Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats
Abstract
:1. Introduction
2. Result
| Group | Tumour weight (g) | Tumour size (mm3) | Inhibition rate (%) |
|---|---|---|---|
| NC | - | - | - |
| HCC | 0.28 ± 0.02 | 315.2 ± 19.5 | - |
| HCC + β-carotene (20 mg/kg b.w.) | 0.21 ± 0.03 c | 291.5 ± 22.4 c | 8.3 |
| HCC + β-carotene (40 mg/kg b.w.) | 0.16 ± 0.02 d | 238.4 ± 15.9 d | 23.6 |
| HCC + β-carotene (60 mg/kg b.w.) | 0.09 ± 0.01 d | 217.1 ± 5.7 d | 31.7 |
| Group | NK (100%) | IL-2 (ng/mL) | TNF-α (ng/mL) |
|---|---|---|---|
| NC | 57.21 ± 4.73 | 4.14 ± 3.28 | 1.21 ± 0.11 |
| HCC | 27.63 ± 2.59 b | 1.38 ± 1.53 b | 2.05 ± 0.18 b |
| HCC + β-carotene (20 mg/kg b.w.) | 36.82 ± 1.84 c | 1.99 ± 1.59 | 2.22 ± 0.15 |
| HCC + β-carotene (40 mg/kg b.w.) | 41.27 ± 3.99 d | 2.62 ± 2.22 d | 2.39 ± 0.13 c |
| HCC + β-carotene (60 mg/kg b.w.) | 50.52 ± 4.18 d | 3.25 ± 2.51 d | 2.56 ± 0.11 d |
| Group | WBC (×109/L) | TP (g/L) | ALB (g/L) | A/G |
|---|---|---|---|---|
| NC | 29.06 ± 1.84 | 70.41 ± 8.34 | 35.86 ± 4.01 | 1.83 ± 1.57 |
| HCC | 17.94 ± 1.48 b | 54.49 ± 3.97 b | 31.21 ± 3.29 b | 1.21 ± 1.11 b |
| HCC + β-carotene (20 mg/kg b.w.) | 20.14 ± 1.77 c | 55.93 ± 6.11 | 33.71 ± 3.33 | 1.31 ± 1.21 c |
| HCC + β-carotene (40 mg/kg b.w.) | 22.64 ± 1.83 c | 59.17 ± 6.52 c | 35.03 ± 2.98 c | 1.52 ± 1.44 d |
| HCC + β-carotene (60 mg/kg b.w.) | 24.03 ± 2.06 d | 63.11 ± 5.98 d | 35.92 ± 3.76 c | 1.69 ± 1.63 d |
| Group | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|
| NC | 48.68 ± 4.22 | 189.53 ± 12.87 | 77.17 ± 8.63 |
| HCC | 92.47 ± 7.83 b | 304.15 ± 29.74 b | 128.49 ± 13.82 b |
| HCC + β-carotene (20 mg/kg b.w.) | 82.49 ± 7.77 c | 287.36 ± 30.11 c | 115.39 ± 13.08 |
| HCC + β-carotene (40 mg/kg b.w.) | 73.58 ± 7.39 d | 254.92 ± 22.86 d | 97.47 ± 8.39 d |
| HCC + β-carotene (60 mg/kg b.w.) | 59.73 ± 4.92 d | 203.17 ± 23.13 d | 80.43 ± 7.29 d |
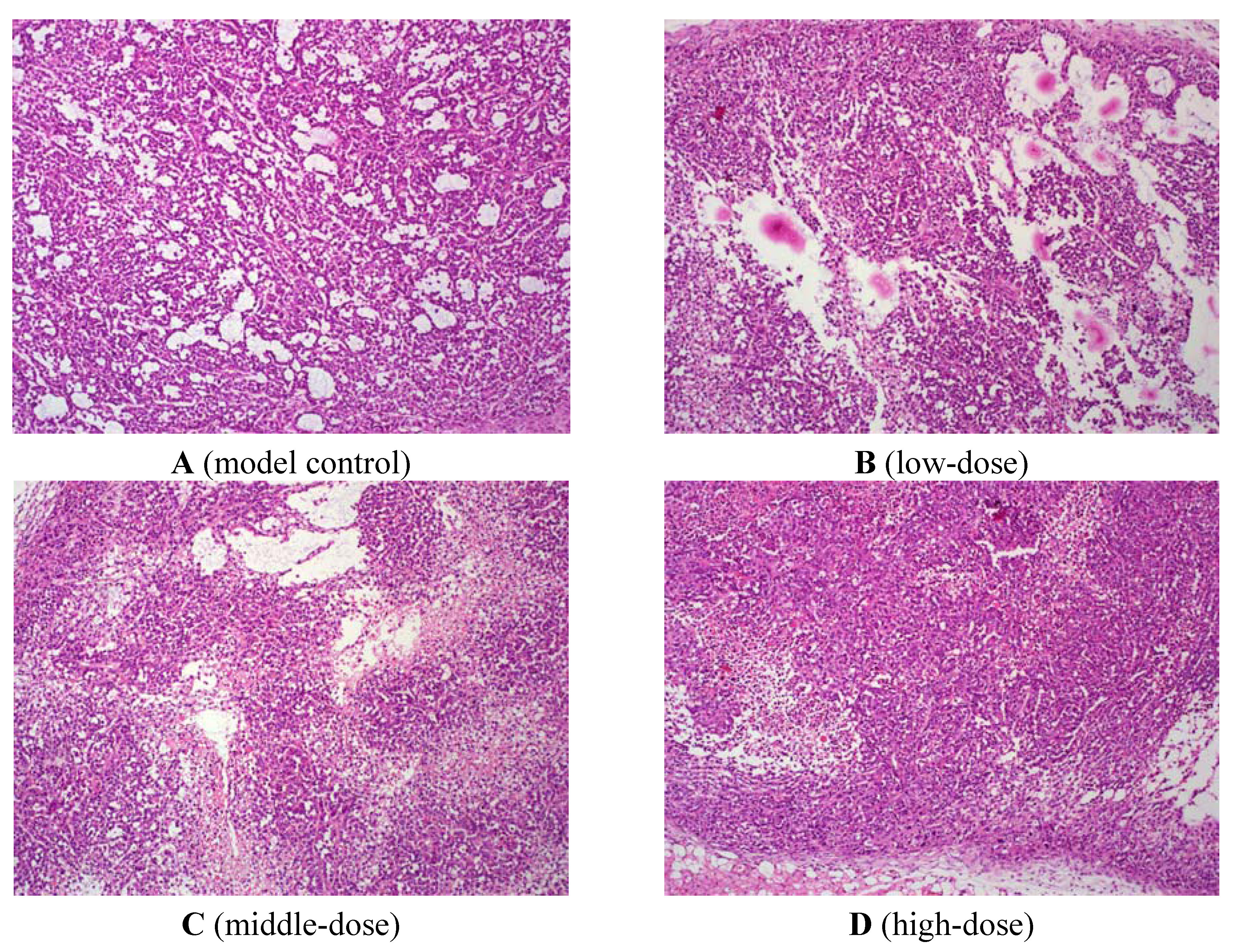
3. Discussion
4. Experimental
4.1. Rats and Cell Lines
4.2. Animal Grouping and Treating
4.3. Biochemical Analysis
4.4. Histological Evaluation
4.5. Statistical Analysis
5. Conclusion
References
- Kuroishi, T.; Hayakawa, N.; Kurihara, M.; Aoki, K. Cancer mortality in 33 countries of the world (1953–1987). In Cancer Mortality and Morbidity Statistics (Japan and the World 1994); Tominaga, S., Fujimoto, I., Kuraihara, M., Eds.; Japan Science Society Press: Tokyo, Japan, 1994; pp. 167–230. [Google Scholar]
- Li, L.D.; Zhang, S.W.; Lu, F.Z.; Mu, R.; Zhang, X.D.; Huang-Pu, X.M. Research on characteristics of mortality spectrum and type composition of malignant tumors in China (in Chinese). Chin. J. Oncol. 1997, 19, 323–328. [Google Scholar]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. Royal Soc. 2010, 7, 3–18. [Google Scholar]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2010, 16, 1126–1132. [Google Scholar]
- Koehn, B.H.; Schoenberger, S.P. Tumor immunotherapy: Making an immortal army. Nat. Med. 2009, 15, 731–732. [Google Scholar]
- Fridlender, Z.G.; Buchlis, G.; Kapoor, V.; Cheng, G.; Sun, J.; Singhal, S.; Cecilia Crisanti, M.; Wang, L.-C.S.; Heitjan, D.; Snyder, L.A.; et al. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010, 70, 109–118. [Google Scholar]
- Sharma, S.V.; Settleman, J. Oncogene addiction: Setting the stage for molecularly targeted cancer therapy. Gene. Dev. 2007, 21, 3214–3231. [Google Scholar] [CrossRef]
- Cross, D.; Burmester, J.K. Gene therapy for cancer treatment: Past, present and future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar]
- Kesavan, K.; Ratliff, J.; Johnson, E.W.; Dahlberg, W.; Asara, J.M.; Misra, P.; Frangioni, J.V.; Jacoby, D.B. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and antiangiogenic effects. J. Biol. Chem. 2010, 285, 4366–4374. [Google Scholar]
- Gerster, H. Anticarcinogenic effect of common carotenoids. Int. J. Vit. Nutr. Res. 1993, 63, 93–121. [Google Scholar]
- Paolini, M.; Abdel-Rahman, S.Z.; Sapone, A.; Pedulli, G.F.; Perocco, P.; Cantelli-Forti, G.; Legator, M.S. Beta-carotene: A cancer chemopreventive agent or a co-carcinogen? Mutat. Res. 2003, 543, 195–200. [Google Scholar] [CrossRef]
- Willett, W.C.; Polk, B.F.; Underwood, B.A.; Stampfer, M.J.; Pressel, S.; Rosner, B.; Taylor, J.O.; Schneider, K.; Hames, C.G. Relation of serum Vitamins A and E and carotenoids to the risk of cancer. N. Engl. J. Med. 1984, 310, 430–434. [Google Scholar] [CrossRef]
- Saffiotti, U.; Montesano, R.; Sellakumar, A.R.; Borg, S.A. Experimental cancer of the lung: Inhibition by Vitamin A of the induction of tracheobronchial and squamousmetaplasma and squamous cell tumors. Cancer 1967, 20, 857–864. [Google Scholar]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary β-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar]
- Alpha Tocopherol Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [CrossRef]
- Omenn, G.S.; Googman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A. Effect of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Van Poppel, G. Carotenoids and cancer: An update with emphasis on human intervention studies. Eur. J. Cancer 1993, 29, 1335–1344. [Google Scholar] [CrossRef]
- Temple, N.J.; Basu, T.K. Protective effects of β-carotene against colon tumours in mice. J. Natl. Cancer Inst. 1987, 78, 1211–1214. [Google Scholar]
- Alam, B.S.; Alam, S.Q. The effect of different levels of dietary β-carotene on DMBA induced salivary gland tumours. Nutr. Cancer 1987, 9, 93–101. [Google Scholar] [CrossRef]
- Suda, D.; Schwartz, J.; Shklar, G. Inhibition of experimental oral carcinogenesis by topical beta-carotene. Carcinogenesis 1986, 7, 711–715. [Google Scholar] [CrossRef]
- Parkin, D.M. Global cancer statistics in the year. Lancet Oncol. 2000, 2, 533–543. [Google Scholar] [CrossRef]
- Tarantino, G.; Saldalamacchia, G.; Conca, P.; Arena, A. Nonalcoholic fatty liver disease: Further expression of the metabolic syndrome. J. Gastroenterol. Hepatol. 2007, 22, 293–303. [Google Scholar]
- Al-Harthi, O.M.; Halawani, E.M.; Abdelkader, H.S. Detection of Salmonella strains in clinical samples from Saudi Arabia by invA and hilA polymerase chain reaction (PCR)-based assays. Afr. J. Microbiol. Res. 2012, 6, 5410–5416. [Google Scholar]
- Adebiyi, O.A.; Adebiyi, O.O.; Ilesanmi, O.R.; Adu, T. Anxiolytic and anti-nociceptive activity of hydroalcholic leaf extract of Cnidoscolous acontifolius. Afr. J. Pharm. Pharmacol. 2012, 6, 1765–1769. [Google Scholar]
- Gassar, S.; Raulet, D.H. Activation and self-tolerance of natural killer cells. Immunol. Rev. 2006, 214, 130–142. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, T. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar]
- Habu, S.; Akamine, K.; Tamaoki, N.; Okumura, K. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J. Immunol. 1984, 133, 2743–2747. [Google Scholar]
- Rager-Zisman, B.; Quan, P.C.; Rosner, M.; Moller, J.R.; Brooks, A.G. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 1987, 138, 884–888. [Google Scholar]
- Rahimi, P.; Kabiri, N.; Asgary, S.; Setorki, M. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2655–2661. [Google Scholar]
- Wipusaree, N.; Sihanonth, P.; Piapukiew, J.; Sangvanich, P.; Karnchanatat, A. Purification and characterization of a xylanase from the endophytic fungus Alternaria alternata isolated from the Thai medicinal plant, Croton oblongifolius Roxb. Afr. J. Microbiol. Res. 2011, 5, 5697–5712. [Google Scholar]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Tsai, T.-H.; Wu, W.-H.; Tseng, T.-P.; Tsai, P.-J. In vitro antimicrobial and anti-inflammatory effects of herbs against Propionibacterium acnes. Food Chem. 2010, 119, 964–968. [Google Scholar]
- Mosa, R.A.; Oyedeji, A.O.; Shode, F.O.; Singh, M.; Opoku, A.R. Triterpenes from the stem bark of Protorhus longifolia exhibit anti-platelet aggregation activity. Afr. J. Pharm. Pharmacol. 2011, 5, 2698–2714. [Google Scholar]
- Finn, O.J. Cancer immunology. N. Engl. J. Med. 2008, 358, 2704–2715. [Google Scholar] [CrossRef]
- Guha Majumdar, D.N. Effect of chronic intake of arsenic contaminated water on liver. Toxicol. Appl. Pharmacol. 2005, 206, 169–175. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Watkin, J.B. Mechanism of formation, hepatic uptake and biliary excretion. Pharmacol. Rev. 1984, 36, 1–67. [Google Scholar]
- Yousef, I.M.; El-Demerdash, M.M.; Radwan, F.M.E. Sodium arsenite induced biochemical perturbations in rats: Ameliorating effect of curcumin. Food Chem. Toxicol. 2008, 48, 3506–3511. [Google Scholar]
- El-Demerdash, F.M.; Yousef, M.I.; Radwan, F.M.E. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem. Toxicol. 2009, 47, 249–254. [Google Scholar] [CrossRef]
- Reynolds, C.W.; Herberman, R.B. In vitro augmentation of rat natural killer (NK) cell activity. J. Immunol. 1981, 126, 1581–1585. [Google Scholar]
- Sample Availability: Samples of the β-carotene are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, B.; Liu, S.; Wang, Q.; Lin, X. Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats. Molecules 2012, 17, 8595-8603. https://doi.org/10.3390/molecules17078595
Cui B, Liu S, Wang Q, Lin X. Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats. Molecules. 2012; 17(7):8595-8603. https://doi.org/10.3390/molecules17078595
Chicago/Turabian StyleCui, Bokang, Su Liu, Qibo Wang, and Xiaojun Lin. 2012. "Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats" Molecules 17, no. 7: 8595-8603. https://doi.org/10.3390/molecules17078595
APA StyleCui, B., Liu, S., Wang, Q., & Lin, X. (2012). Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats. Molecules, 17(7), 8595-8603. https://doi.org/10.3390/molecules17078595




