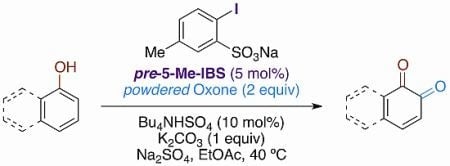
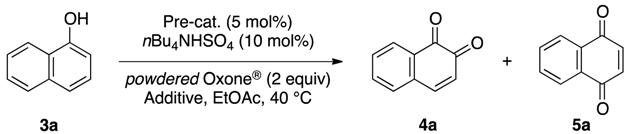
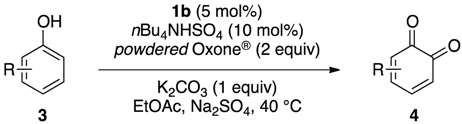IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone®
Abstract
:1. Introduction
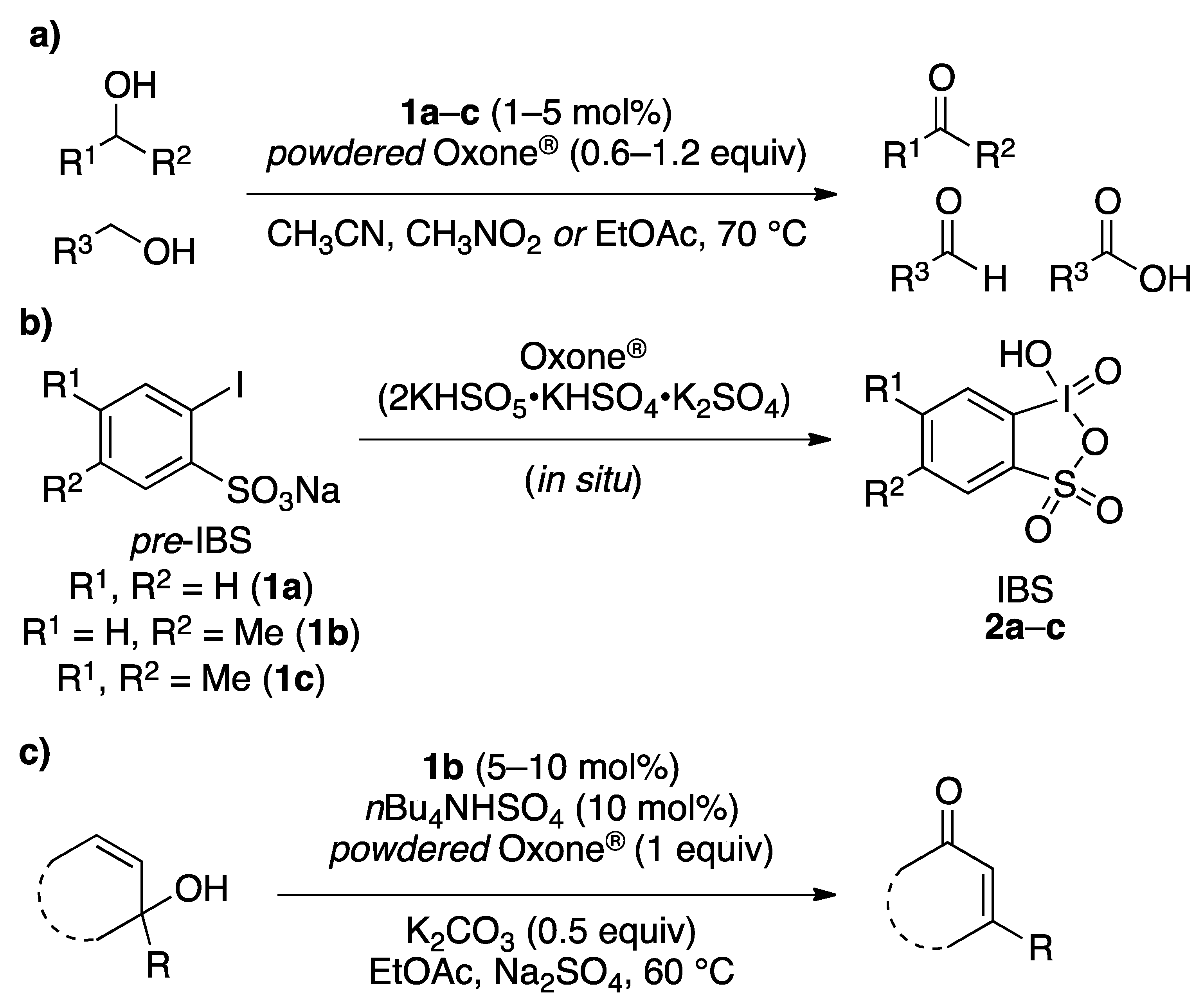
2. Results and Discussion
| Entry | Pre-cat. | Additive (equiv.) | Time (h) | 4a, Yield (%) a | 5a, Yield (%) a |
|---|---|---|---|---|---|
| 1 | – | – | 24 | trace b | trace b |
| 2 | PhI | – | 24 | trace b | trace b |
| 3 | 6 f | – | 24 | trace b | trace b |
| 4 | 7 g | – | 24 | 5 b | 5 b |
| 5 | 1a | – | 11 | 64 | 5 |
| 6 | 1b | – | 8 | 69 | 6 |
| 7 | 1c | – | 9 | 67 | 6 |
| 8 c | 1b | – | 3.5 | trace b | 51 |
| 9 d | 1b | K2CO3 (1) | 1 | 78 | 6 |
| 10 e | 1b | K2CO3 (1) | 24 | trace b | trace b |
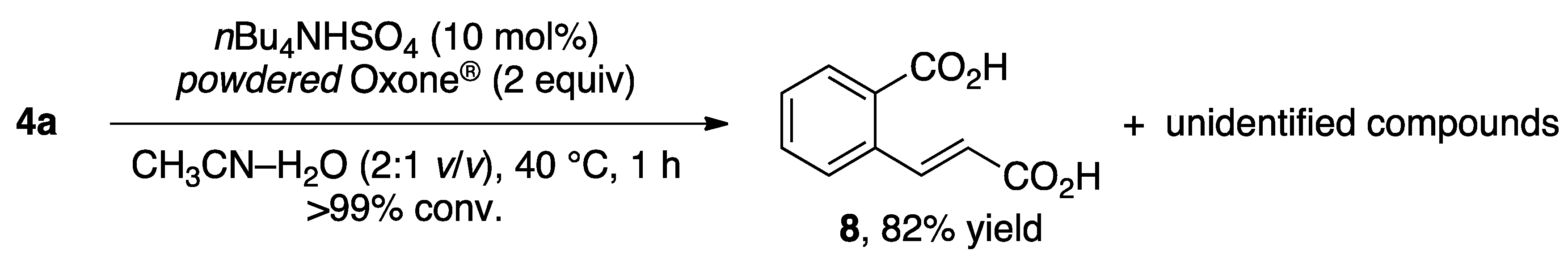
| Entry | 3 | 4 | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | 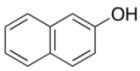 | 4a | 4 | 84 |
| 3b | ||||
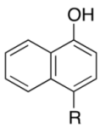 | 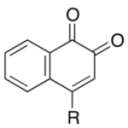 | |||
| 2 | 3c (R = Cl) | 4c | 5 | 80 |
| 3 | 3d (R = Br) | 4d | 3 | 75 |
| 4 | 3e (R = OMe) | 4e | 2 | 50 c |
| 5 | 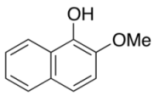 | 4a | 2 | 72 |
| 3f | ||||
| 6 | 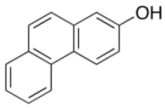 | 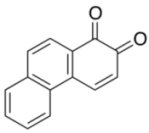 | 2 | 90 |
| 3g | 4g | |||
| 7 | 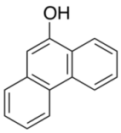 |  | 2 | 97 |
| 3h | 4h | |||
| 8 | 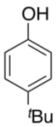 |  | 5 | 63 |
| 3i | 4i | |||
| 9 |  | 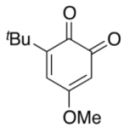 | 5 | 66 d |
| 3j | 4j | |||
| 10 | 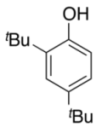 |  | 24 | 73 |
| 3k | 4k |
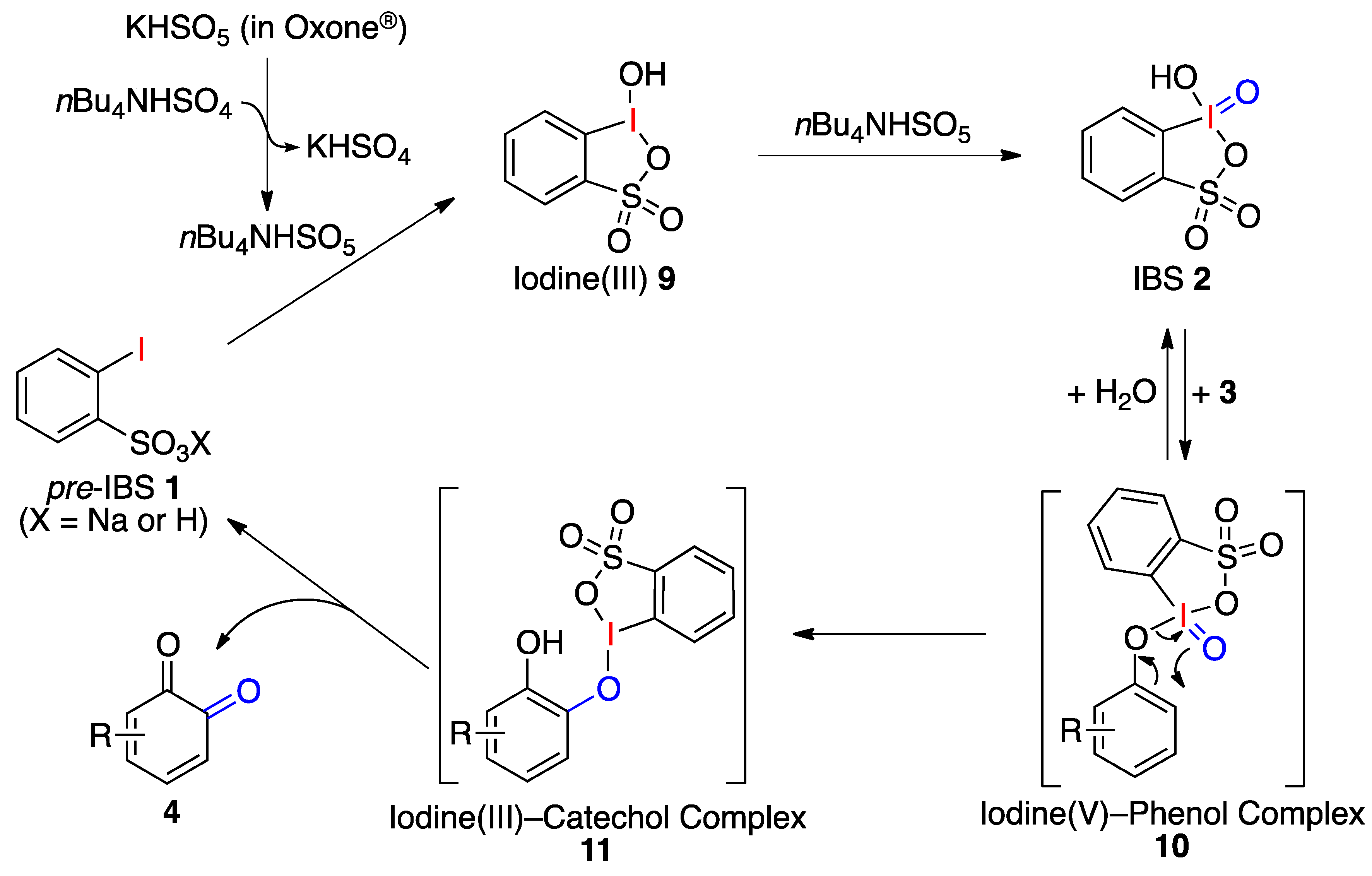
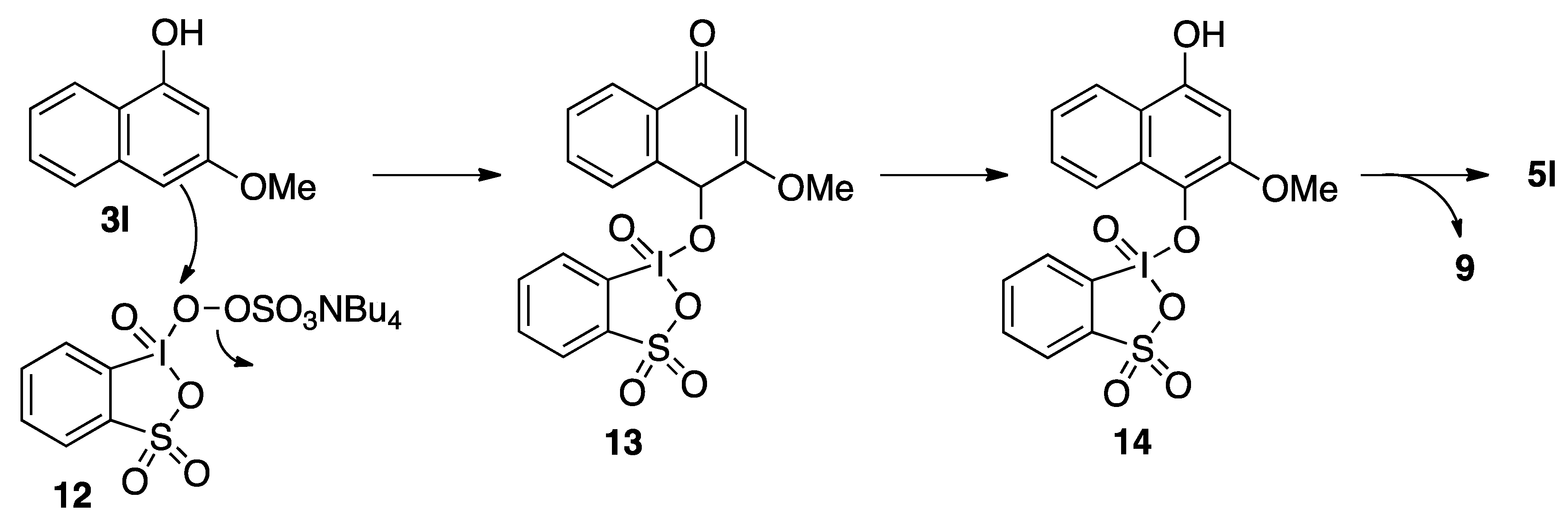
3. Experimental
3.1. General
3.2. General Procedure for the Oxidation Phenol to Quinone
4. Conclusions
Acknowledgments
References
- Quideau, S.; Pouységu, L. Synthetic uses of orthoquinonemonoketals and their orthoquinols variants. A review. Org. Prep. Proced. Int. 1999, 31, 617–680. [Google Scholar] [CrossRef]
- Scott, J.D.; Williams, R.M. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 2002, 102, 1669–1730. [Google Scholar]
- Magdziak, D.; Meek, S.J.; Pettus, T.R.R. Cyclohexadienoneketals and quinols: Four building blocks potentially useful for enantioselective synthesis. Chem. Rev. 2004, 104, 1383–1430. [Google Scholar]
- Pouységu, L.; Deffieux, D.; Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 2010, 66, 2235–2261. [Google Scholar]
- Danishefsky, S.J.; Mazza, S.; McCurry, P. Diels-Alder reactions of o-benzoquinones. J. Org. Chem. 1974, 39, 3610–3611. [Google Scholar]
- Omote, Y.; Tomotake, A.; Kashima, C. Reaction of 1,2-benzoquinones with enamines. J. Chem. Soc. Perkin Trans. 1 1988, 2, 151–156. [Google Scholar]
- Nair, V.; Maliakal, D.; Treesa, P.M.; Rath, N.P.; Eigendorf, G.K. [4+2] Cycloaddition reactions of o-benzoquinones with styrenes: A facile synthesis of bicyclo[2.2.2]octenediones. Synthesis 2000, 850–856. [Google Scholar]
- Stavber, S.; Zupan, M. The effect of heteroatoms on the reactions of organic molecules with cesium fluoroxysulphate. Tetrahedron 1992, 48, 5875–5882. [Google Scholar] [CrossRef]
- Osman, F.H.; Abd El-Rahman, N.M.; El-Samahy, F.A. Reaction of phosphonium ylides with 4-triphenylmethyl-1,2-benzoquinone. Tetrahedron 1993, 49, 8691–8704. [Google Scholar]
- Viallon, L.; Reinaud, O.; Capdevielle, P.; Maumy, M. Synthesis of new 4-alkylamino-5-methoxy-2H-pyran-2-ones. Tetrahedron Lett. 1995, 36, 6669–6672. [Google Scholar]
- Takuwa, A.; Kai, R.; Kawasaki, K.I.; Nishigaichi, Y.; Iwamoto, H. New formal [3+2] photoaddition of vinyl ethers to o-benzoquinones. J. Chem. Soc. Chem. Commun. 1996, 703–704. [Google Scholar]
- Dudfield, P.J. Synthesis of quinones. In Comprehensive Organic Synthesis; Trost, B.M., Flemming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume 7, pp. 345–356. [Google Scholar]
- Gallagher, P.T. The synthesis of quinones. Contemp. Org. Synth. 1996, 3, 433–446. [Google Scholar]
- Akai, S.; Kita, Y. Review of recent progress in the synthesis of p-quinones and p-dihydroquinones through oxidation of phenol derivatives. Org. Prep. Proced. Int. 1998, 30, 603–629. [Google Scholar]
- Deya, P.M.; Dopico, M.; Raso, A.G.; Morey, J.; Saa, J.M. On the regioselectivity of the Fremy’s salt oxidation of phenols. Tetrahedron 1987, 43, 3523–3532. [Google Scholar]
- Saladino, R.; Neri, V.; Mincione, E.; Marini, S.; Coletta, M.; Fiorucci, C.; Filippone, P. A new and efficient synthesis of ortho- and para-benzoquinones of cardanol derivatives by the catalytic system MeReO3-H2O2. J. Chem. Soc. Perkin Trans. 1 2000, 4, 581–586. [Google Scholar]
- Crandall, J.K.; Zucco, M.; Kirsch, R.S.; Coppert, D.M. The formation of orthoquinones in the dimethyldioxirane oxidation of phenols. Tetrahedron Lett. 1991, 32, 5441–5444. [Google Scholar]
- Barton, D.H.R.; Finet, J.P.; Thomas, M. Comparative oxidation of phenols with benzeneseleninic anhydride and with benzeneseleninic acid. Tetrahedron 1988, 44, 6397–6406. [Google Scholar]
- Magdziak, D.; Rodriguez, A.A.; van de Water, R.W.; Pettus, T.R.R. Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid (IBX). Org. Lett. 2002, 4, 285–288. [Google Scholar]
- Pezzella, A.; Lista, L.; Napolitano, A.; d’Ischia, M. An expedient one-pot entry to catecholestrogens and other catechol compounds via IBX-mediated phenolic oxygenation. Tetrahedron Lett. 2005, 46, 3541–3544. [Google Scholar]
- Bernini, R.; Crisante, F.; Barontini, M.; Fabrizi, G. A new and efficient route for the synthesis of naturally occurring catecholamines. Synthesis 2009, 2009, 3838–3842. [Google Scholar]
- Bernini, R.; Mincione, E.; Crisante, F.; Barontini, M.; Fabrizi, G. A novel use of the recyclable polymer-supported IBX: An efficient chemoselective and regioselective oxidation of phenolic compounds. The case of hydroxytyrosol derivatives. Tetrahedron Lett. 2009, 50, 1307–1310. [Google Scholar]
- Barontini, M.; Bernini, R.; Crisante, F.; Fabrizi, G. Selective and efficient oxidative modifications of flavonoids with 2-iodoxybenzoic acid (IBX). Tetrahedron 2010, 66, 6047–6053. [Google Scholar]
- Wu, A.; Duan, Y.; Xu, D.; Penning, T.M.; Harvey, R.G. Regiospecific oxidation of polycyclic aromatic phenols to quinones by hypervalent iodine reagents. Tetrahedron 2010, 66, 2111–2118. [Google Scholar]
- Richardson, R.D.; Wirth, T. Hypervalent iodine goes catalytic. Angew. Chem. Int. Ed. Engl. 2006, 45, 4402–4404. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Stang, P.J. Chemistry of polyvalent iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef]
- Ochiai, M.; Miyamoto, K. Catalytic version of and reuse in hypervalentorgano-λ3- and -λ5-iodane oxidation. Eur. J. Org. Chem. 2008, 2008, 4229–4239. [Google Scholar] [CrossRef]
- Dohi, T.; Kita, Y. Hypervalent iodine reagents as a new entrance to organocatalysts. Chem. Commun. 2009, 2009, 2073–2085. [Google Scholar] [CrossRef]
- Uyanik, M.; Ishihara, K. Catalysis with insitu-generated (hypo)iodite ions for oxidative coupling reactions. ChemCatChem 2012, 4, 177–185. [Google Scholar] [CrossRef]
- Yakura, T.; Konishi, T. A novel catalytic hypervalent iodine oxidation of p-alkoxyphenols to p-quinones using 4-iodophenoxyacetic acid and Oxone®. Synlett 2007, 2007, 765–768. [Google Scholar] [CrossRef]
- Yakura, T.; Yamauchi, Y.; Tian, Y.; Omoto, M. Catalytic hypervalent iodine oxidation of p-dialkoxybenzenes to p-quinones using 4-iodophenoxyacetic acid and Oxone®. Chem. Pharm. Bull. 2008, 56, 1632–1634. [Google Scholar]
- Yakura, T.; Tian, Y.; Yamauchi, Y.; Omoto, M.; Konishi, T. Catalytic hypervalent iodine oxidation using 4-iodophenoxyacetic acid and Oxone®: Oxidation of p-alkoxyphenols to p-benzoquinones. Chem. Pharm. Bull. 2009, 57, 252–256. [Google Scholar]
- Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzenesulfonic acid as an extremely active catalyst for the selective oxidation of alcohols to aldehydes, ketones, carboxylic acids, and enones with Oxone®. J. Am. Chem. Soc. 2009, 131, 251–262. [Google Scholar] [CrossRef]
- Uyanik, M.; Ishihara, K. Hypervalent iodine-mediated oxidation of alcohols. Chem. Commun. 2009, 2009, 2086–2099. [Google Scholar] [CrossRef]
- Uyanik, M.; Ishihara, K. 2-Iodoxybenzenesulfonic acid (IBS) catalyzed oxidation of alcohols. Aldrichim. Acta 2010, 43, 83–91. [Google Scholar]
- Uyanik, M.; Ishihara, K. 2-Iodoxy-5-methylbenzenesulfonic acid-catalyzed selective oxidation of 4-bromobenzyl alcohol to 4-bromobenzaldehyde or 4-bromobenzoic acid with Oxone®. Org. Syn. 2012, 89, 105–114. [Google Scholar]
- Uyanik, M.; Fukatsu, R.; Ishihara, K. IBS-catalyzed oxidative rearrangement of tertiary allylic alcohols to enones with Oxone®. Org. Lett. 2009, 11, 3470–3473. [Google Scholar]
- Cui, L.C.; Liu, K.; Zhang, C. Effective oxidation of benzylic and alkane C-H bonds catalyzed by sodium o-iodobenzenesulfonate with Oxone® as a terminal oxidant under phase-transfer conditions. Org. Biomol. Chem. 2011, 9, 2258–2265. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ishihara, T.; Konno, T. A new entry for the oxidation of fluoroalkyl-substituted methanol derivatives: Scope and limitation of the organoiodine(V) reagent-catalyzed oxidation. J. Fluor. Chem. 2012, 137, 99–104. [Google Scholar] [CrossRef]
- Giurg, M.; Syper, L.; Mlochowski, J. Hydrogen peroxide oxidation of naphthalene derivatives catalyzed by poly(bis-1,2-diphenylene) diselenide. Pol. J. Chem. 2004, 78, 231–238. [Google Scholar]
- Carreño, M.C.; Gonzáles-López, M.; Urbano, A. Oxidative de-aromatization of para-alkyl phenols into para-peroxyquinols and para-quinols mediated by Oxone® as a source of singlet oxygen. Angew. Chem. Int. Ed. Engl. 2006, 45, 2737–2741. [Google Scholar]
- Ojha, L.R.; Kudugunti, S.; Maddukuri, P.P.; Kommareddy, A.; Gunna, M.R.; Dokuparthi, P.; Gottam, H.B.; Botha, K.K.; Parapati, D.R.; Vinod, T.K. Benzylic carbon oxidation by an in situ formed o-iodoxybenzoic acid (IBX) derivative. Synlett 2009, 2009, 117. [Google Scholar]
- Ding, Z.; Xue, S.; Wulff, W.D. A succinct synthesis of the vaulted biarylkigandvanol via a dienone-phenol rearrangement. Chem. Asian J. 2011, 6, 2130–2146. [Google Scholar]
- Ludwik, S. The Baeyer-Villiger oxidation of aromatic aldehydes and ketones with hydrogen peroxide catalyzed by selenium compounds. A convenient method for the preparation of phenols. Synthesis 1989, 1989, 167–172. [Google Scholar] [CrossRef]
- Bell, K.H.; McCaffery, L.F. Regioselectivemonomethylation of unsymmetrical naphthalenediols with methanolic hydrogen chloride. Aust. J. Chem. 1993, 46, 731–737. [Google Scholar] [CrossRef]
- Crich, D.; Zou, Y. Catalytic oxidation adjacent to carbonyl groups and at benzylic positions with a fluorousseleninic acid in the presence of iodoxybenzene. J. Org. Chem. 2005, 70, 3309–3311. [Google Scholar] [CrossRef]
- Suchard, O.; Kane, R.; Roe, B.J.; Zimmermann, E.; Jung, C.; Waske, P.A.; Mattay, J.; Oelgemöller, M. Photooxygenations of 1-naphthols: An environmentally friendly access to 1,4-naphthoquinones. Tetrahedron 2006, 62, 1467–1473. [Google Scholar]
- Perumal, P.T.; Bhatt, M.V. Oxidation of halophenols and highly substituted phenols with lead(IV) acetate. Synthesis 1980, 1980, 943–945. [Google Scholar] [CrossRef]
- Ogata, T.; Okamoto, I.; Kotani, E.; Takeya, T. Biomimetic synthesis of the dinaphthofuranquinone violet-quinone, utilizing oxidative dimerization with the ZrO2/O2 system. Tetrahedron 2004, 60, 3941–3948. [Google Scholar] [CrossRef]
- Bernd, P.; Meike, N.; Anja, P. The acid accelerated ruthenium-catalyzed dihydroxylation. Scope and limitations. Org. Biomol. Chem. 2004, 2, 1116–1124. [Google Scholar]
- Ratnikov, M.O.; Farkas, L.E.; McLaughlin, E.C.; Chiou, G.; Choi, H.; El-Khalafy, S.H.; Doyle, M.P. Dirhodium-catalyzed phenol and aniline oxidations with T-HYDRO. Substrate scope and mechanism of oxidation. J. Org. Chem. 2011, 76, 2585–2593. [Google Scholar]
- Miyamura, H.; Shiramizu, M.; Matsubara, R.; Kobayashi, S. Aerobic Oxidation of Hydroquinone Derivatives Catalyzed by Polymer-Incarcerated Platinum Catalyst. Angew. Chem. Int. Ed. Engl. 2008, 47, 8093–8095. [Google Scholar] [CrossRef]
- Lebrasseur, N.; Fan, G.J.; Oxoby, M.; Looney, M.A.; Quideau, S. λ3-Iodane-mediated arenol dearomatization. Synthesis of five-membered ring-containing analogues of the aquayamycin ABC tricyclic unit and novel access to the apoptosis inducer menadione. Tetrahedron 2005, 61, 1551–1562. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1a–c are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Uyanik, M.; Mutsuga, T.; Ishihara, K. IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone®. Molecules 2012, 17, 8604-8616. https://doi.org/10.3390/molecules17078604
Uyanik M, Mutsuga T, Ishihara K. IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone®. Molecules. 2012; 17(7):8604-8616. https://doi.org/10.3390/molecules17078604
Chicago/Turabian StyleUyanik, Muhammet, Tatsuya Mutsuga, and Kazuaki Ishihara. 2012. "IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone®" Molecules 17, no. 7: 8604-8616. https://doi.org/10.3390/molecules17078604
APA StyleUyanik, M., Mutsuga, T., & Ishihara, K. (2012). IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone®. Molecules, 17(7), 8604-8616. https://doi.org/10.3390/molecules17078604