Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia lanceolata
Abstract
:1. Introduction
2. Results
3. Discussion
4. Experimental
4.1. Plant Material
4.2. Sample Preparation
4.3. Animal
4.4. Drugs and Chemicals
4.5. HPLC Fingerprint Analysis
4.6. Acute Toxicity Test
4.7. Acetic Acid-Induced Writhing Test [12]
4.8. Hot Plate Latency Test [13]
4.9. Carrageenan Induced Paw Oedema Test [14,15]
4.9.1. Edema Inhibitory Activity
4.9.2. Content of COX-2 in Blood Serum
4.9.3. Content of PEG2 in Paw Edema Tissue
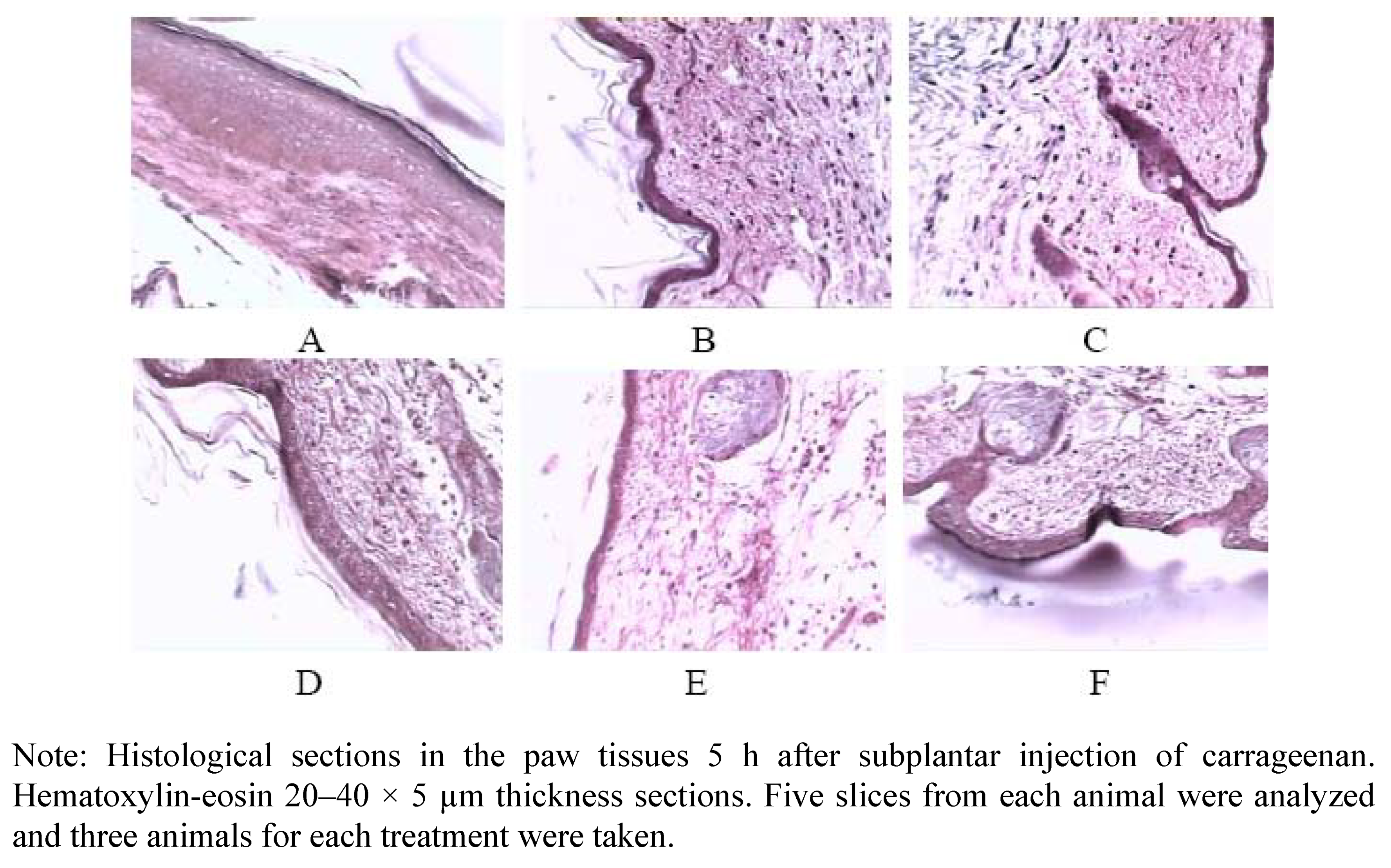
4.9.4. Histological Assessment
4.10. Formalin Test [16]
4.11. Statistical Analysis
5. Conclusions
References
- Nanjing New Medicine College. The Dictionary of Chinese Herbal Medicine; Shanghai Science and Technology Press: Shanghai, China, 1977. [Google Scholar]
- Ye, Z.; Lin, W.; Chen, W.; Yu, X. Chemical components and antimicrobial activity of essential oils in Cunninghamia lanceolata heartwood. Ying Yong Sheng Tai Xue Bao 2005, 16, 2394–2398. [Google Scholar] [PubMed]
- Cheng, S.S.; Chung, M.J.; Lin, C.Y.; Wang, Y.N.; Chang, S.T. Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J. Agric. Food. Chem. 2012, 60, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Liu, P.; Liu, J.P.; Xin, H.L.; Zhang, L.; Wang, Y.L.; Tang, K.X. Study on chemical constituents of the branches and leaves of Cunninghamia lanceolata. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2011, 29, 67–71. [Google Scholar]
- Zhang, M.; Liu, J.P.; Liu, J.; Liu, P.; Xin, H.L.; Zhang, L.; Wang, Y.L. Amentoflavone content of leaves and branches of Cunninghamia lanceolata from different origins. Pharm. Care. Res. 2011, 11, 149–150. [Google Scholar] [CrossRef]
- Santos, A.R.S.; Calixto, J.B. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides 1997, 31, 381–389. [Google Scholar] [CrossRef]
- Marrassini, C.; Acevedo, C.; Miño, J.; Ferraro, G.; Gorzalczany, S. Evaluation of antinociceptive, antiinfl-amatory activities and phytochemical analysis of aerial parts of Urtica urens L. Phytother. Res. 2010, 24, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Lorenzetti, B.B.; Castro, M.S.A.; Correa, F.M.A. Antialgic Effect of Aspirin-Like Drugs and the Inhibition of Prostaglandin Synthesis. In The Recognition of Anti-rheumatic Drugs; Dumonde, D.C., Jasani, M.K., Eds.; MTP Press Limited: Lancaster, UK, 1978; pp. 25–37. [Google Scholar]
- Gene, R.M.; Segura, L.; Adzet, T.; Marin, E.; Inglesias, J. Heterotheca inuloides: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 1998, 60, 157–162. [Google Scholar] [CrossRef]
- Panthong, A.; Kanjanapothi, D.; Taesotikul, T.; Wongcome, T.; Reutrakul, V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J. Ethnopharmacol. 2003, 85, 151–156. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: an evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Saha, A.; Ahmed, M. The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pak. J. Pharm. Sci. 2009, 22, 74–77. [Google Scholar] [PubMed]
- Franzotti, E.M.; Santos, C.V.; Rodrigues, H.M.; Mourão, R.H.; Andrade, M.R.; Antoniolli, A.R. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J. Ethnopharmacol. 2000, 72, 273–277. [Google Scholar] [CrossRef]
- Amresha, G.; Singh, P.N.; Rao, C.V. Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J. Ethnopharmacol. 2007, 111, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.B.; Damre, A.S.; Kulkarni, K.R.; Saraf, M.N. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine 2002, 9, 433–437. [Google Scholar]
- Santos, A.R.S.; Calixto, J.B. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides 1997, 31, 381–389. [Google Scholar] [CrossRef]
- Rajnarayana, K.; Sripal Reddy, M.; Chaluvadi, M.R. Bioflavanoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
Sample Availability: Samples of the compounds are available from the author Hai-Liang Xin. |
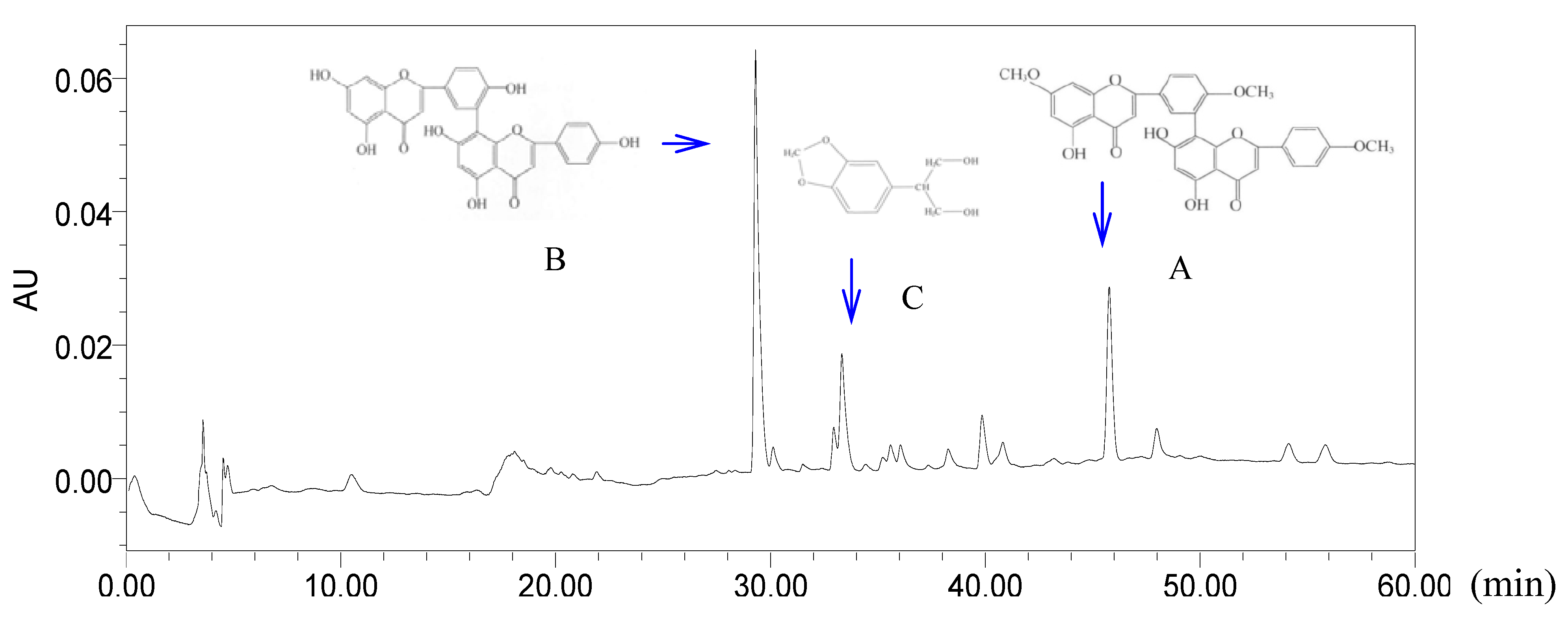
| Groups | Acetic acid-induced writhing (number of writhings) | Hot plate latency test (duration on the plate) | Formalin test | |
|---|---|---|---|---|
| Paw licking time in early phase (s) | Paw licking time in late phase (s) | |||
| Normal saline (10 mL/kg. o.p.) | 37.41 ± 4.91 | 14.36 ± 5.10 | 134.5 ± 28.3 | 171.4 ± 24.8 |
| TFC (100 mg/kg. o.p.) | 29.66 ± 5.02 * | 13.27 ± 4.80 | 117.6 ± 20.4 | 132.1 ± 37.5 * |
| TFC (200 mg/kg. o.p.) | 24.24 ± 3.81 ** | 15.31 ± 6.46 | 121.4 ± 18.5 | 110.3 ± 26.7 ** |
| TFC (400 mg/kg. o.p.) | 18.35 ± 4.54 ** | 18.39 ± 3.02 * | 108.5 ± 20.0 * | 96.2 ± 23.1 ** |
| ASA (100 mg/kg. o.p.) | 13.27 ± 5.49 ** | / | / | / |
| Morphine (5 mg/kg. s.c.) | / | 36.40 ± 1.69 ** | 19.3 ± 3.2 ** | 10.4 ± 8.3 ** |
| Group | Increase in paw size (edema) (mm) | Inhibition (%) | ||
|---|---|---|---|---|
| 3 h | 5 h | 3 h | 5 h | |
| Normal saline (10 mL/kg. o.p.) | 6.72 ± 0.34 | 4.80 ± 0.22 | / | / |
| TFC (100 mg/kg. o.p.) | 5.10 ± 0.21 ** | 3.49 ± 0.37 ** | 33.47 | 44.63 |
| TFC (200 mg/kg. o.p.) | 4.67 ± 0.42 ** | 3.04 ± 0.19 ** | 41.43 | 58.39 |
| TFC (400 mg/kg. o.p.) | 3.61 ± 0.36 * | 2.86 ± 0.24 ** | 73.27 | 62.75 |
| ASA (10 mg/kg. o.p.) | 3.06 ± 0.30 ** | 2.13 ± 0.16 ** | 62.45 | 87.92 |
| Group | Content (OD value/g) |
|---|---|
| Normal saline (10 mL/kg. o.p.) | 0.94 ± 0.12 |
| TFC (100 mg/kg. o.p.) | 0.47 ± 0.16 * |
| TFC (200 mg/kg. o.p.) | 0.31 ± 0.20 ** |
| TFC (400 mg/kg. o.p.) | 0.28 ± 0.11 ** |
| ASA (10 mg/kg. o.p.) | 0.21 ± 0.14 ** |
| Group | Content (pg/mL) |
|---|---|
| Normal saline (10 mL/kg. o.p.) | 874.64 ± 142.51 |
| TFC (100 mg/kg. o.p.) | 521.70 ± 193.65 * |
| TFC (200 mg/kg. o.p.) | 328.75 ± 123.27 ** |
| TFC (400 mg/kg. o.p.) | 275.91 ± 105.38 ** |
| ASA (10 mg/kg. o.p.) | 217.61 ± 149.64 ** |
© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xin, H.-L.; Zhai, X.-F.; Zheng, X.; Zhang, L.; Wang, Y.-L.; Wang, Z. Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia lanceolata. Molecules 2012, 17, 8842-8850. https://doi.org/10.3390/molecules17088842
Xin H-L, Zhai X-F, Zheng X, Zhang L, Wang Y-L, Wang Z. Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia lanceolata. Molecules. 2012; 17(8):8842-8850. https://doi.org/10.3390/molecules17088842
Chicago/Turabian StyleXin, Hai-Liang, Xiao-Feng Zhai, Xu Zheng, Lei Zhang, Yu-Liang Wang, and Zhuo Wang. 2012. "Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia lanceolata" Molecules 17, no. 8: 8842-8850. https://doi.org/10.3390/molecules17088842
APA StyleXin, H.-L., Zhai, X.-F., Zheng, X., Zhang, L., Wang, Y.-L., & Wang, Z. (2012). Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia lanceolata. Molecules, 17(8), 8842-8850. https://doi.org/10.3390/molecules17088842




