2. Results and Discussion
The proximate composition of
I. hederacea and
L. sativum seeds (
Table 1) indicates the presence of appreciable amounts of protein (23.36 ± 1.02% to 24.18 ± 1.54%), fibre (16.55 ± 0.31% to 6.75 ± 1.02%), lipids (14.09 ± 0.66% to 28.03 ± 1.05%), ash (3.65 ± 1.10% to 3.92 ± 1.06%), moisture (5.29 ± 1.02% to 3.92 ± 1.06%) and carbohydrates (37.06 ± 0.89% to 32.87 ± 0.29%). Proximate composition varies depending upon plant variety, agronomic practices, stage of collection of seeds and climatic and geological condition of area from where seeds are collected. It is an important indicator and first step during nutritional evaluation of fruits and seeds of plants and crops and it dictates further studies on components which seem more interesting [
14,
16].
Ash contents indicate the seeds are an appreciable source of minerals. The low moisture content is an index of stability, quality and increased shelf life of seeds [
19]. Our results are in partial agreement with those reported earlier for
I. hederacea [
20,
21] and
L. sativum [
22]. Higher protein and lipid contents indicate that both seeds have high food energy, however seeds cannot be considered safe for consumption since the toxicity levels for the seeds are not established yet.
Table 1.
Proximate chemical composition of seeds (%).
Table 1.
Proximate chemical composition of seeds (%).
| Component | Ipomoea hederacea | Lepidium sativum |
|---|
| Crude protein | 23.36 ± 1.02 b | 24.18 ± 1.54 a |
| Total lipids | 14.09 ± 0.66 b | 28.03 ± 1.05 a |
| Total carbohydrates | 37.06 ± 0.89 a | 32.87 ± 0.29 b |
| Crude fiber | 16.55 ± 0.31 a | 6.75 ± 1.02 b |
| Moisture | 5.29 ± 1.02 a | 3.92 ±1.06 b |
| Ash | 3.65 ± 1.10 a | 4.25 ± 0.13 a |
The relatively higher contents of proteins and lipids motivated us to undertake in-depth studies on both types of biomolecules. Data concerning qualitative and quantitative amino acids composition is presented in
Table 2. Amino acid composition indicates the nutritional quality of protein. Glutamic acid and aspartic acid were found to be the major non-essential amino acids in the samples tested. Results indicated that all essential amino acids, except S-containing types and tryptophan, are present in high amounts in both species. The amino acid profile showed that methionine and cysteine are present in the lowest concentration. Results are comparable to those of earlier workers for
L. sativum [
23,
24] while there is no previous report on amino acid composition of
I. hederacea seeds.
Table 2.
Percentage composition of amino acids in seeds.
Table 2.
Percentage composition of amino acids in seeds.
| Amino acid | Ipomoea hederacea | Lepidium sativum |
|---|
| Isoleucine | 5.03 ± 0.36 a | 4.19 ± 0.03 b |
| Leucine | 6.59 ± 0.85 b | 7.03 ± 0.08 a |
| Lysine | 4.25 ± 0.54 b | 5.98 ± 0.03 a |
| Methionine | 1.17 ± 0.62 a | 0.51 ± 0.01 b |
| Phenylaniline | 6.24 ± 0.92a | 5.39 ± 0.17 b |
| Threonine | 3.07 ± 0.31 a | 3.76 ± 0.08 a |
| Tryptophan | 1.88 ± 0.05 a | 0.92 ± 0.07 b |
| Valine | 7.10 ± 0.94 a | 6.21 ± 0.02 b |
| Arginine | 5.50 ± 0.23 a | 3.44 ± 0.11 b |
| Histidine | 3.55 ± 0.92 a | 3.87 ± 0.14 a |
| Alanine | 3.99 ± 0.37 b | 4.59 ± 0.19 a |
| Aspartic acid | 10.82 ± 0.86 b | 12.07 ± 0.31 a |
| Cystine | 0.90 ± 0.01 a | 0.21 ± 0.11 a |
| Glutamic acid | 22.71 ± 0.41 b | 24.29 ± 0.75 a |
| Glycine | 5. 36 ± 0.39 a | 5.08 ± 0.12 a |
| Proline | 4.46 ± 0.53 a | 4.63 ± 0.36 a |
| Serine | 4.02 ± 0.46 a | 4.18 ± 0.55 a |
| Tyrosine | 2.58 ± 0.59 a | 2.88 ± 0.69 a |
Mineral contents of seeds (
Table 3) varied between both species, but potassium constituted the major mineral in both cases, ranging from 978.46 mg/100 g in
I. hederacea to 1,236.51 mg/100 g in
L. sativum. Zinc was found in the lowest quantity in
I. hederacea (4.0 mg/100 g), while
L. sativum had the lowest manganese content (2.0 mg/100 g) content. Both species contained good amounts of calcium, phosphorus and magnesium. Mineral content for
L. sativum is in agreement with those reported earlier for
L. sativum [
22], while the metal content of
I. hederacea has not been reported previously. The relatively high amount of sodium in
I. hederacea seeds indicates that its high consumption might cause problems for hypertensive patients. Considering the different elements present in seeds of both plants, both plants have potential for providing essential nutrients for human and other animals, since the nutritional activity of any plant is usually related to the particular elements it contains [
25].
Table 3.
Mineral content (mg/100 g) of seeds.
Table 3.
Mineral content (mg/100 g) of seeds.
| Metals | Ipomoea hederacea | Lepidium sativum |
|---|
| Calcium | 317.41 ± 1.72 b | 266.35 ± 1.44 a |
| Copper | 4.62 ± 0.64 b | 5.73 ± 2.11 a |
| Iron | 9.85 ± 1.02 a | 8.31 ± 0.36 a |
| Magnesium | 179.14 ± 1.23 b | 339.23 ± 2.13 a |
| Manganese | 6.37 ± 0.68 a | 2.00 ± 1.08 b |
| Phosphorus | 596.19 ± 2.36 a | 608.63 ± 1.39 a |
| Potassium | 978.46 ± 1.44 b | 1236.51 ± 1.67 a |
| Sodium | 106.32 ± 1.33 a | 19. 65 ± 0.98 b |
| Zinc | 4.01 ± 0.69 b | 6.99 ± 0.54 a |
| Na:K | 0.11 | 0.01 |
| Ca:P | 0.53 | 0.43 |
Physico-chemical parameters provide important information regarding storage, structural stability and quality of seed oils. The physico-chemical properties of the investigated oils (
Table 4) are in agreement with those reported earlier for
L. sativum [
26,
27]. The color of an oil is an important feature which often determines the consumers’ acceptability of the product. The oil color observed for
L. sativum is dirty yellow, while that of
I. hederacea is light yellow. Oil color is mainly due to the presence of some pigments like chlorophyll and carotenoids which are unintentionally co-extracted during the oil extraction process. The high refractive index values (1.47 ± 0.03 to 1.47 ± 0.08) are an indication of substantial unsaturation in these oils. The refractive index also provides useful information about the purity of oils. Each oil has certain range for this parameter and deviation of the data from the set specification may indicate adulteration of oil [
28]. Like other parameters there is limited information on oil physico-chemical characteristics of
I. hederacea seed oil however our results are comparable to some investigated parameters [
29]. Oil composition (
Table 5) showed a high content of triacylglycerols,
i.e., 80.2 ± 0.51 and 90.00 ± 0.62 g/100g for
I. hederacea and
L. sativum respectively. Hydrocarbons and waxes were observed to be present in the lowest amount in both oils. Seed oils can be separated into the various classes on the basis of their relative polarity by using various chromatographic methods, mostly by thin-layer chromatography (TLC). Although this technique is time-consuming, yet it is the most used to study oil classes. Free fatty acids are the products of hydrolysis, formed either due to chemical (due to presence of moisture) or lypolytic hydrolysis (due to presence of natural enzymes, especially lipases). Lower free fatty acid contents (1.20 ± 0.42 to 0.58 ± 0.35 g/100g for
I. hederacea and
L. sativum, respectively, indicate a low degree of hydrolysis and thus good oil quality. The low free fatty acid value of both oils also indicates that oils are rich in triglycerides which is a basic and desirable property of edible oils [
30].
Table 4.
Physico-chemical parameters of oils of I. hederacea and L. sativum.
Table 4.
Physico-chemical parameters of oils of I. hederacea and L. sativum.
| Parameter | Ipomoea hederacea | Lepidium sativum |
|---|
| Color | Light yellow | Dirty Yellow |
| Refractive index | 1.47 ± 0.03 a | 1.47 ± 0.08 a |
| Specific gravity | 0.92 ± 0.04 a | 0.82 ± 0.06 a |
| Unsaponifiable matter | 1.73 ± 0.11 a | 0.57 ± 0.02 b |
| Acid value | 2.98 ± 0.08 a | 1.04 ± 0.05 b |
| Saponification value | 190.48 ± 0.67 a | 179.03 ± 0.73 b |
Table 5.
Composition (%) of oils of I. hederacea and L. sativum.
Table 5.
Composition (%) of oils of I. hederacea and L. sativum.
| Oil class | Ipomoea hederacea | Lepidium sativum |
|---|
| Hydrocarbons + waxes | 0.40 ± 0.23 a | 0.30 ± 0.04 a |
| Steryl esters | 0.60 ±0.34 a | 0.70 ± 0.07 a |
| Triacylglycerols | 80.20 ± 0.51 b | 90.00 ± 0.62 a |
| Free fatty acid | 1.20 ± 0.42 a | 0.58 ± 0.35 b |
| Diglycerols | 0.70 ± 0.08 a | 0.60 ± 0.58 a |
| Monoglycerols | 1.00 ± 0.37 a | 0.10 ± 0.63 b |
| Polar lipids | 6.50 ± 0.91 a | 1.70 ± 0.21 b |
| Phospholipids | 4.80 ± 0.82 a | 1.40 ± 0.36 b |
| Unidentified | 4.60 ± 0.57 a | 4.63 ± 0.67 a |
The fatty acid composition of
I. hederacea seed oil is in agreement to that reported earlier [
31]. Unsaturated fatty acids are found in higher amounts in both oils. Palmitic acid was observed as the most abundant saturated fatty acid, in amounts of 17.03 ± 0.89 and 10.3 ± 0.12 g/100g for
I. hederacea and
L. sativum, respectively. Similarly palmitoleic acid was observed as being the least abundant unsaturated fatty acid, with values of 1.10 ± 0.62 and 0.70 ± 0.30 g/100g for
I. hederacea and
L. sativum respectively. Fatty acid composition (
Table 6) revealed high content of linoleic acid (52%) in
I. hederacea, while α-linoleic acid (32.18%) and oleic acid (30.5%) were the most abundant fatty acids in the oil of
L. sativum. Higher intake of oleic acid is associated with decreased risk of coronary heart disease caused by high cholesterol level in blood [
32]. The fatty acid composition of the seed oils of these two plant species is interesting from the nutritional point of view for their higher contents of unsaturated fatty acids, especially
L. sativum rich in ω-3 fatty acids which are is beneficial for health.
Table 6.
Fatty acid profile (%) of I. hederacea and L. sativum.
Table 6.
Fatty acid profile (%) of I. hederacea and L. sativum.
| Fatty acid | Ipomoea hederacea | Lepidium sativum |
|---|
| Palmitic acid (16:0) | 17.03 ± 0.89 a | 10.30 ± 0.12 b |
| Palmitoleic acid (16:1) | 1.10 ± 0.62 a | 0.70 ± 0.30 b |
| Stearic acid (18:0) | 6.00 ± 0.56 a | 1.90 ± 0.19 b |
| Oleic acid (18:1) | 19.50 ± 0.37 b | 30.50 ± 0.16 a |
| Linoleic acid (18:2) | 52.09 ± 0.48 a | 8.60 ± 0.38 b |
| α-Linolenic acid (18:3) | 4.28 ± 0.92 b | 32.18 ± 0.59 a |
| Arachidic acid (20:0) | - | 2.10 ± 0.57 |
| Eicosaenoic acid (20:1) | - | 13.40 ± 0.66 |
Tocopherols are natural antioxidants that inhibit oil oxidation. Tocopherols act as biological scavengers of free radicals and could prevent diseases, besides possessing an important nutritional function for human beings as a source of vitamin E [
33,
34]. Total tocopherol contents observed were 33.10 ± 0.56 and 139.73 ± 0.91 mg/100g for
I. hederacea and
L. sativum, respectively. γ-Tocopherol was found in the highest amount in seed oil of
I. hederacea, while δ-tocopherol was the most abundant in the seed oil of
L. sativum (
Table 7). High amounts of tocopherols can be interesting for the stabilization of fats and oils against oxidative deterioration and for applications in dietary, pharmaceutical, or biomedical products. Results presented are in agreement with those reported earlier [
35,
36,
37].
Table 7.
Tocopherol profile (mg/100g) of oils of I. hederacea and L. sativum.
Table 7.
Tocopherol profile (mg/100g) of oils of I. hederacea and L. sativum.
| Tocopherol | Ipomoea hederacea | Lepidium sativum |
|---|
| α-Tocopherol | 0.50 ± 0.21 b | 17.19 ± 0.52 a |
| β-Tocopherol | 1.60 ± 0.21 a | 0.10 ± 0.08 b |
| γ-Tocopherol | 28.70 ± 0.14 a | 10.88 ± 0.72 b |
| δ-Tocopherol | 2.30 ± 0.34 b | 111.56 ± 0.37 a |
| Total | 33.10 ± 0.56 b | 139.73 ± 0.91 a |
The samples were subjected to antioxidant capacity screening, using three different testing methods. The extracts of
I. hederacea and
L. sativum showed good antioxidant capacity, reducing different types of radicals (
Table 8). In general, traditional spectrophotometric assays provide simple and fast screening methods to quantify total phenolic compounds in plant samples. However, due to the complexity of plant phenolics and different reactivity of phenols toward assay reagents, these results should be reinforced by the HPLC analysis, currently the most popular and reliable technique for analysis of phenolic compounds. Using this technique, coupled with mass spectrometer, tentative identification of phenolic compounds based on their mass spectra has been performed (
Table 9).
Table 8.
Antioxidant activity of plant extract of I. hederacea and L. sativum.
Table 8.
Antioxidant activity of plant extract of I. hederacea and L. sativum.
| Antioxidant Assay | Ipomoea hederacea | Lepidium sativum |
|---|
| Total phenol content (mg CAE/g) | 14.33 ± 0.09 b | 120.26 ± 1.52 a |
| TEAC (µmol TE/g) | 93.57 ± 2.42 b | 168.10 ± 1.32 a |
| FRAP (µmol Fe++/g) | 278.24 ± 9.74 b | 1317.04 ± 5.74 a |
| TRAP (µmol TE/g) | 69.02 ± 2.92 b | 506.40 ± 14.87 a |
Table 9.
Tentative identification of phenolic compounds based on their mass spectral characteristics present in I. hederacea and L. sativum.
Table 9.
Tentative identification of phenolic compounds based on their mass spectral characteristics present in I. hederacea and L. sativum.
| Compound | [M−H]− (
m/z) | Qualifier ions (
m/z) | I. hederacea | L. sativum |
|---|
| Gallic acid | 169 | 125 | - | + |
| Ferulic acid | 193 | 134 | + | - |
| Protocatechuic acid | 153 | 109 | - | + |
| Coumaric acid | 163 | 119 | - | + |
| Caffeic acid | 179 | 135 | - | + |
| Coumaric acid-hexoside | 325 | 163, 119 | + | + |
| Caffeic acid-hexoside | 341 | 179, 135 | - | + |
| Ferulic acid-hexoside | 355 | 193, 134 | + | + |
| Sinapic acid-hexoside | 385 | 223,149 | + | - |
| Vanillic acid-hexoside | 329 | 167 | - | + |
| Caffeoylquinic acid | 353 | 191, 179, 173, 135 | - | + |
| Coumaroylquinic acid | 337 | 191, 173 | - | + |
| Kaemferol-hexoside | 447 | 285 | - | + |
| Quercetin-hexoside | 463 | 301 | - | + |
| Kaemferol-glucuronide | 461 | 285 | - | + |
| Quercetin | 301 | 151 | - | + |
More phenolics have been identified in
L. sativum than
I. hederacea. In
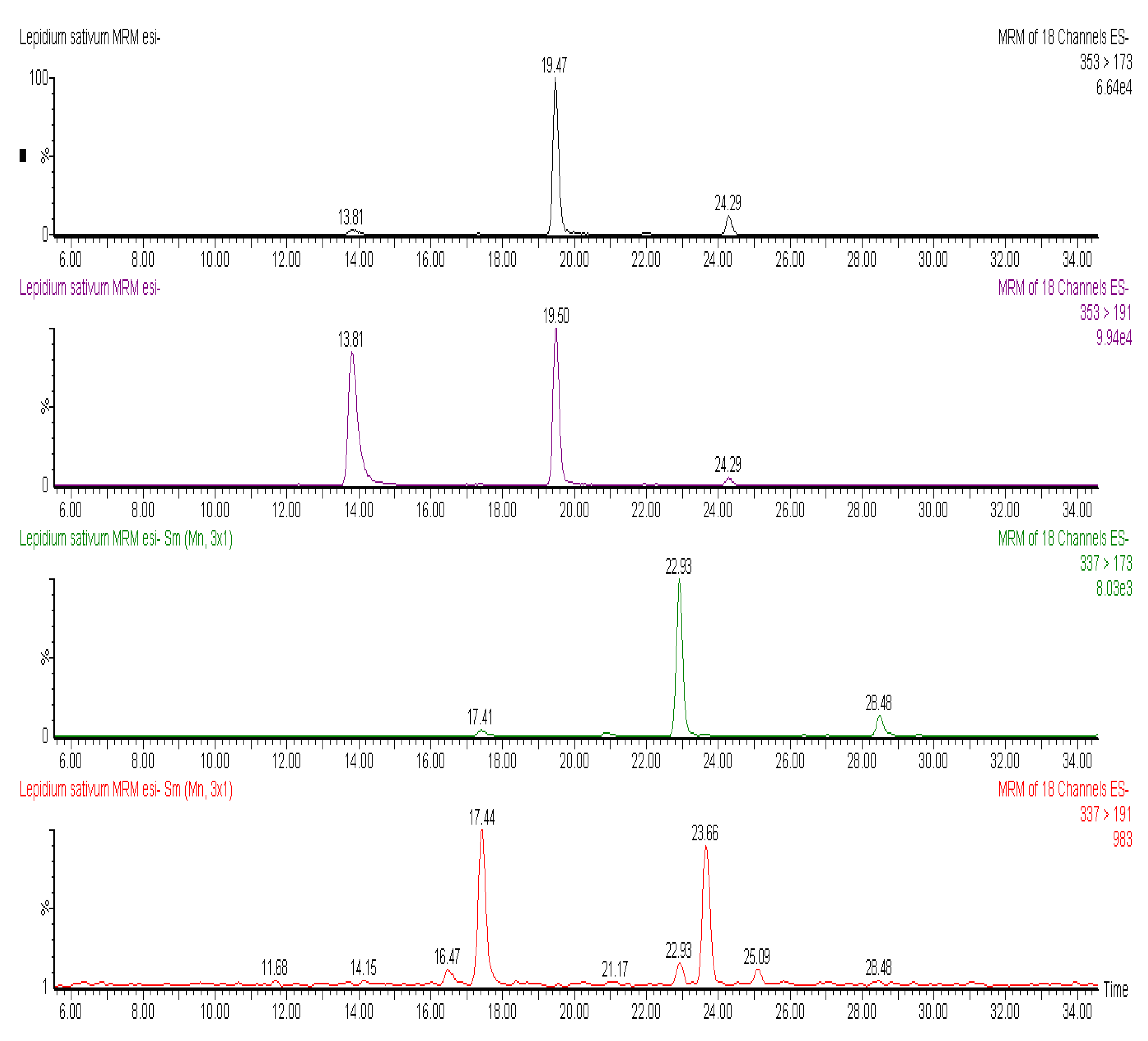
Figure 1, the chromatographic profile of some polyphenols identified in
L. sativum is reported. At least three isomers of caffeoylquinic acid were identified in the seeds of
L. sativum. These isomers had a negatively charged molecular ion ([M−H]
−) at
m/z 353 and MS2 ions deriving from loss of the caffeoyl moiety and resulting ionization of free quinic acid (
m/z 191) and quinic acid with a loss of water molecule (
m/z 173). Among chlorogenic acids, coumaroylquinic acids showed a [M−H]
− at
m/z 337, and were characterized by the same fragmentation patterns of caffeoylquinic acids, involving the breakdown of hydroxycinnamate-quinic acid linkages. Moreover, coumaric acid was recovered in esterified form with hexose groups, with a [M−H]
− at
m/z 325, that generated by tandem mass fragmentation the free coumaric acid after loss of a hexose moiety (
m/z 163) and further fragmentation of free hydroxycinnamic acid (
m/z 119) (
Figure 2).
Figure 1.
MRM chromatograms of caffeoylquinic acid. It is possible to note at least three isomers (at 13.81, 19.47 and 24.29 min), identified through the loss and ionization quinic acid (353 > 191) and (353 > 173) derived from loss of H2O from formed quinic acid. MRM chromatograms of coumaroylquinic acid, identified through the loss and ionization quinic acid (337 > 191) and (337 > 173) derived from loss of H2O from formed quinic acid.
Figure 1.
MRM chromatograms of caffeoylquinic acid. It is possible to note at least three isomers (at 13.81, 19.47 and 24.29 min), identified through the loss and ionization quinic acid (353 > 191) and (353 > 173) derived from loss of H2O from formed quinic acid. MRM chromatograms of coumaroylquinic acid, identified through the loss and ionization quinic acid (337 > 191) and (337 > 173) derived from loss of H2O from formed quinic acid.
Besides phenolic acids,
L. sativum contained several flavonoids, including two quercetin-hexosides that shared [M−H]
− at
m/z 463, identified through the loss of sugar moieties (probably glucose and galactose units) and resultant ionization of quercetin at
m/z 301, as reported in
Figure 2. As for the phenolic profile of
I. hederacea, only free ferulic acid and sinapic acid-hexosides were identified in this plant, while ferulic and coumaric acids were presented in esterified form with hexose groups, like in
L. sativum.
Total antioxidant capacity, measured by three assays, and total phenolic content, measured using the Folin–Ciocalteu colorimetric method, of analyzed plant extracts are reported in
Table 8.
L. sativum seeds had a higher content of TPC (120.26 mg CAE/g) than
Ipomea hederacea seeds and this is consistent with the higher number of phenolics identified in these seeds (
Table 9). Few studies have evaluated the content of TPC in these plants. Compared to our results [
38], analyzing the hydroalcoholic extracts of edible plants from Calabria (Italy), we found a lower content of TPC in
L. sativum leaves, whereas Aziz-ur-Rehman
et al. found a higher TPC value in
I. hederacea extracted with different solvents and collected from the Kotli region in Pakistan [
39]. Due to the higher content of TPC and total tocopherols,
L. sativum showed higher values of total antioxidant capacity (TAC), regardless of the applied assay (
Table 8), with respect to
I. hederacea. TEAC and FRAP values of the analyzed
L. sativum were high when compared to those found in Indian
L. sativum seeds, which, accordingly, showed a lower content of TPC than that found in our sample [
40]. Similarly, FRAP values of
I. hederacea were higher than those reported by Aziz-ur-Rehman
et al. [
39]. It is well known that the antioxidant capacity and phenolic content of plants depend on several factors such as different genotype, growing condition, agronomic practices employed, season, maturity, post-harvest storage and processing conditions and solvent used for extraction
. These factors may explain the differences found among our samples and those analyzed in previous studies.
Figure 2.
MRM chromatograms of coumaric acid-hexosides. It is possible to note at least two isomers (at 15.28 and 17.71 min), identified through the loss of hexose moiety (325 > 163) and further fragmentation of coumaric acid (163 > 119). MRM chromatogram of quercetin-hexosides (463 > 301), identified through the loss of sugar moiety and resultant ionization of quercetin.
Figure 2.
MRM chromatograms of coumaric acid-hexosides. It is possible to note at least two isomers (at 15.28 and 17.71 min), identified through the loss of hexose moiety (325 > 163) and further fragmentation of coumaric acid (163 > 119). MRM chromatogram of quercetin-hexosides (463 > 301), identified through the loss of sugar moiety and resultant ionization of quercetin.