Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane
Abstract
:1. Introduction
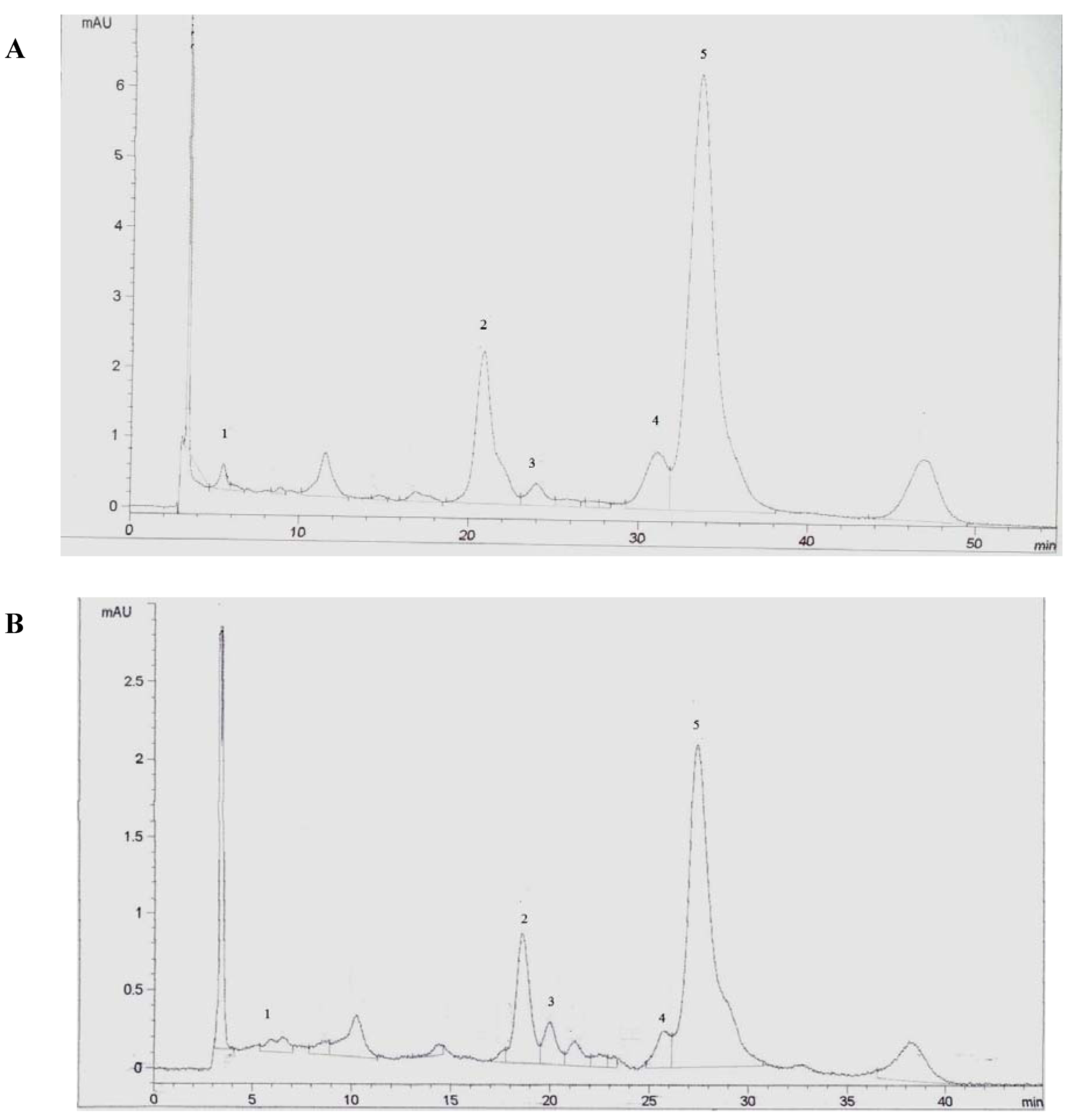
2. Results and Discussion
2.1. Effect of Matrix Composition
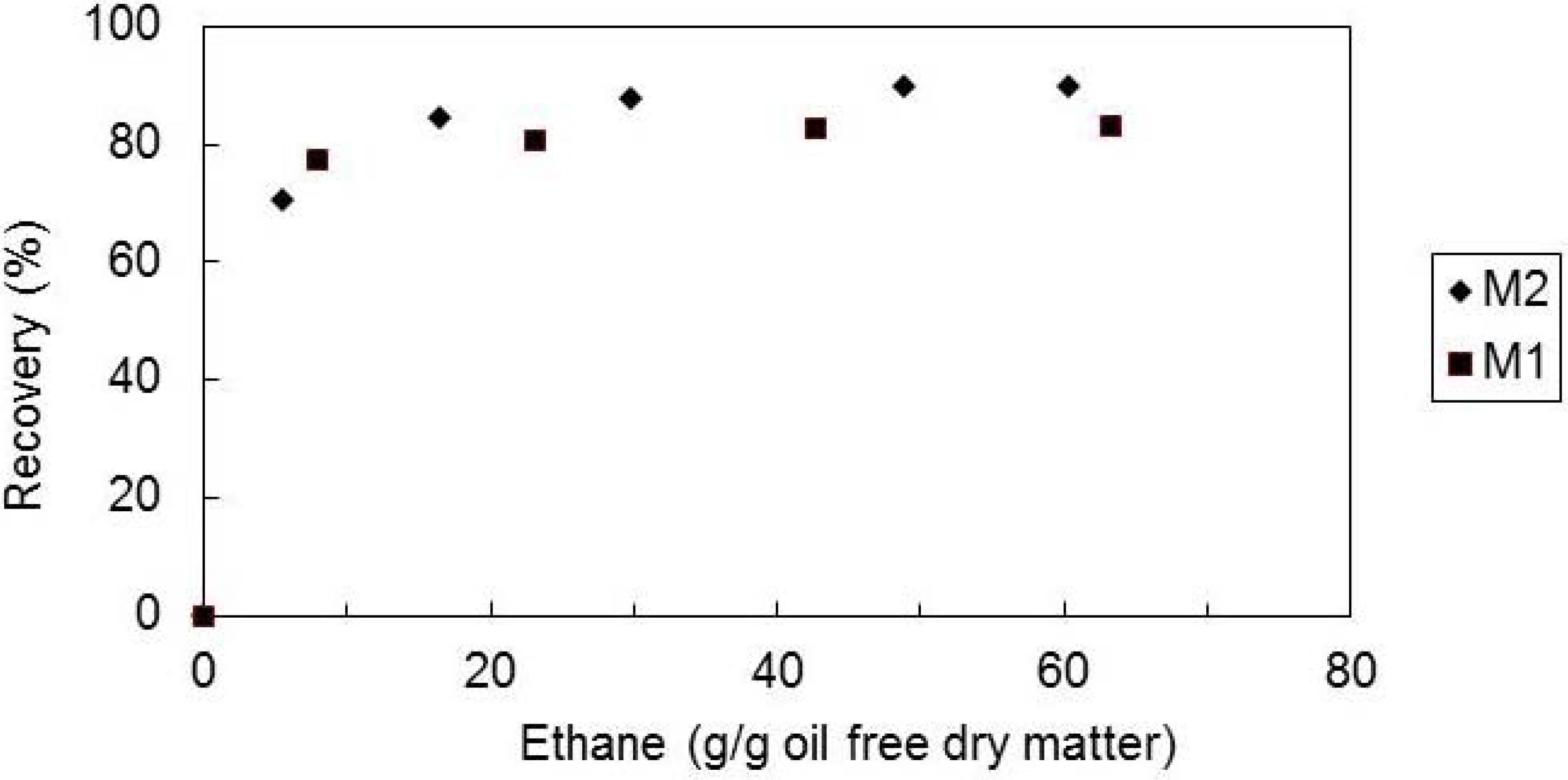
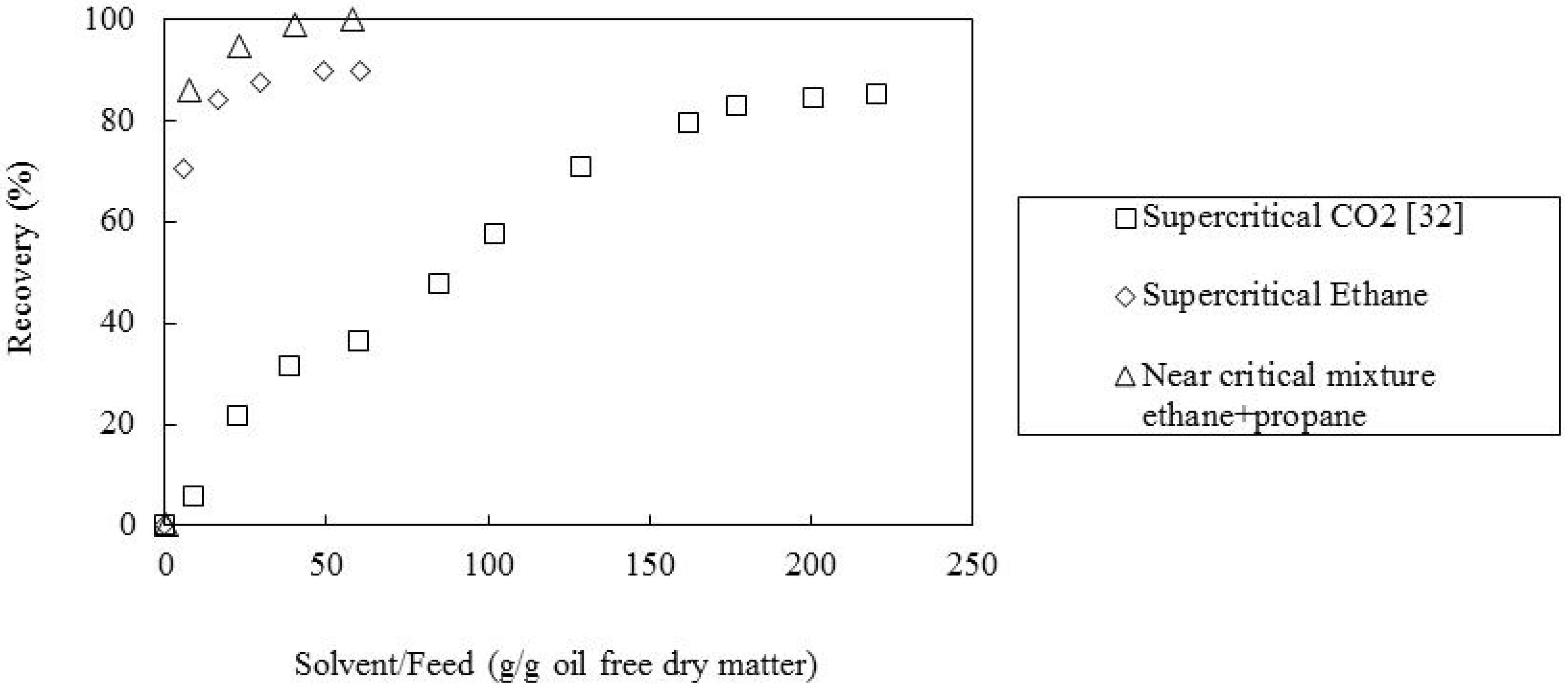
2.2. Effect of the Supercritical Solvent
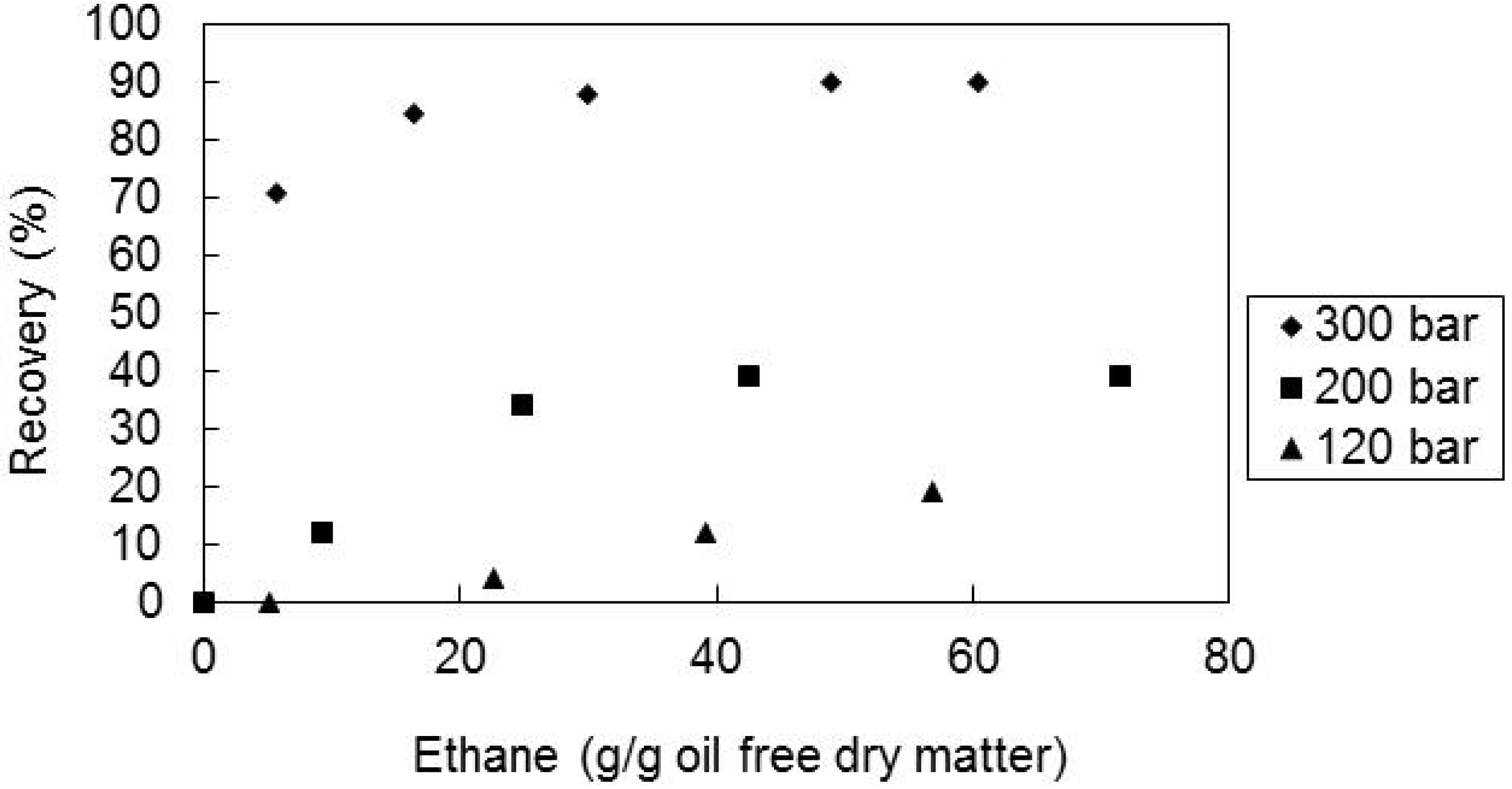
2.3. Effect of the Pressure and Temperature
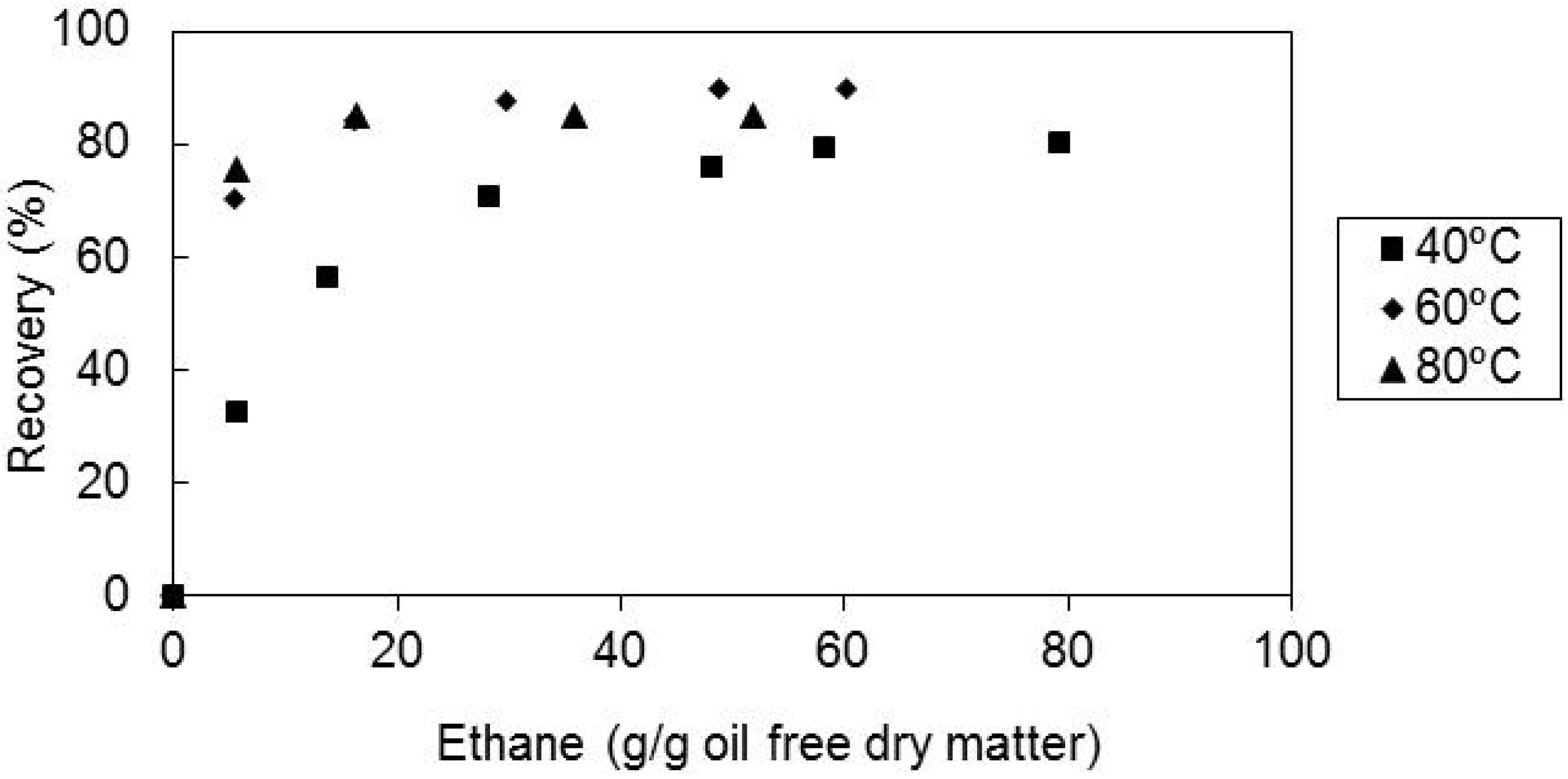
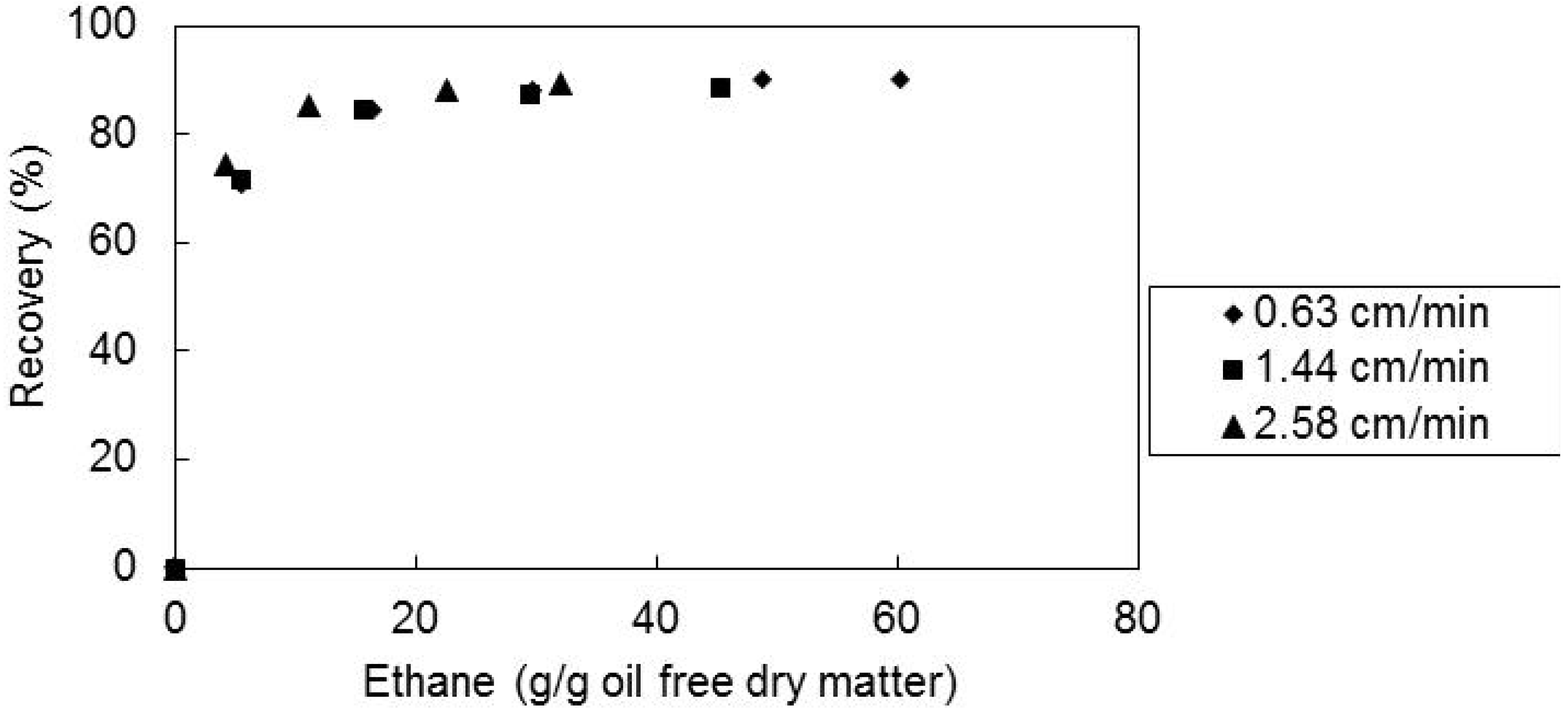
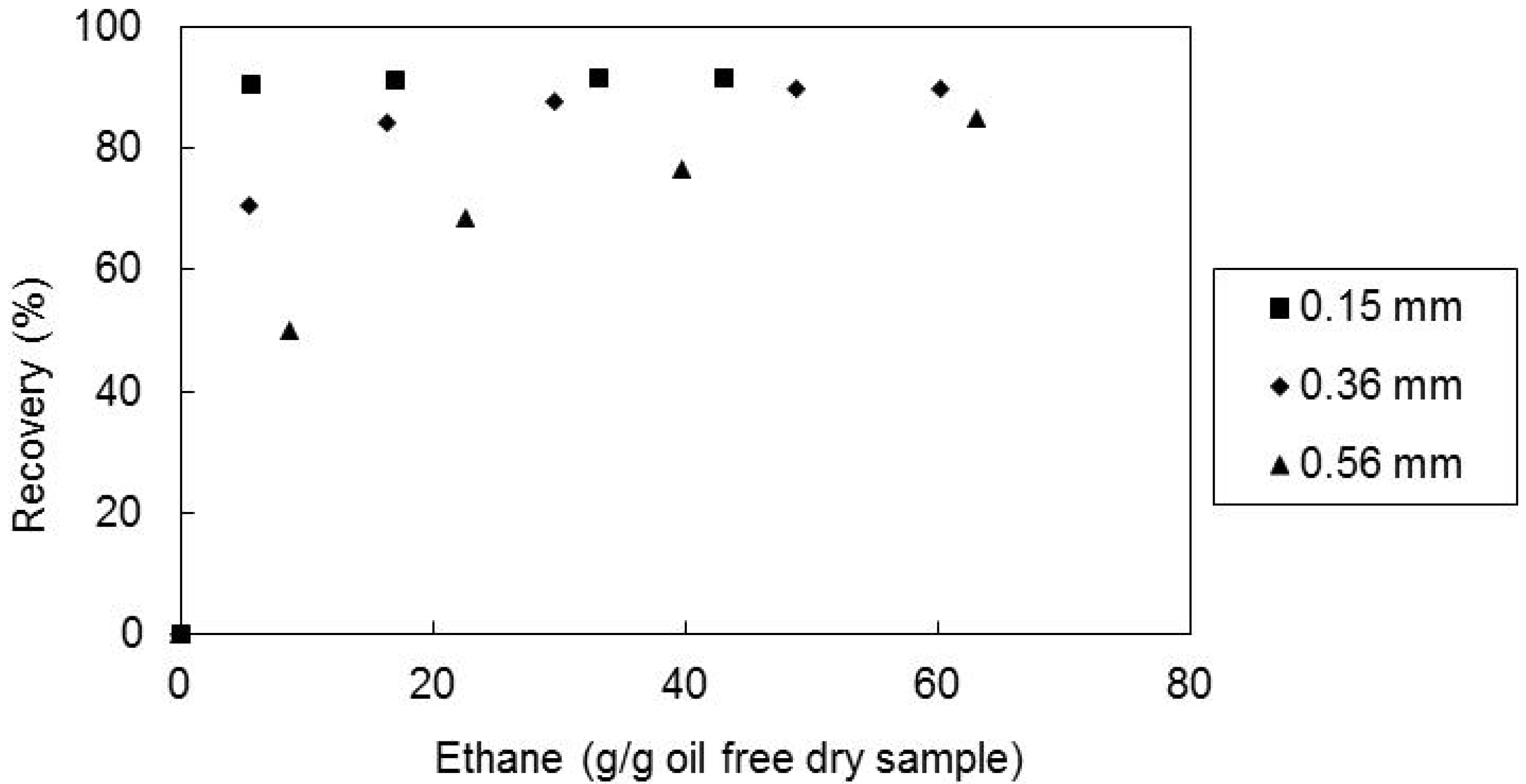
2.4. Effect of the Solvent Superficial Velocity and Particle Size
3. Experimental
3.1. Materials
3.2. Sample Preparation
3.3. Experimental Procedure
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- Perkins-Veazie, P.; Collins, J.K. Flesh quality and lycopene stability of fresh-cut watermelon. Postharvest Biol. Technol. 2004, 31, 159–166. [Google Scholar] [CrossRef]
- Böhm, V.; Fröhlich, K.; Bitsch, R. Rosehip—A “new” source of lycopene? Mol. Aspects Med. 2003, 24, 385–389. [Google Scholar] [CrossRef]
- Pol, J.; Hyitylainen, T.; Ranta-Aho, O.; Riekkola, M. Determination of lycopene in food by on-line SFE coupled to HPLC using a single monolithic column for trapping and separation. J. Chromatogr. A 2004, 1052, 25–31. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.; Katz, N.B. Chemistry, distribution and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Tejada, M.L.S. Application of a UV-Vis detection-HPLC method for a rapid determination of lycopene and beta-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Nieto-Lopéz, M.J.; Costa, J.; Peiro, E.; Méndez, E.; Rodrigues-saiz, M. Biotechnological lycopene production by mated fermentation of Blakeslea trispora. Appl. Microbiol. Biotechnol. 2004, 66, 153–159. [Google Scholar] [CrossRef]
- Kerr, S.; Calé, C.; Cabral, J.M.S.; van Keulen, F. Factors enhancing lycopene production by a new Mycobacterium aurum mutant. Biotechnol. Lett. 2004, 26, 103–108. [Google Scholar] [CrossRef]
- Anonymous. Rapid growth anticipated for canthaxanthin & lycopene usage. Focus Pigment 2003, 6, 3. [Google Scholar]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Zelkha, M.; Ben-Yehuda, M.; Hartal, D.; Raveh, Y.; Garti, N. Industrial processing of tomatoes and lycopene extraction. WO Patent 97/48287, 1997. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Review: Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar]
- Brandt, S.; Pék, Z.; Barna, E.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Sharma, S.K.; Le Maguer, M. Licopene in tomatoes and tomato pulp fractions. Ital. J. Food Sci. 1996, 2, 107–113. [Google Scholar]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Britton, G.; Gambelli, L.; Dunphy, P.; Pudney, P.; Gildey, M. Physical state of carotenoids in chromoplasts of tomato and carrots: Consequences and bioavailability. In Proceedings of the 2nd International Congress on Pigments in Food, Lisbon, Portugal, 11–14 June 2002.
- Leoni, C. “Scarti” in the tomato processing industry: A contribution to disentanglement among culls rejected tomatoes. Production rejected and processing waste. Indust. Cons. 1997, 73, 278–290. [Google Scholar]
- Al-Wandawi, H.; Abul-rahman, M.; Al-Shaikhly, K. Tomato processing wastes as essential raw material sources. J. Agric. Food Chem. 1985, 33, 804–807. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Bruno, T.J.; Castro, C.A.N.; Hamel, J.F.P.; Palavra, A.M.F. Supercritical fluid extraction of biological products. In Recovery Process for Biological Materials; Kennedy, J.F., Cabral, M.S., Eds.; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Saldana, M.A.D.; Zefel, C.; Mohamed, M.S.; Brunner, G. Extraction of caffeine, theobromine and coca butter from brazilian cocoa beans using supercritical CO2 and ethane. Chem. Eng. Trans. 2002, 2, 447–482. [Google Scholar]
- Knez, Z.; Skerget, M. Phase equilibria of the vitamins D2, D3 and K3 in binary systems with CO2 and propane. J. Supercrit. Fluids 2001, 20, 131–144. [Google Scholar] [CrossRef]
- McHugh, M.A.; Krukonis, V.J. Supercritical Fluid Extraction-Principles and Practice; Brenner, H., Ed.; Butterworth-Heinemann: Stoneham, MA, USA, 1994. [Google Scholar]
- Rozzi, N.L.; Sing, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar]
- Baysal, T.; Ersus, S.; Starmans, D.A.J. Supercritical CO2 Extraction of beta-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar]
- Favati, F.; Pietrafesa, A.; Galgano, F. Extraction of natural antioxidants (carotenoids and tocopherols) from by-products of the tomato processing industry. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Vasapollo, G.; Longo, L.; Rescio, L.; Ciurlia, L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids 2004, 29, 87–96. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Espinosa, V.M.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and beta-carotene extraction from tomato processing waste using supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Suogi, Z.; Yungxiang, H.; Guopeng, Q.; Renan, W. Extracting lycopene from tomato powders by supercritical propane and carbon dioxide with industrial scale pilot. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Paolo, A.; Ireneo, K.; Angelo, C.; Alessia, F.; Marisa, T.; Crucil, I. Lycopene extraction from processed tomatoes using supercriticas CO2. In Proceedings of the 7th Italian Conference on Supercritical Fluids/9th Meeting on Supercritical Fluids, Triest, Italy, 13–16 June 2004.
- Ollanketo, M.; Hartanen, K.; Riekkola, M.; Holm, Y.; Hiltunen, R. Supercritical carbon dioxide extraction of lycopene in tomato skins. Eur. Food Res. Technol. 2001, 212, 561–565. [Google Scholar]
- Nobre, B.P.; Palavra, A.F.; Pessoa, F.L.P.; Mendes, R.L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 2009, 116, 680–685. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Coelho, J.P.; Palavra, A.F. Solubility of Beta-Carotene in Supercritical Carbon Dioxide and Ethane. J. Supercrit. Fluids 1999, 16, 99–106. [Google Scholar] [CrossRef]
- Nobre, B.P.; Palavra, A.F.; Mendes, R.L. Solubility of Beta-Carotene in Near-Critical Mixtures of (Ethane + Propane). J. Chem. Eng. Data 2002, 47, 1159–1163. [Google Scholar]
- Mendes, R.L.; Coelho, J.P.; Fernandes, H.L.; Marrucho, I.J.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Tech. Biotechnol. 1995, 62, 53–59. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nobre, B.P.; Gouveia, L.; Matos, P.G.S.; Cristino, A.F.; Palavra, A.F.; Mendes, R.L. Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane. Molecules 2012, 17, 8397-8407. https://doi.org/10.3390/molecules17078397
Nobre BP, Gouveia L, Matos PGS, Cristino AF, Palavra AF, Mendes RL. Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane. Molecules. 2012; 17(7):8397-8407. https://doi.org/10.3390/molecules17078397
Chicago/Turabian StyleNobre, Beatriz P., Luisa Gouveia, Patricia G. S. Matos, Ana F. Cristino, António F. Palavra, and Rui L. Mendes. 2012. "Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane" Molecules 17, no. 7: 8397-8407. https://doi.org/10.3390/molecules17078397
APA StyleNobre, B. P., Gouveia, L., Matos, P. G. S., Cristino, A. F., Palavra, A. F., & Mendes, R. L. (2012). Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane. Molecules, 17(7), 8397-8407. https://doi.org/10.3390/molecules17078397



