Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii
Abstract
:1. Introduction
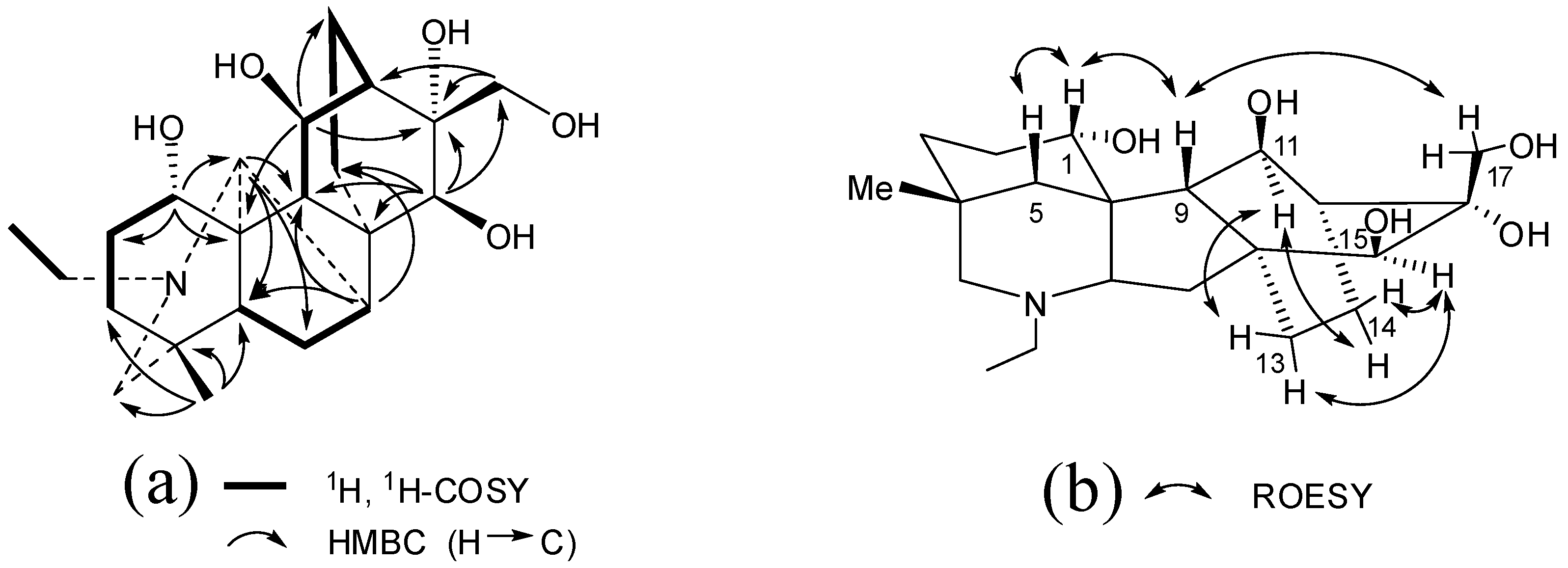
2. Results and Discussion
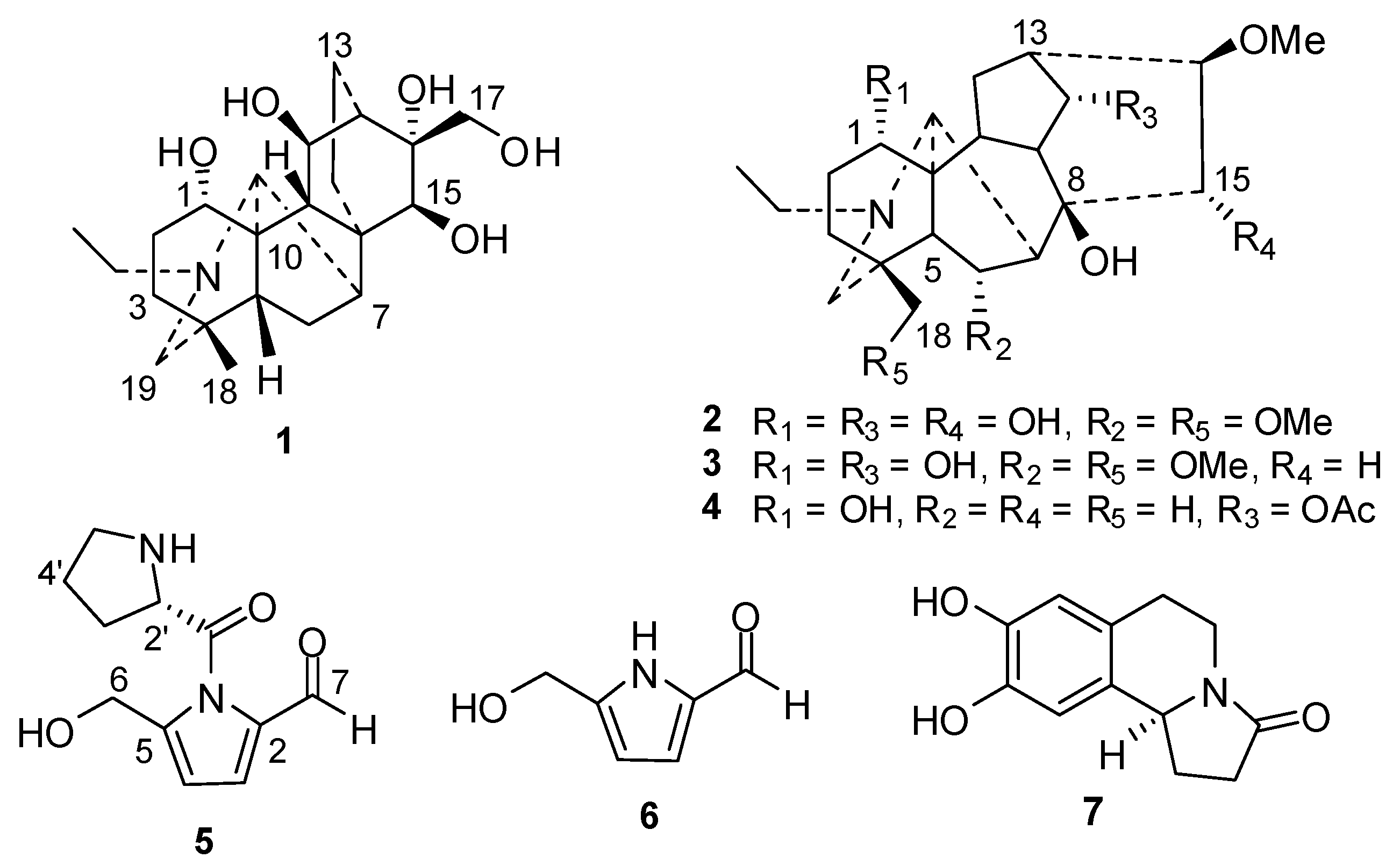
| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 1 | 4.18 dd (10.8, 6.4) | 71.3 | 12 | 1.60 m | 46.2 |
| 2 | 1.78 m, 2.40 m | 31.8 | 13 | 1.42 m, 1.92 m | 21.6 |
| 3 | 1.39 m, 1.61 m | 39.7 | 14 | 1.03 m, 1.84 m | 28.3 |
| 4 | – | 34.6 | 15 | 3.83 s | 86.4 |
| 5 | 1.37 d (8.0) | 54.3 | 16 | – | 80.3 |
| 6 | 1.26 dd (14.0, 5.2) | 24.5 | 17 | 3.79 d (12.0) | 69.2 |
| 2.86 dd (14.0, 8.0) | – | 4.03 d (12.0) | – | ||
| 7 | 2.12 d (5.2) | 43.5 | 18 | 0.75 s | 26.4 |
| 8 | – | 44.9 | 19 | 2.31 d (11.2), 2.58 d (11.2) | 58.1 |
| 9 | 1.82 d (9.6) | 51.8 | 20 | 3.75 br s | 68.5 |
| 10 | – | 52.1 | 21 | 2.48 m, 2.60 m | 52.1 |
| 11 | 4.56 d (9.6) | 72.3 | 22 | 1.10 t (7.2) | 13.7 |
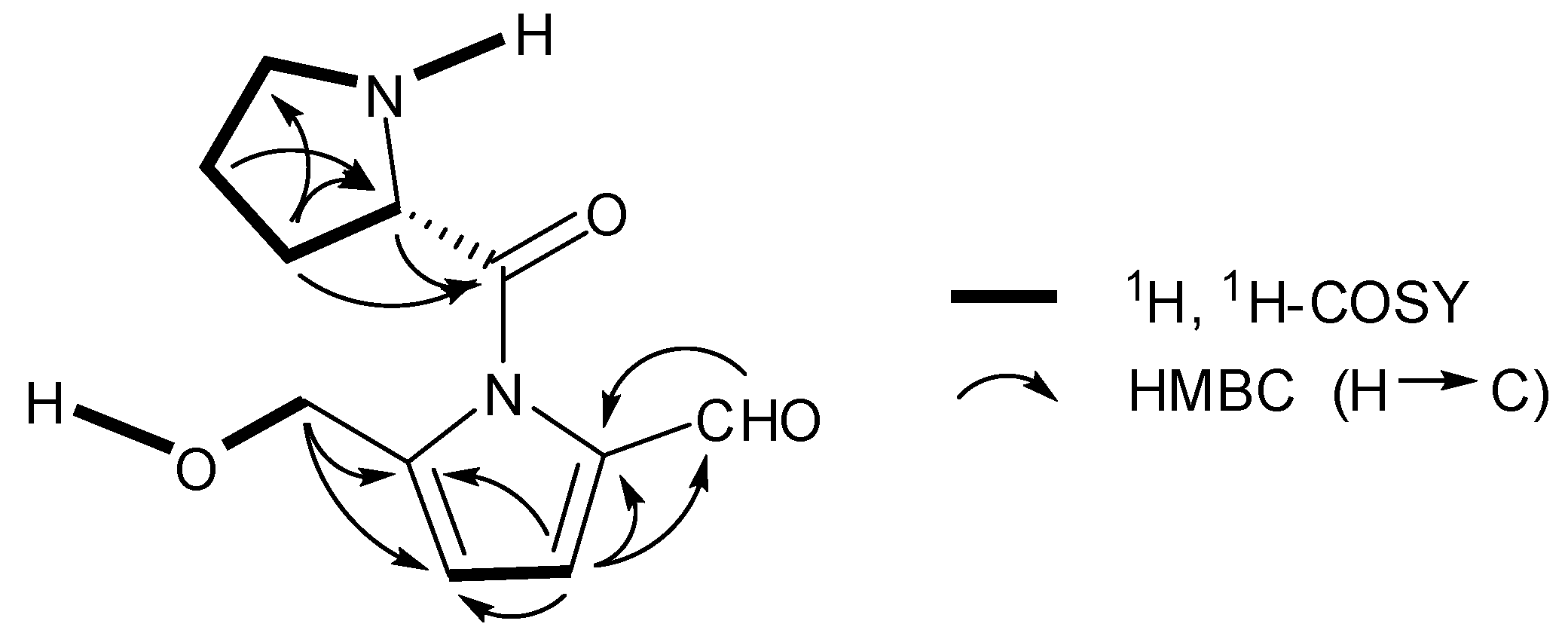
| No. | δH | δC | |||
|---|---|---|---|---|---|
| 5 | 6 | 5 | 6 | ||
| 1 | – | 10.88 s | – | – | |
| 2 | – | – | 133.4 | 132.6 | |
| 3 | 6.95 d (4.0) | 6.91 d (4.0) | 125.5 | 125.7 | |
| 4 | 6.19 d (4.0) | 6.22 d (4.0) | 110.0 | 108.5 | |
| 5 | – | – | 144.5 | 142.0 | |
| 6 | 4.65 s | 4.64 s | 56.7 | 56.8 | |
| 7 | 9.37 s | 9.47 s | 178.8 | 178.1 | |
| OH-6 | 4.39 s | 4.29 s | – | ||
| 1′ | 6.69 ( s) | – | |||
| 2′ | 5.00 dd (10.8, 5.6) | 57.5 | |||
| 3′ | 1.97 m, 2.23 m | 30.1 | |||
| 4′ | 1.97 m, 2.29 m | 23.6 | |||
| 5′ | 3.31 m, 3.63 m | 42.7 | |||
| 6′ | – | 169.2 | |||
| Compound | Increase of the cell viability (%) | ||
|---|---|---|---|
| 10 μM | 1 μM | 0.1 μM | |
| 2 | 65.44 | 64.14 | 63.09 |
| 3 | 73.82 | 72.51 | 47.64 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cardiomyocyte Protection Assay
3.5. Antibacterial Activity Experiments
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1995.
- Shen, Y.; Zuo, A.X.; Jiang, Z.Y.; Zhang, X.M.; Wang, H.L.; Chen, J.J. Two new C20-diterpenoid alkaloids from Aconitum carmichaelii. Helv. Chim. Acta 2011, 94, 122–126. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Lin, S.; Zhu, C.G.; Wang, S.J.; Wang, Y.N.; Chen, M.H.; Zhang, J.J.; Hu, J.F.; Chen, N.H.; Yang, Y.C.; et al. Diterpenoid alkaloids from the lateral root of Aconitum carmichaelii. J. Nat. Prod. 2012, 75, 1145–1159. [Google Scholar]
- Shim, S.H.; Lee, S.Y.; Kim, J.S.; Son, K.H.; Kang, S.S. Norditerpenoid alkaloids and other components from the processed tubers of Aconitum carmichaeli. Arch. Pharm. Res. 2005, 28, 1239–1243. [Google Scholar] [CrossRef]
- Han, G.Y.; Liang, H.Q.; Zhang, W.D.; Chen, H.S.; Wang, J.Z.; Liao, Y.Z.; Liu, M.Z.; Shen, Q.H. Studies on the alkaloids and a new cardiac principle isolated from Jiangyou Fuzi (Aconitum carmichaelii Debx.). Nat. Prod. Res. Dev. 1997, 9, 30–33. [Google Scholar]
- Csupor, D.; Wenzig, E.M.; Zupkó, I.; Wölkart, K.; Hohmann, J.; Bauer, R. Qualitative and quantitative analysis of aconitine-type and lipo-alkaloids of Aconitum carmichaelii roots. J. Chromatogr. A 2009, 1216, 2079–2086. [Google Scholar] [CrossRef]
- Yue, H.; Pi, Z.; Song, F.; Liu, Z.; Cai, Z.; Liu, S. Studies on the aconitine-type alkaloids in the roots of Aconitum Carmichaeli Debx. by HPLC/ESIMS/MSn. Talanta 2009, 77, 1800–1807. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Mody, N.V.; Varughese, K.I. Fuziline, A new alkaloid from the Chinese drug “Fuzi” (Aconitum carmichaeli Debx). Heterocycles 1982, 18, 47–49. [Google Scholar] [CrossRef]
- Khetwal, K.S.; Desai, H.K.; Joshi, B.S.; Pelletier, S.W. Norditerpenoid alkaloids from the aerial parts of Aconitum balfourii stapf. Heterocycles 1994, 38, 833–842. [Google Scholar] [CrossRef]
- Hikino, H.; Kuroiwa, Y.; Konno, C. Structure of hokbusine A and B, diterpenic alkaloids of Aconitum carmichaeli roots from Japan. J. Nat. Prod. 1983, 46, 178–182. [Google Scholar] [CrossRef]
- Hiermann, A.; Kedwani, S.; Schramm, H.W.; Seger, C. A new pyrrole alkaloid from seeds of Castanea sativa. Fitoterapia 2002, 73, 22–27. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.M.; Wang, W.; Wang, R.F.; Ding, Y.; Du, L.J. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Wada, K.; Kawahara, N. Diterpenoid and norditerpenoid alkaloids from the roots of Aconitum yesoense var. macroyesoense. Helv. Chim. Acta 2009, 92, 629–637. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, S.L.; Liao, X.; Yu, K.B.; Ding, L.S. Three new diterpene alkaloids from the roots of Aconitum nagarum var. lasiandrum. Planta Med. 2005, 71, 1073–1076. [Google Scholar] [CrossRef]
- Batsuren, D.; Tunsag, J.; Batbayar, N.; Mericli, A.M.; Mericli, F.; Teng, Q.; Desai, H.K.; Joshi, B.S.; Pelletier, S.W. Alkaloids of some mongolian Ranunculaceae species: detailed 1H and 13C-NMR studies of denudatine and lepenine. Heterocycles 1998, 49, 327–341. [Google Scholar] [CrossRef]
- Sudhakar, G.; Kadam, V.D.; Bayya, S.; Pranitha, G.; Jagadeesh, B. Total synthesis and stereochemical revision of acortatarins A and B. Org. Lett. 2011, 13, 5452–5455. [Google Scholar] [CrossRef]
- Lu, D.F.; Gong, Y.F.; Wang, W.Z. Prolylprolinol catalyzed asymmetric michael addition of aliphatic aldehydes to nitroalkenes. Adv. Synth. Catal. 2010, 352, 644–650. [Google Scholar] [CrossRef]
- Fadel, A.; Lahrache, N. An efficient synthesis of enantiomerically pure (R)-pipecolic acid, (S)-proline, And their N-alkylated derivatives. J. Org. Chem. 2007, 72, 1780–1784. [Google Scholar] [CrossRef]
- Fitzi, R.; Seebach, D. Resolution and use in α-amino acid synthesis of imidazolidinone glycine derivatives. Tetrahedron 1988, 44, 5277–5292. [Google Scholar] [CrossRef]
- Sample Availability: Not availbale.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, L.; Peng, C.; Xie, X.-F.; Guo, L.; He, C.-J.; Geng, Z.; Wan, F.; Dai, O.; Zhou, Q.-M. Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii. Molecules 2012, 17, 9939-9946. https://doi.org/10.3390/molecules17089939
Xiong L, Peng C, Xie X-F, Guo L, He C-J, Geng Z, Wan F, Dai O, Zhou Q-M. Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii. Molecules. 2012; 17(8):9939-9946. https://doi.org/10.3390/molecules17089939
Chicago/Turabian StyleXiong, Liang, Cheng Peng, Xiao-Fang Xie, Li Guo, Cheng-Jun He, Zhao Geng, Feng Wan, Ou Dai, and Qin-Mei Zhou. 2012. "Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii" Molecules 17, no. 8: 9939-9946. https://doi.org/10.3390/molecules17089939
APA StyleXiong, L., Peng, C., Xie, X.-F., Guo, L., He, C.-J., Geng, Z., Wan, F., Dai, O., & Zhou, Q.-M. (2012). Alkaloids Isolated from the Lateral Root of Aconitum carmichaelii. Molecules, 17(8), 9939-9946. https://doi.org/10.3390/molecules17089939




