Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Endophyte Isolation and Initial Screening
| Strain | SL (cm) | CC (SPAD) | SFW (g) | SDW (g) |
|---|---|---|---|---|
| Control (GF) | 8.3 + 0.51 b | 28.5 + 0.67 b | 0.1386 + 0.1 b | 0.0319 + 0.81 ab |
| Control (DW) | 7.3 + 0.21 c | 22.86 + 1.3 c | 0.0986 + 0.07 c | 0.0357 + 0.11 b |
| CSH-5C | 9.3 + 0.35 a | 29.43 + 0.89 b | 0.155 + 0.104 a | 0.0373 + 0.01 a |
| CSC-1A | 9.6 + 1.6 a | 31.4 + 0.36 a | 0.1622 + 0.08 a | 0.0405 + 0.109 a |
| Strain | SL (cm) | CC (SPAD) | SFW (g) | SDW (g) |
|---|---|---|---|---|
| Control (DW) | 8.4 ± 0.44 c | 15.26 ± 3.88 c | 0.1094 ± 0.07 c | 0.0357 ± 0.13 b |
| CSH-5C | 11.5 ± 0.25 b | 24.13 ± 1.95 a | 0.1596 ± 0.104 a | 0.0493 ± 0.06 a |
| CSC-1A | 12.66 ± 1.2 a | 19.96 ± 0.64 b | 0.1283 ± 0.08 b | 0.0395 ± 0.102 b |
2.2. Identification and Phylogenetic Analysis
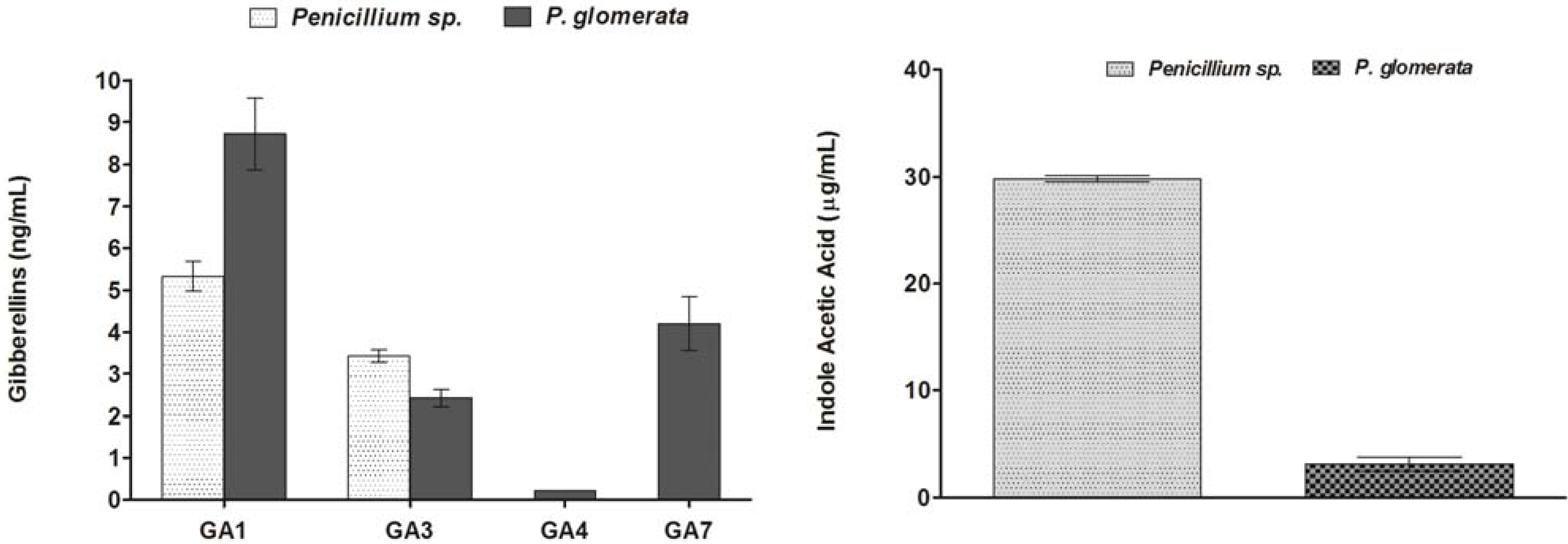
2.3. Analysis of Culture Filtrates for Screening Gibberellins
2.4. Quantification of IAA in Culture Filtrate
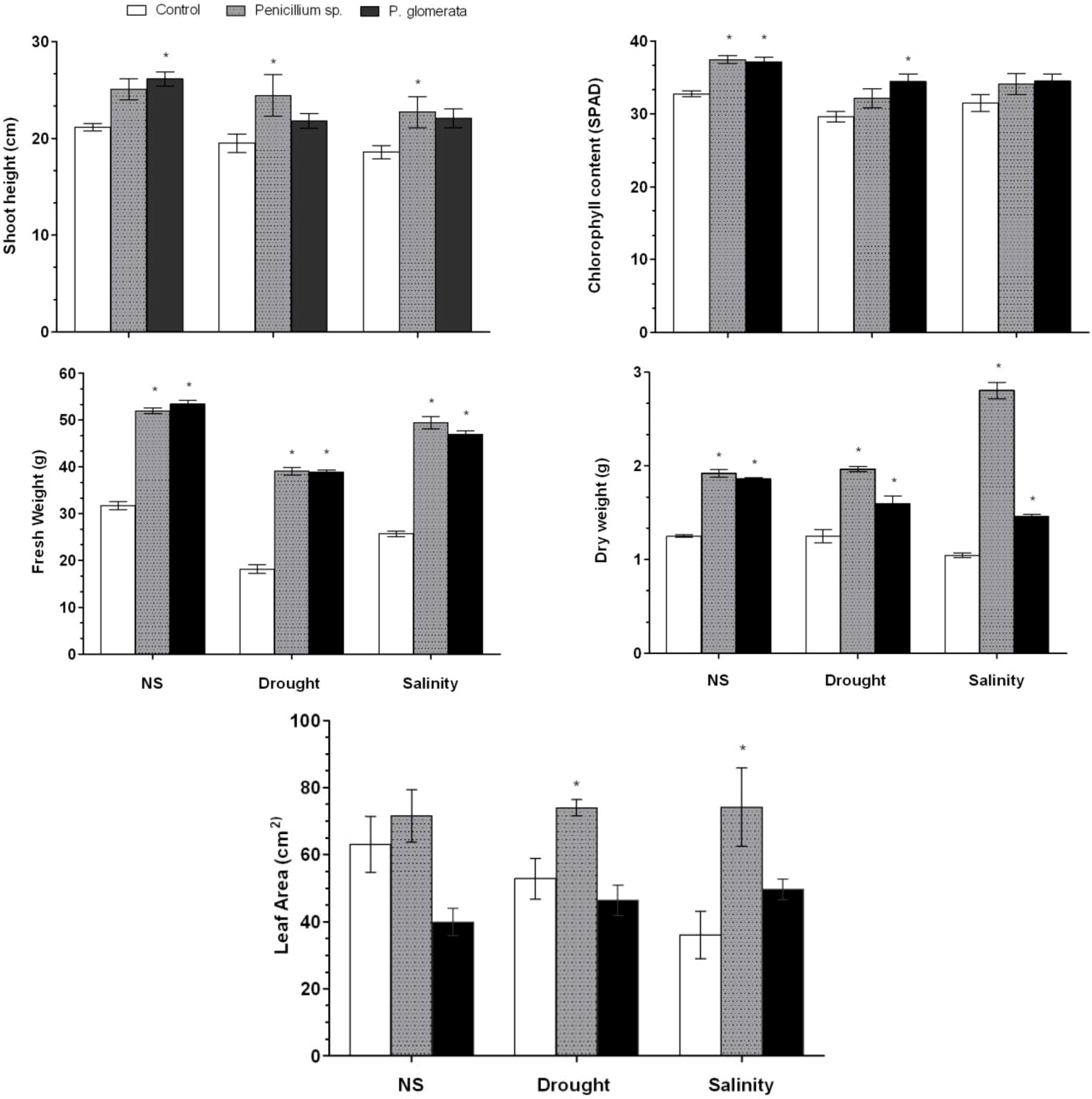
2.5. Response of Endophytes to Salinity and Drought Stress
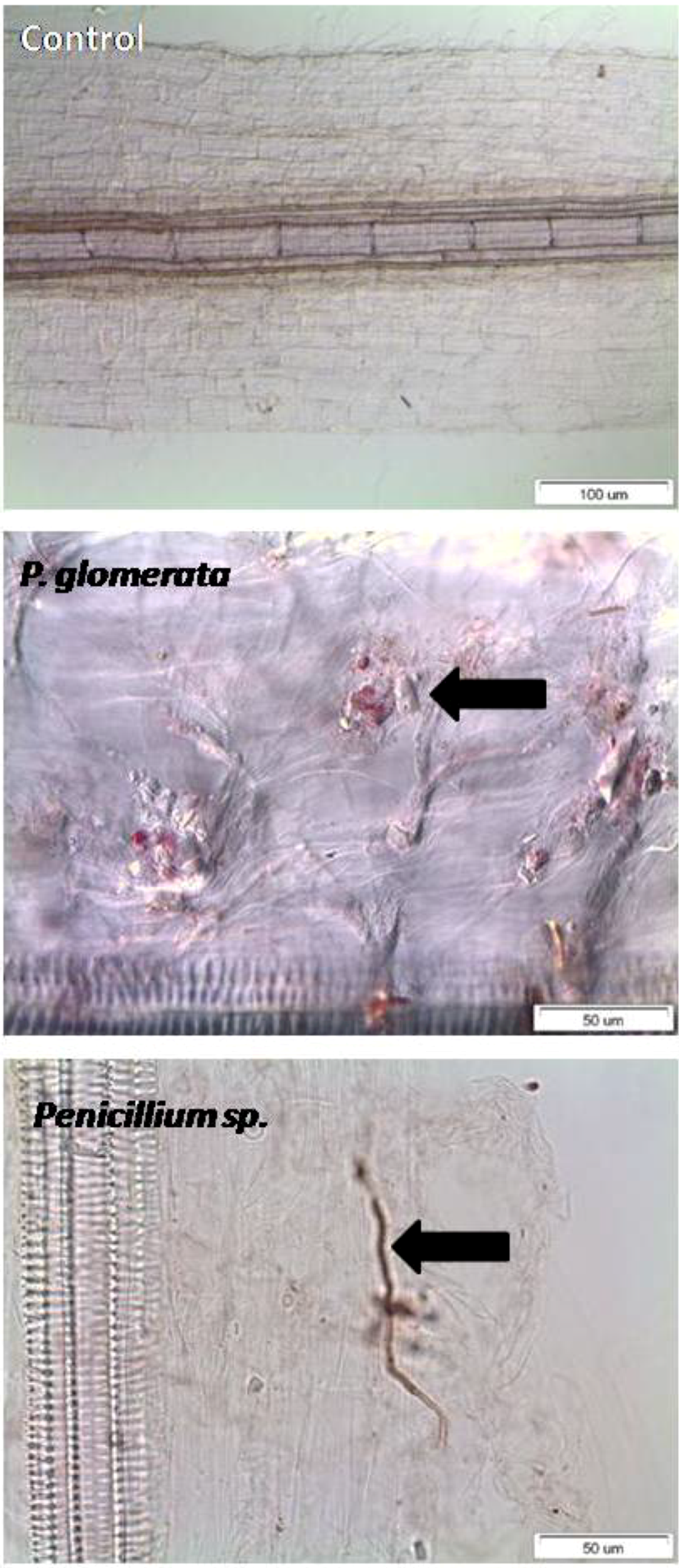
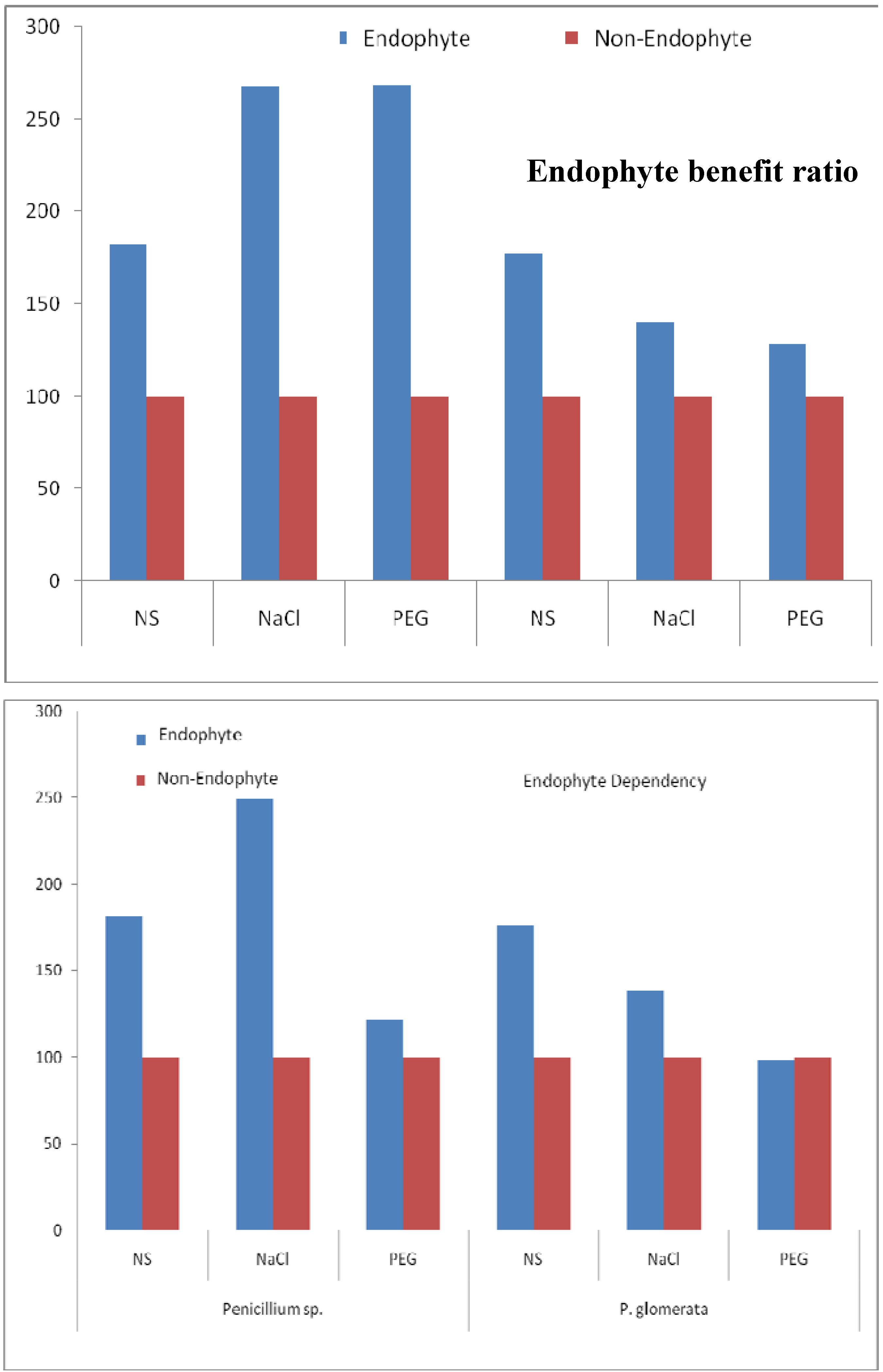
2.6. Endophyte Benefit Ratio, and Dependency
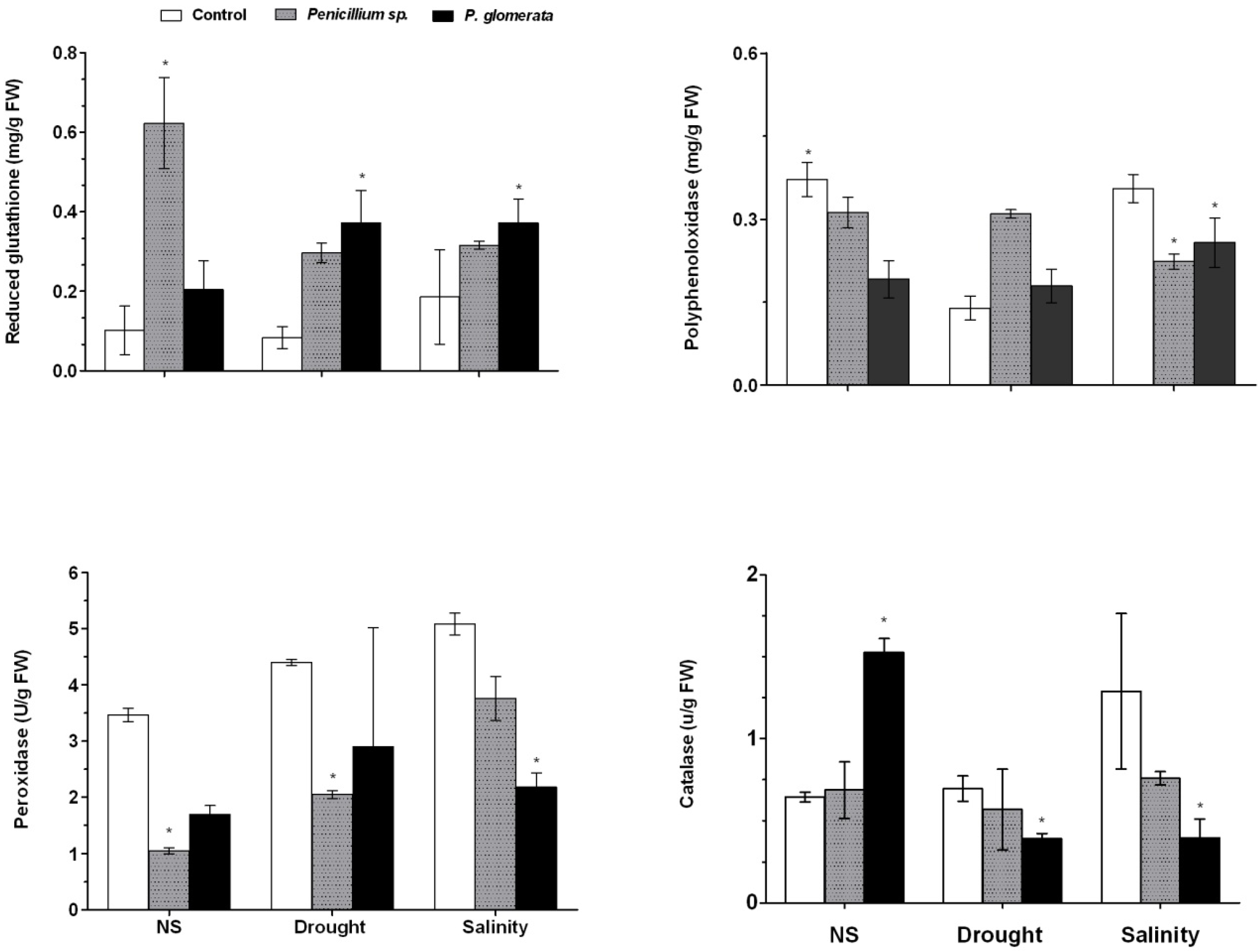
2.7. Symbiotic Association and Effect on Oxidative Stress during Abiotic stress
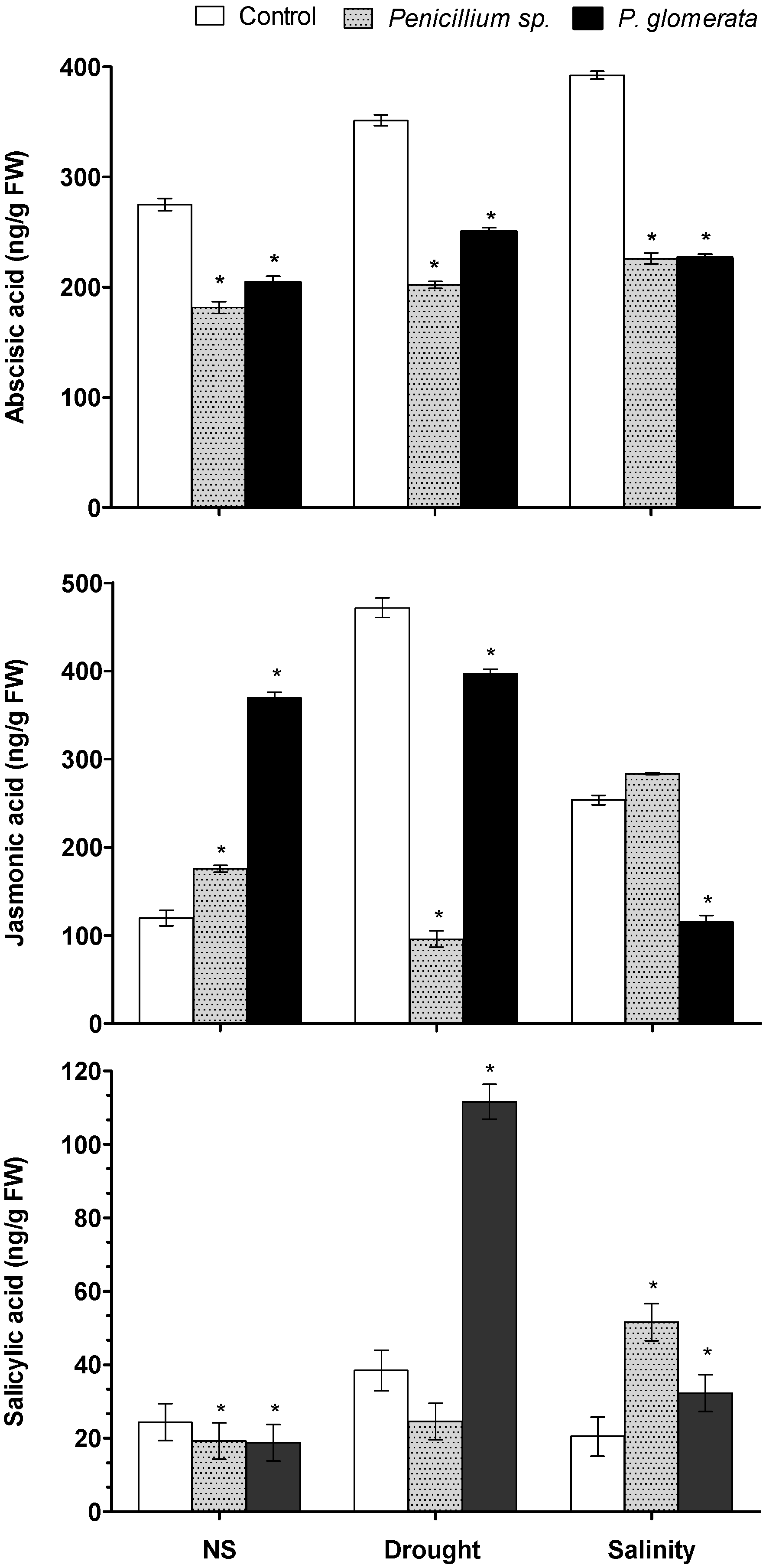
2.8. Effect of Symbiotic Association on Host Plant Hormones ABA, JA and SA
2.9. Elemental Analysis in Salinity Stress

3. Experimental
3.1. Sample Collection and Endophyte Isolation
3.2. Endophyte Identification
3.3. Gibberellins Deficient Mutant and Normal Rice Bioassay
3.4. Gibberellins Analysis of Pure Culture
3.5. IAA Analysis of Pure Culture
3.6. Host Plant and Endophytes Association in Salinity and Drought Stress
3.7. Hormonal Analysis (ABA and JA) of Host Plant
3.8. Antioxidants Activities of Host Plant
3.9. Elemental Analysis
3.10. Endophyte Benefit (%), Endophyte Dependency
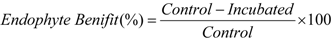
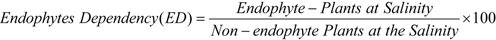
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgements
References
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Thompson, J.E.; Ledge, R.L.; Barber, R.F. The role of free radicals in senescence and wounding. New Phytol. 1987, 105, 317–344. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1985. [Google Scholar]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integ. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A. Evaluation of oxidative stress tolerance in two wheat (Triticum aestivum) cultivars in response to drought. Int. J. Agric. Biol. 2009, 11, 7–12. [Google Scholar]
- Shao, H.B.; Liang, Z.S.; Shao, M.A. Changes of some anti-oxidative enzymes under soil water deficits among 10 wheat genotypes at maturation stage. Colloid. Surfaces B 2005, 45, 7–13. [Google Scholar] [CrossRef]
- Schwanz, P.; Picon, C.; Vivin, P.; Dreyer, E.; Guehl, J.; Polle, A. Responses of antioxidative systems to drought stress in Pendunculate Oak and Maritim Pine as modulated by elevated CO2. Plant Physiol. 1996, 110, 393–402. [Google Scholar]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Brodersen, P.; Petersen, M.; Nielsen, H.B.; Zhu, S.; Newman, M.A.; Shokat, K.M.; Rietz, S.; Parker, J.; Mundy, J. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 2006, 47, 532–546. [Google Scholar] [CrossRef]
- Balbi, V.; Devoto, A. Jasmonate signaling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2008, 177, 301–318. [Google Scholar] [CrossRef]
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defence responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; Frei dit Frey, N.; Leung, J. An Update on Abscisic Acid Signaling in Plants and More. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J. Cell signalling during cold, Drought and salt stresses. Plant Cell 2002, 14, 163–183. [Google Scholar]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Woodward, C.J.; Redman, R.S. Fungal Influence on Plant Tolerance to Stress. In Biocomplexity of Plant-Fungal Interactions; Southworth, D., Ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Krings, M.; Taylor, T.N.; Hass, H.; Kerp, H.; Dotzler, N.; Hermsen, E.J. Fungal endophytes in a 400-million-yr-old land plant: Infection pathways, Spatial distribution, And host response. New Phytol. 2007, 174, 648–657. [Google Scholar]
- Sun, X.; Guo, L.D.; Hyde, K.D. Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers. 2011, 47, 85–95. [Google Scholar]
- Saikkonen, K.; Saari, S.; Helander, M. Defensive mutualism between plants and endophytic fungi? Fungal Divers. 2010, 41, 101–113. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Zhang, C.L.; Lin, F.C. Role of Diverse Non-Systemic Fungal Endophytes in Plant Performance and Response to Stress: Progress and Approaches. J. Plant Growth Regul. 2010, 29, 116–126. [Google Scholar] [CrossRef]
- Arnold, A.E.; Henk, D.A.; Eells, R.L.; Lutzoni, F.; Vilgalys, R. Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 2007, 99, 185–206. [Google Scholar] [CrossRef]
- Mei, C.; Flinn, B.S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat. Biotechnol. 2010, 4, 81–95. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, L.; Wang, J.; Shan, T.; Zhong, L.; Liu, X.; Gao, X. Endophytic fungi for producing bioactive compounds originally from their host plants. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2012; Volume 1, pp. 567–576. [Google Scholar]
- Liu, C.; Liu, T.; Yuan, F.; Gu, Y. Isolating endophytic fungi from evergreen plants and determining their antifungal activities. Afr. J. Microbiol. Res 2010, 4, 2243–2248. [Google Scholar]
- Soon-Ok, R.; Lee, J.H.; Khan, S.A.; Lee, I.J.; Rhee, I.K.; Lee, K.S.; Kim, J.G. Isolation and Identification of Fungal Strains Producing Gibberellins from the Root of plants. Kor. J. Microbiol. Biotechnol. 2007, 35, 357–363. [Google Scholar]
- Nishijima, T.; Katsura, N. A Modified Micro-Drop Bioassay Using Dwarf Rice for Detection of Femtomol Quantities of Gibberellins. Plant Cell Physiol. 1989, 30, 324–327. [Google Scholar]
- Higgs, R.E.; Zahn, J.A.; Gygi, J.D.; Hilton, M.D. Rapid Method to Estimate the Presence of Secondary Metabolites in Microbial Extracts. Appl. Environ. Microbiol. 2001, 67, 371–376. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef]
- Kawaide, H. Biochemical and molecular analysis of gibberellins biosynthesis in fungi. Biosci. Biotechnol. Biochem. 2006, 70, 583–590. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Fierro, A.C.; Wiedemann, G.; Reski, R.; Straeten, D.V.D. Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol. 2007, 7, 65. [Google Scholar] [CrossRef]
- Barrow, J.R.; Lucero, M.E.; Reyes-Vera, I.; Havstad, K.M. Do symbiotic microbes have a role in plant evolution, Performance and response to stress? Commun. Integr. Biol. 2008, 1, 69–73. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; de Pascale, S. Contrasting Effects of GA3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, Ionic partitioning, Photosynthesis, Yield and hormonal homeostasis. Environ. Exp. Bot. 2010. [Google Scholar]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance conferred to plant host and fungal endophyte during mutualistic symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Dai, C.C.; Yu, B.Y.; Li, X. Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr. J. Biotechnol. 2008, 7, 3505–3510. [Google Scholar]
- Zandavalli, R.B.; Dillenburg, L.R.; Paulo, V.D. Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl. Soil Ecol. 2004, 25, 245–255. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Hammad, R.; Rusan, M. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 2001, 11, 41–47. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. The reactive oxygen gene network in plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Iqbal, I.; Ahmad, B.; Lee, I.J. Isolation of a Gibberellin-producing fungus (Penicillium sp. MH7) and growth promotion of crown daisy (Chrysanthemum coronarium). J. Microbiol. Biotechnol. 2010, 20, 202–207. [Google Scholar]
- Agrawal, G.K.; Tamogami, S.; Iwahashi, H.; Agrawal, V.P.; Rakwal, R. Transient regulation of jasmonic acid-inducible rice MAP kinase gene (OsBWMK1) by diverse biotic and abiotic stresses. Plant Physiol. Biochem. 2003, 41, 355–361. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; Wettstein, D.V.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, Disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.D.A.; Greer, C.; Espino, L.; Sharon, L.D.; Rodriguez, R.J. Increased Fitness of Rice Plants to Abiotic Stress Via Habitat Adapted Symbiosis: A Strategy for Mitigating Impacts of Climate Change. PLoS One 2011, 6, e14823. [Google Scholar]
- Hasan, H.A.H. Gibberellin and auxin production plant root fungi and their biosynthesis under salinity-calcium interaction. Rostlinna Vyroba 2002, 48, 101–106. [Google Scholar]
- Khan, S.A.; Hamayun, M.; Yoon, H.J.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231–239. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 12 January 2012).
- Lee, I.J.; Foster, K.; Morgan, P.W. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998, 116, 1003–1011. [Google Scholar] [CrossRef]
- Shahab, S.; Ahmed, N.; Khan, N.S. Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. Afr. J. Agric. Res. 2009, 4, 1312–1316. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Qi, Q.G.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression and very long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef]
- McCloud, E.S.; Baldwin, I.T. Herbivory and caterpillar regurgitants amplify the wound induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 1997, 203, 430–435. [Google Scholar] [CrossRef]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. heat seed priming in relation to salt tolerance; Growth, Yield and levels of free salicylic acid and polyamines. Ann. Bot. Fennici 2006, 43, 250–259. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–127. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and polyphenoloxidase activites during rice leaf senescenc. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Raju, P.S.; Clark, R.B.; Ellis, J.R.; Duncan, R.R.; Marvanville, J.W. Benefit and cost analysis and phosphorus efficiency of VA mycorrhizal fungi colonization with sorghum (Sor-ghum bicolor) genotypes grown at varied phosphorus levels. Plant Soil 1990, 124, 199–204. [Google Scholar] [CrossRef]
- Gerdemann, J.W. Vesicular arbuscular mycorrhizal. In The Development and Function of Roots; Torrey, D.G., Clarkson, D.T.C., Eds.; Academic Press: London, UK, 1975; pp. 575–591. [Google Scholar]
- Sample Availability: Samples of the Gibberellins and indoleacetic acid are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules 2012, 17, 10754-10773. https://doi.org/10.3390/molecules170910754
Waqas M, Khan AL, Kamran M, Hamayun M, Kang S-M, Kim Y-H, Lee I-J. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules. 2012; 17(9):10754-10773. https://doi.org/10.3390/molecules170910754
Chicago/Turabian StyleWaqas, Muhammad, Abdul Latif Khan, Muhammad Kamran, Muhammad Hamayun, Sang-Mo Kang, Yoon-Ha Kim, and In-Jung Lee. 2012. "Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress" Molecules 17, no. 9: 10754-10773. https://doi.org/10.3390/molecules170910754
APA StyleWaqas, M., Khan, A. L., Kamran, M., Hamayun, M., Kang, S.-M., Kim, Y.-H., & Lee, I.-J. (2012). Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules, 17(9), 10754-10773. https://doi.org/10.3390/molecules170910754





