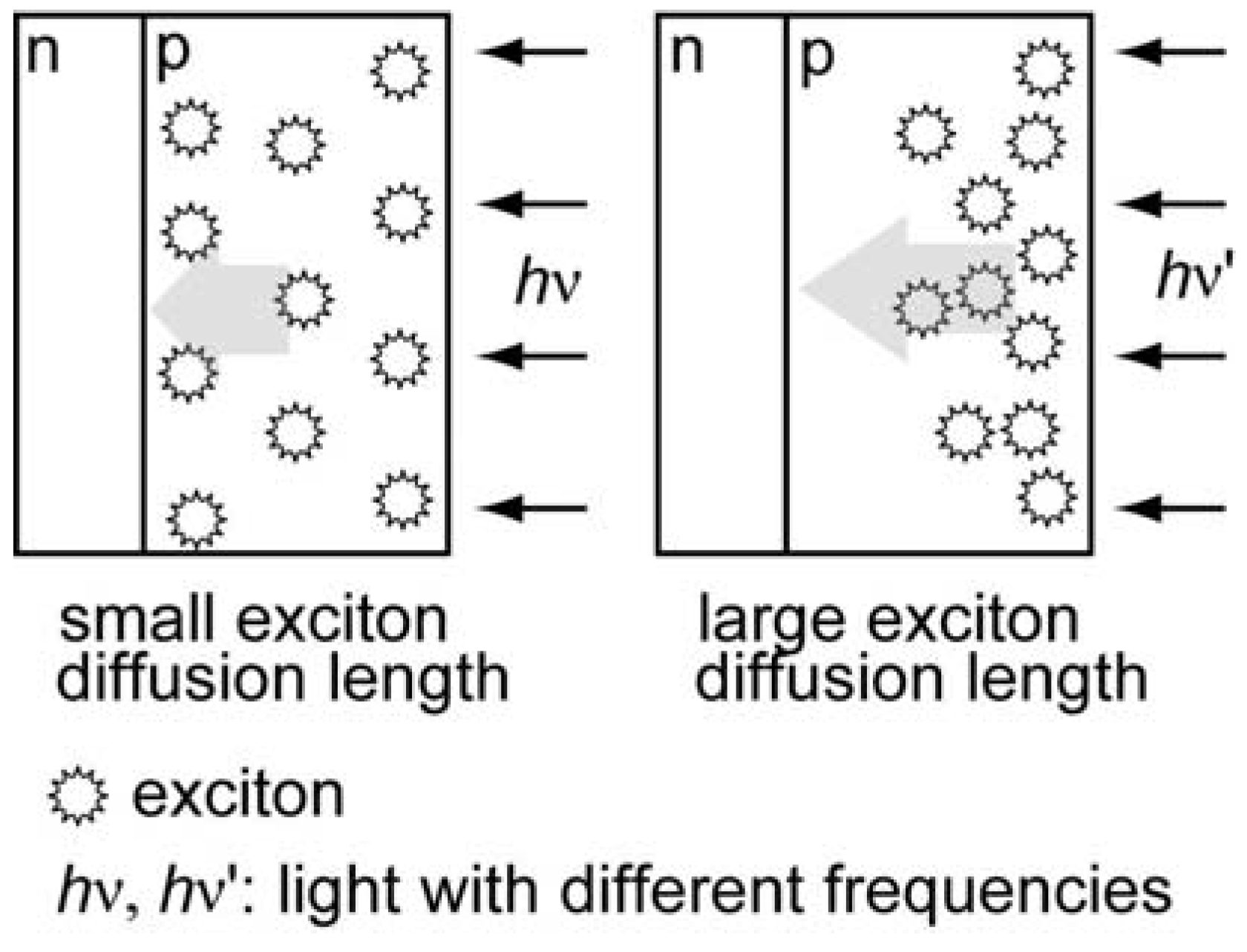
2.4. Comparison of Photocathodic Characteristics for Vapor-Deposited Electrodes
2.5. Comparison of Photocathodic Characteristics for Electrodes Composed of Different Nanoparticles
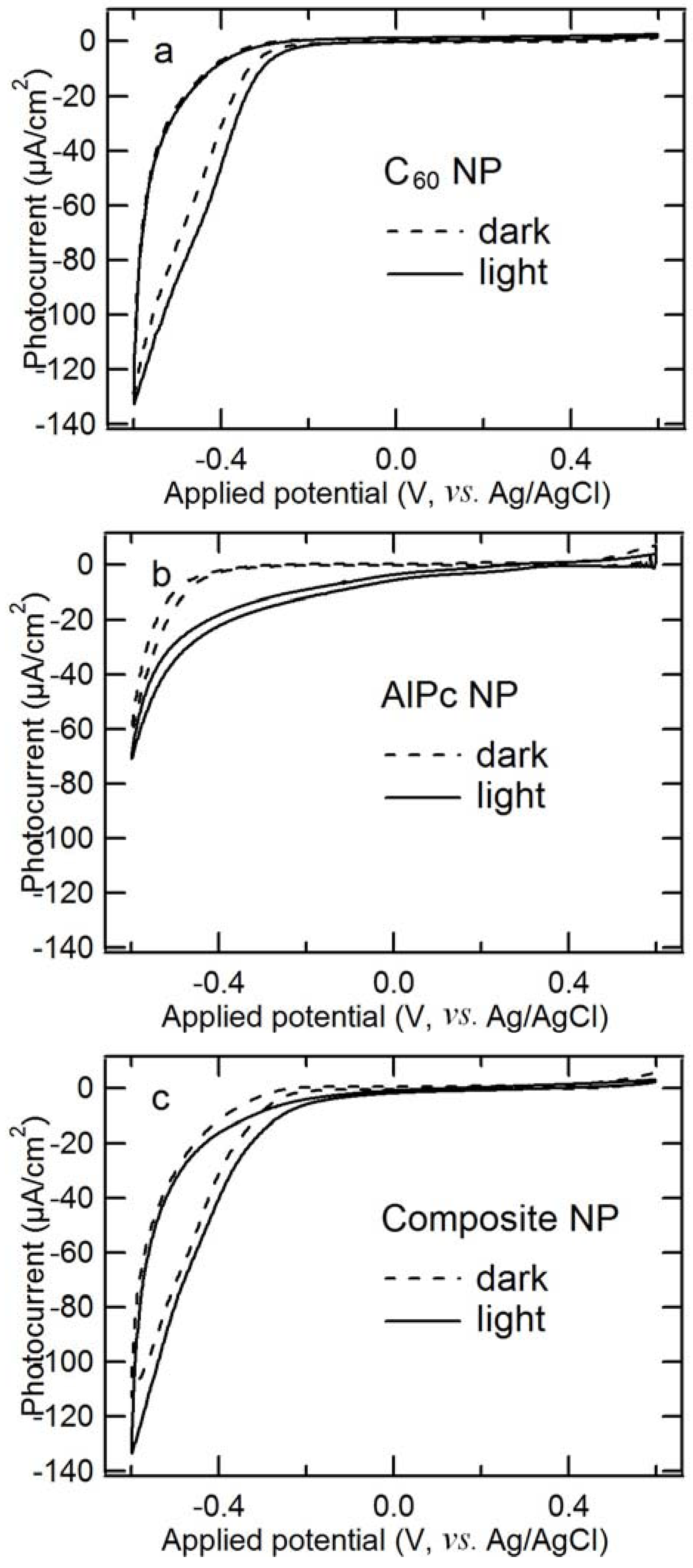
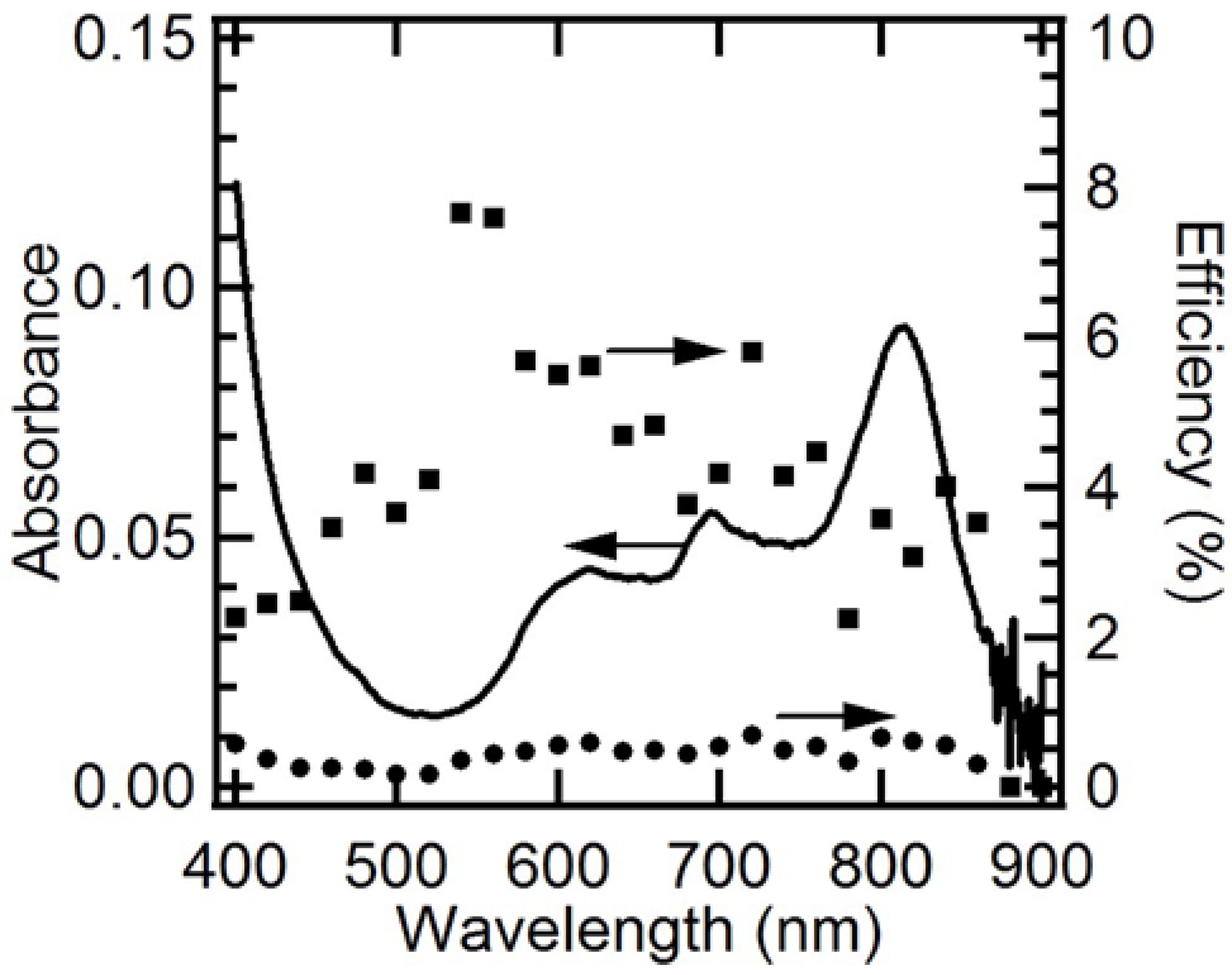
2.6. EQE and IQE Spectra for Steady-State Photocathodic Current
3. Experimental
3.1. Materials
3.2. Particle Synthesis and Electrode Preparation
3.3. Photoelectrochemical Measurements


4. Conclusions
Supplementary Materials
Acknowledgments
References
- Bottari, G.; de la Torre, G.; Guldi, D.M.; Torres, T. Covalent and noncovalent phthalocyanine-carbon nanostructure systems: Synthesis, photoinduced electron transfer, and application to molecular photovoltaics. Chem. Rev. 2010, 110, 6768–6816. [Google Scholar] [CrossRef]
- Shaabani, A.; Farhangi, E.; Rahmati, A. Aerobic oxidation of alkyl arenes and alcohols using cobalt(II) phthalocyanine as a catalyst in 1-buty-3-methyl-imidazolium bromide. Appl. Catal. A-Gen. 2008, 338, 14–19. [Google Scholar] [CrossRef]
- Kotronarou, A.; Hoffmann, M.R. Catalytic autoxidation of hydrogen sulfide in wastewater. Environ. Sci. Technol. 1991, 25, 1153–1160. [Google Scholar] [CrossRef]
- Joseph, J.K.; Jain, S.L.; Sain, B. Covalently anchored polymer immobilized Co(II) phthalocyanine as efficient catalyst for oxidation of mercaptans using molecular oxygen as oxidant. Ind. Eng. Chem. Res. 2010, 49, 6674–6677. [Google Scholar] [CrossRef]
- Kasuga, K.; Tsuboi, K.; Handa, M.; Sugimori, T.; Sogabe, K. Oxygen-oxygenation of cyclohexene catalyzed by manganese(III), iron(III) and cobalt(II) complexed of tetra-tert-butylphthalocyanine in the presence of iso-butyraldehyde. Inorg. Chem. Commun. 1999, 2, 507–509. [Google Scholar] [CrossRef]
- Parton, R.F.; Neys, P.E.; Jacobs, P.A.; Sosa, R.C.; Rouxhet, P.G. Iron-phthalocyanine immobilized on active carbon black: A selective catalyst for alkane oxidation. J. Catal. 1996, 164, 341–346. [Google Scholar]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Peumans, P.; Uchida, S.; Forrest, S.R. Efficient bulk heterojuction photovoltaic cells using small-molecular-weight organic thin films. Nature 2003, 425, 158–159. [Google Scholar]
- Hiramoto, M.; Fujiwara, H.; Yokoyama, M. Three-layered organic solar cell with a photoactive interlayer of codeposited pigments. Appl. Phys. Lett. 1991, 58, 1062–1064. [Google Scholar] [CrossRef]
- Wöhrle, D.; Kreienhoop, L.; Schnurpfeil, G.; Elbe, J.; Tennigkeit, B.; Hiller, S.; Schlettwein, D. Investigation of n/p-junction photovoltaic cells of perylenetetraacarboxylic acid diimides and phthalocyanines. J. Mater. Chem. 1995, 5, 1819–1829. [Google Scholar] [CrossRef]
- Loi, M.A.; Denk, P.; Hoppe, H.; Neugebauer, H.; Winder, C.; Meissner, D.; Brabec, C.; Sariciftci, N.S.; Gouloumis, A.; Vázquez, P.; Torres, T. Long-lived photoinduced charge separation for solar cell applications in phthalocyanine-fulleropyrrolidine dyad thin films. J. Mater. Chem. 2003, 13, 700–704. [Google Scholar] [CrossRef]
- de la Escosura, A.; Martínez-Díaz, M.V.; Torres, T.; Grubbs, R.H.; Guldi, D.M.; Neugebauer, H.; Winder, C.; Drees, M.; Sariciftci, N.S. New donor-acceptor materials based on random polynorbornenes bearing pendant phthalocyanine and fullerene units. Chem. Asian. J. 2006, 1-2, 148–154. [Google Scholar]
- Ito, O.; D’Souza, F. Recent advances in photoinduced electron transfer processes of fullerene-based molecular assemblies and nanocomposites. Molecules 2012, 17, 5816–5835. [Google Scholar] [CrossRef]
- López-Duarte, I.; Wang, M.; Humphry-Baker, R.; Ince, M.; Martínez-Díaz, M.V.; Nazzeeruddin, M.K.; Torres, T.; Grätzel, M. Molecular engineering of zinc phthalocyanines with phosphinic acid anchoring groups. Angew. Chem. Int. Ed. 2011, 50, 1–5. [Google Scholar]
- O’Regan, B.C.; López-Duarte, I.; Martínez-Díaz, M.V.; Forneli, A.; Albero, J.; Morandeira, A.; Palomares, E.; Torres, T.; Durrant, J.R. Catalysis of recombination and its limitation on open circuit voltage for dye sensitized photovoltaic cells using phthalocyanine dyes. J. Am. Chem. Soc. 2008, 130, 2906–2907. [Google Scholar]
- Imahori, H.; Umeyama, T.; Ito, S. Large π-aromatic molecules as potential sensitizers for highly efficient dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1809–1818. [Google Scholar] [CrossRef]
- He, J.; Benko, G.; Korodi, F.; Polívka, T.; Lomoth, R.; Åkermark, B.; Sun, L.; Hagfeldt, A.; Sundström, V. Modified phthalocyanines for efficient near-IR sensitization of nanostructured TiO2 electrode. J. Am. Chem. Soc. 2002, 124, 4922–4932. [Google Scholar]
- Clifford, J.N.; Palomares, E.; Nazeeruddin, M.K.; Grätzel, M.; Nelson, J.; Li, X.; Long, N.J.; Durrant, J.R. Molecular control of recombination dynamics in dye-sensitized nanocrystalline TiO2 films: Free energy vs. distance dependence. J. Am. Chem. Soc. 2004, 126, 5225–5233. [Google Scholar]
- de la Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 2000–2015. [Google Scholar]
- Morandeira, A.; López-Duarte, I.; Martínez-Díaz, M.V.; O’Regan, B.; Shuttle, C.; Haji-Zainulabidin, N.A.; Torres, T.; Palomares, E.; Durrant, J.R. Slow electron injection on Ru-phthalocyanine sensitized TiO2. J. Am. Chem. Soc. 2007, 129, 9250–9251. [Google Scholar]
- Takanabe, K.; Kamata, K.; Wang, X.; Antonietti, M.; Kubota, J.; Domen, K. Photocatalytic hydrogen evolution on dye-sensitized mesoporous carbon nitride photocatalyst with magnesium phthalocyanine. Phys. Chem. Chem. Phys. 2010, 12, 13020–13025. [Google Scholar]
- Chen, F.; Deng, Z.; Li, X.; Zhang, J.; Zhao, J. Visible light detoxification by 2,9,16,23-tetracarboxyl phthalocyanine copper modified amorphous titania. Chem. Phys. Lett. 2005, 415, 85–88. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef]
- Abe, T.; Nagai, K.; Kabutomori, S.; Kaneko, M.; Tajiri, A.; Norimatsu, T. An organic photoelectrode working in the water phase: Visible-light-induced dixoygen evolution by a perylene derivative/cobalt phthalocyanine bilayer. Angew. Chem. Int. Ed. 2006, 45, 2778–2781. [Google Scholar]
- Abe, T.; Tobinai, S.; Taira, N.; Chiba, J.; Itoh, T.; Nagai, K. Molecular hydrogen evolution by organic p/n bilayer film of phthalocyanine/fullerene in the entire visible-light energy region. J. Phys. Chem. C 2011, 115, 7701–7705. [Google Scholar]
- Nagai, K.; Abe, K.; Kaneyasu, Y.; Yasuda, Y.; Kimishima, I.; Iyoda, T.; Imaya, H. A Full-spectrum visible-light-responsive organophotocatalyst film for removal of trimethylamine. ChemSusChem 2011, 4, 727–730. [Google Scholar] [CrossRef]
- Zhang, S.; Sakai, R.; Abe, T.; Iyoda, T.; Norimatsu, T.; Nagai, K. Photoelectrochemical and photocatalytic properties of biphasic organic p- and n-type semiconductor nanoparticles fabricated by a reprecipitation process. ACS Appl. Mater. Interfaces 2011, 3, 1902–1909. [Google Scholar] [CrossRef]
- Zhang, S.; Arunachalam, P.; Abe, T.; Iyoda, T.; Nagai, T. Photocatalytic decomposition of N-methyl-2-pyrrolidone, aldhydes, and thiol by bisphase and p/n junction-like organic semiconductor composite nanoparticles responsive to nearly full spectrum of visible light. J. Photochem. Photobiol. A 2012, 244, 18–23. [Google Scholar] [CrossRef]
- Nagai, K.; Morishita, K.; Yoshida, H.; Norimatsu, T.; Miyanaga, N.; Izawa, Y.; Yamanaka, T. Photo-reflection and laser-ablation properties of phthalocyanine/perylene derivative bilayer. Synth. Met. 2001, 121, 1445–1446. [Google Scholar] [CrossRef]
- Nagai, K.; Yoshida, H.; Norimatsu, T.; Miyanaga, N.; Izawa, Y.; Yamanaka, T. Uniform laser ablation via photovoltaic effect of phthalocyanine/perylene derivative. Appl. Surf. Sci. 2002, 197-198, 808–813. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Abe, T.; Nagai, K.; Kaneko, M.; Okubo, T.; Sekimoto, K.; Tajiri, A.; Norimatsu, T. A novel and efficient system of a visible-light-responsive organic photoelectrocatalyst working in a water phase. ChemPhysChem 2004, 5, 716–720. [Google Scholar] [CrossRef]
- Abe, T.; Miyakushi, S.; Nagai, K.; Norimatsu, T. Study of the factors affecting the photoelectrode characteristics of a perylene/phthalocyanine bilayer working in the water phase. Phys. Chem. Chem. Phys. 2008, 10, 1562–1568. [Google Scholar]
- Abe, T.; Nagai, K.; Sekimoto, K.; Tajiri, A.; Norimatsu, T. Novel characteristics at a fullerene/water interface in an organic bilayer photoelectrode of phthalocyanine/fullerene. Electrochem. Commun. 2005, 7, 1129–1132. [Google Scholar] [CrossRef]
- Abe, T.; Nagai, K.; Ichinohe, H.; Shibata, T.; Tajiri, A.; Norimatsu, T. An efficient oxidation at photofunctional interface of phthalocyanine in combination with fullerene. J. Electroanal. Chem. 2007, 599, 65–71. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Corrigan, D.A. Photoelectrochemistry of nickel hydroxide thin films. J. Electrochem. Soc. 1989, 136, 1022–1026. [Google Scholar] [CrossRef]
- Abe, T.; Kaneko, M. Reduction catalysis by metal complex confined in a polymer matrix. Prog. Polym. Sci. 2003, 28, 1441–1448. [Google Scholar] [CrossRef]
- Sobbi, A.K.; Wöhrle, D.; Schlettwein, D. Photochemical stability of various porphyrins in solution and as thin film electrodes. J. Chem. Soc. Perkin Trans. 2 1993, 481–488. [Google Scholar]
- Karmann, E.; Schlettwein, D.; Jaeger, N.I. Photoelectrochemical oxidation of 2-mercaptoethanol at the surface of octacyanophthalocyanine thin film electrodes. J. Electroanal. Chem. 1996, 405, 149–158. [Google Scholar] [CrossRef]
- Abe, T.; Ichinohe, H.; Kakuta, S.; Nagai, K. Organic photoanode of fullerene/phthalocyanine working in the water phase with respect to preparation methods of the bilayer film. Jpn. J. Appl. Phys. 2010, 49, 015101. [Google Scholar] [CrossRef]
- Haufler, R.E.; Conceicao, J.; Chibante, L.P.F.; Chai, Y.; Byrne, N.E.; Flanagan, S.; Haley, M.M.; O’Brien, S.C.; Pan, C.; Xiao, Z.; et al. Efficient production of C60 (buckminsterfullerene), C60H36, and the solvated buckide ion. J. Phys. Chem. 1990, 94, 8634–8636. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, S.; Abe, T.; Iyoda, T.; Nagai, K. Photoelectrode Characteristics of Partially Hydrolyzed Aluminum Phthalocyanine Chloride/Fullerene C60 Composite Nanoparticles Working in a Water Phase. Molecules 2012, 17, 10801-10815. https://doi.org/10.3390/molecules170910801
Zhang S, Abe T, Iyoda T, Nagai K. Photoelectrode Characteristics of Partially Hydrolyzed Aluminum Phthalocyanine Chloride/Fullerene C60 Composite Nanoparticles Working in a Water Phase. Molecules. 2012; 17(9):10801-10815. https://doi.org/10.3390/molecules170910801
Chicago/Turabian StyleZhang, Shuai, Toshiyuki Abe, Tomokazu Iyoda, and Keiji Nagai. 2012. "Photoelectrode Characteristics of Partially Hydrolyzed Aluminum Phthalocyanine Chloride/Fullerene C60 Composite Nanoparticles Working in a Water Phase" Molecules 17, no. 9: 10801-10815. https://doi.org/10.3390/molecules170910801
APA StyleZhang, S., Abe, T., Iyoda, T., & Nagai, K. (2012). Photoelectrode Characteristics of Partially Hydrolyzed Aluminum Phthalocyanine Chloride/Fullerene C60 Composite Nanoparticles Working in a Water Phase. Molecules, 17(9), 10801-10815. https://doi.org/10.3390/molecules170910801