Design, Synthesis, and Anti-Proliferative Evaluation of [1,1′-biphenyl]-4-ols as Inhibitor of HUVEC Migration and Tube Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
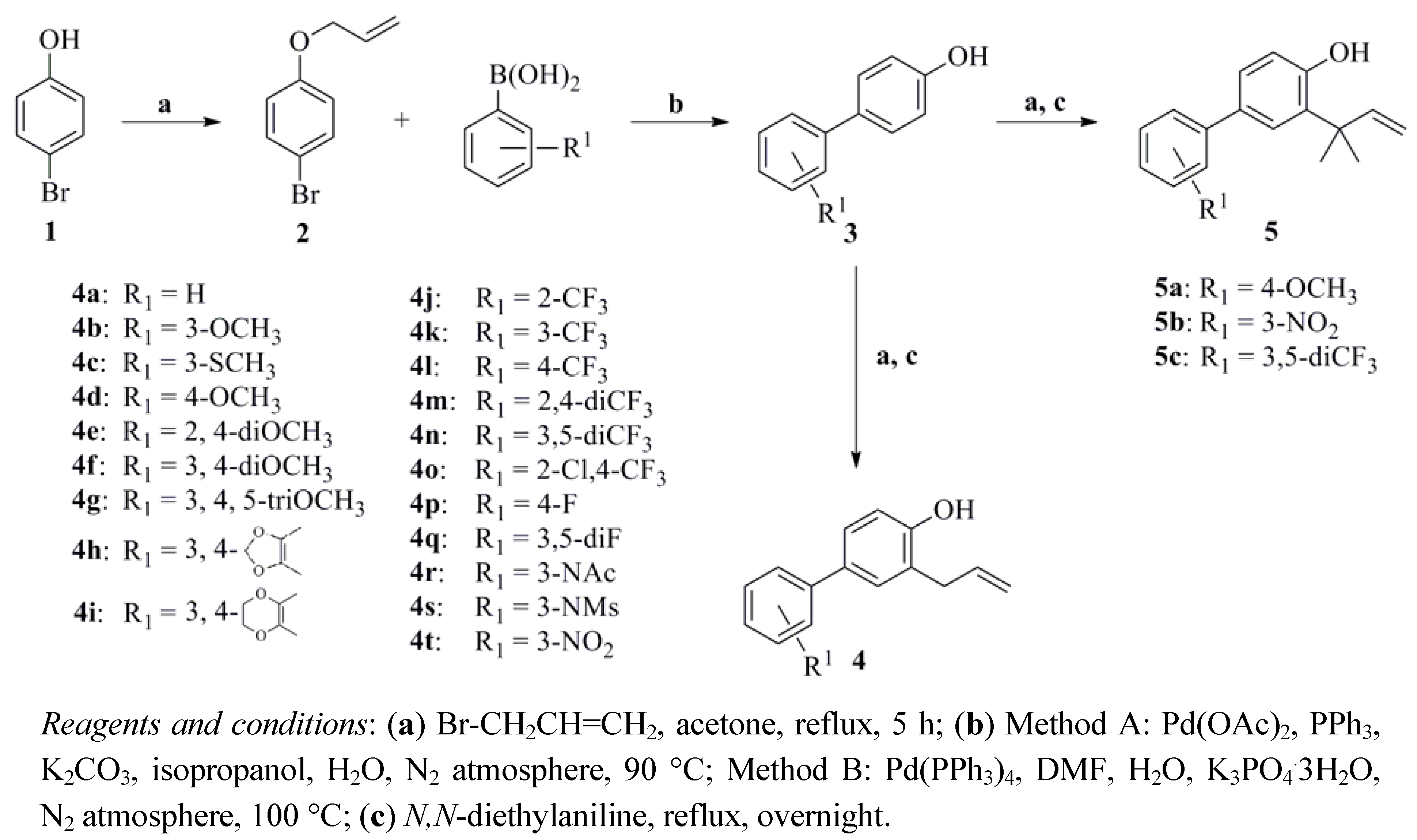
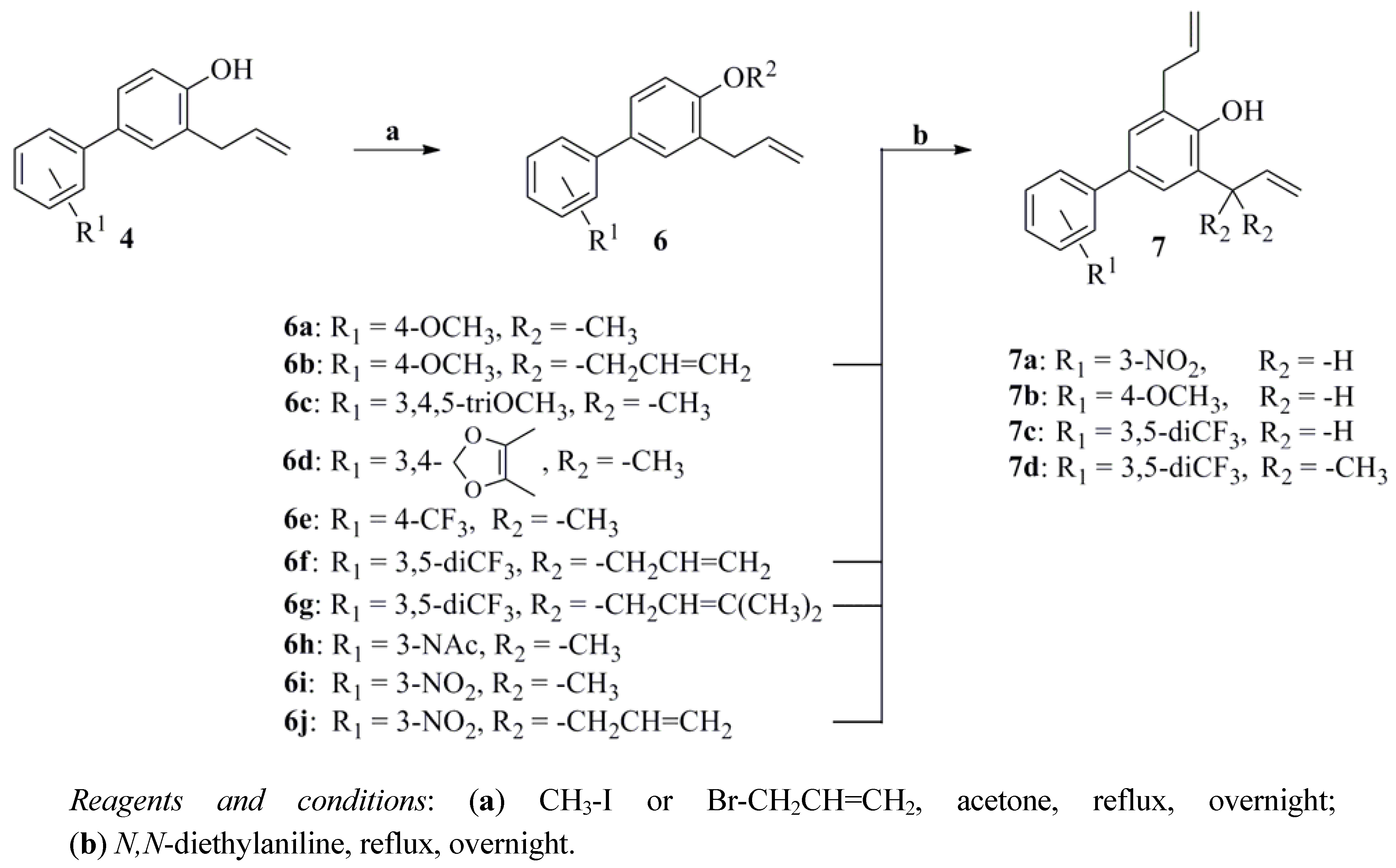
2.2. Anti-Proliferative Activity and SAR Study
| Compd | MW a | cLogP b | miLogP c | xLogP d | IC50 (μM) | ||
|---|---|---|---|---|---|---|---|
| C26 | Hela | K562 | |||||
| Honokiol | 266.33 | 5.03 | 5.01 | 4.98 | 65.1 | 62.0 | 42.0 |
| 4a | 210.10 | 4.37 | 4.47 | 4.26 | 69.0 | 96.0 | NI e |
| 4b | 240.12 | 4.25 | 4.51 | 4.23 | >100.0 | 82.0 | NI |
| 4c | 256.36 | 4.81 | 4.88 | 4.78 | 63.0 | 62.0 | 71.2 |
| 4d | 240.30 | 4.21 | 4.53 | 4.23 | 67.0 | 81.5 | NI |
| 4e | 270.03 | 4.12 | 4.51 | 4.21 | 96.0 | 58.0 | 90.0 |
| 4f | 270.13 | 4.12 | 4.12 | 4.21 | 90.0 | >100.0 | NI |
| 4g | 300.35 | 3.99 | 4.10 | 4.18 | 74.0 | 95.5 | 110.5 |
| 4h | 254.09 | 4.15 | 4.36 | 4.08 | 80.0 | 89.5 | NI |
| 4i | 268.31 | 3.88 | 3.98 | 3.98 | >100.0 | 76.5 | NI |
| 4j | 278.27 | 5.29 | 5.32 | 5.15 | 53.1 | 52.3 | 41.6 |
| 4k | 278.09 | 5.29 | 5.34 | 5.15 | 54.0 | 70.1 | 61.5 |
| 4l | 278.09 | 5.29 | 5.37 | 5.15 | 61.7 | 68.5 | 55.8 |
| 4m | 346.08 | 6.21 | 6.19 | 6.03 | 45.1 | 48.7 | 65.8 |
| 4n | 346.08 | 6.21 | 6.19 | 6.03 | 34.5 | 36.0 | 56.3 |
| 4o | 312.05 | 5.85 | 5.97 | 5.78 | 59.5 | 50.0 | 62.4 |
| 4p | 228.26 | 4.53 | 4.64 | 4.36 | 57.1 | 94.5 | 82.0 |
| 4q | 246.25 | 4.69 | 4.73 | 4.46 | 88.1 | 72.5 | 78.0 |
| 4r | 267.13 | 3.28 | 3.67 | 3.44 | 67.2 | >100.0 | NI |
| 4s | 303.09 | 2.59 | 3.68 | 3.23 | >100.0 | 80.0 | NI |
| 4t | 255.09 | 3.95 | 4.41 | 4.09 | 48.5 | 56.5 | 71.0 |
| 5a | 268.35 | 5.05 | 5.50 | 5.11 | 74.0 | 95.0 | 80.0 |
| 5b | 283.12 | 4.96 | 5.38 | 4.97 | 58.0 | 55.0 | 53.0 |
| 5c | 374.32 | 7.01 | 7.16 | 6.91 | 15.0 | 25.0 | 21.2 |
| 6a | 254.32 | 4.51 | 4.60 | 4.56 | >100.0 | >100.0 | NI |
| 6b | 280.15 | 5.20 | 5.24 | 5.20 | >100.0 | >100.0 | NI |
| 6c | 314.15 | 4.26 | 4.17 | 4.50 | 76.0 | >100.0 | NI |
| 6d | 268.11 | 4.41 | 4.43 | 4.40 | >100.0 | >100.0 | NI |
| 6e | 292.11 | 5.56 | 5.44 | 5.47 | >100.0 | >100.0 | NI |
| 6f | 386.11 | 7.17 | 6.90 | 7.00 | >100.0 | >100.0 | NI |
| 6g | 414.14 | 7.17 | 7.94 | 7.86 | >100.0 | >100.0 | NI |
| 6h | 281.35 | 3.54 | 3.74 | 3.77 | >100.0 | >100.0 | 58.0 |
| 6i | 269.11 | 4.47 | 4.48 | 4.42 | >100.0 | >100.0 | NI |
| 6j | 295.12 | 5.03 | 5.12 | 5.06 | >100.0 | >100.0 | NI |
| 7a | 295.12 | 5.04 | 4.95 | 5.16 | 44.7 | 51.3 | 55.2 |
| 7b | 280.15 | 5.30 | 5.07 | 5.31 | 65.0 | 72.0 | 73.4 |
| 7c | 386.33 | 7.27 | 6.74 | 7.10 | 25.5 | 36.0 | 37.3 |
| 7d | 414.38 | 8.07 | 7.71 | 7.98 | 29.0 | 40.0 | 42.1 |
| Compd. | IC50 (μM) | ||
|---|---|---|---|
| HUVEC | A549 | HepG2 | |
| Honokiol | 40.0 | 75.0 | 55.4 |
| 4j | 82.0 | 59.7 | 52.4 |
| 4k | 76.0 | 73.0 | 58.3 |
| 4l | 74.0 | 76.0 | 60.3 |
| 4n | >100.0 | 50.0 | 37.5 |
| 4o | >100.0 | 72.0 | 68.0 |
| 5b | 77.0 | 64.0 | 56.0 |
| 5c | 47.0 | 29.5 | 13.0 |
| 7c | 48.0 | 30.0 | 26.5 |
| 7d | 70.0 | 32.0 | 33.0 |
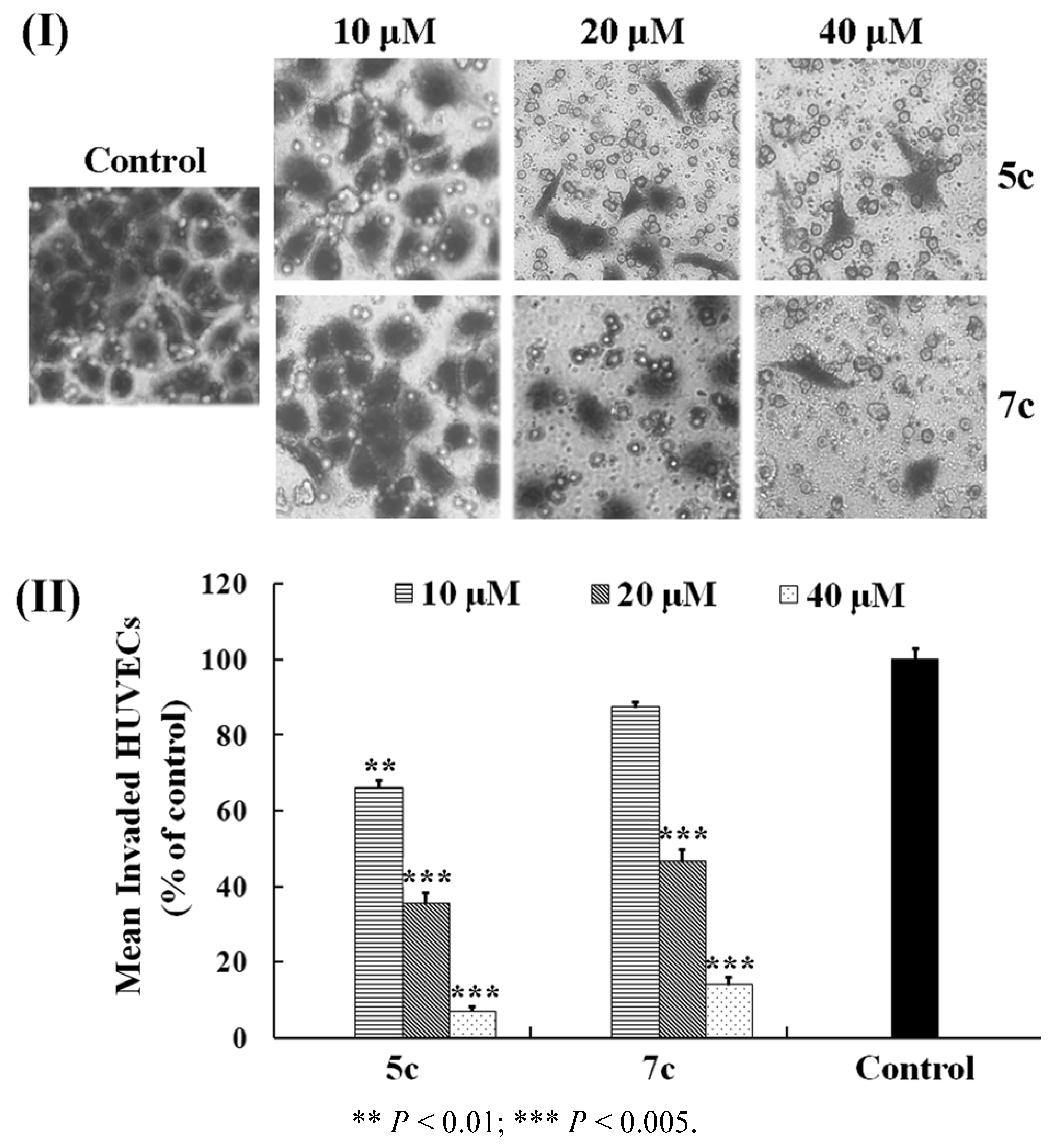
2.3. Effects on HUVEC Migration

3. Experimental
3.1. Chemistry
3.1.1. General Procedure Step I for the Preparation of 1-Allyloxy-4-Bromobenzene (2)
3.1.2. Step II for the Preparation of Intermediates 3 by Suzuki-Coupling Reaction
3.1.3. Step III for the Preparation of 4a–t
3.1.4. Syntheses of 5a–c followed the general procedure
3.1.5. Syntheses of 6a–j Following Step III of the General Procedure
3.1.6. Syntheses of 7a–d Following Step III of the General Procedure
4. Conclusions
Acknowledgment
References
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- Feng, X.D.; Ofstad, W.; Hawkins, D. Antiangiogenesis therapy: A new strategy for cancer treatment. US Pharm. 2010, 35, 4–9. [Google Scholar]
- Yang, S.P.; Cai, Y.J.; Zhang, B.L.; Tong, L.J.; Xie, H.; Wu, Y.; Lin, L.P.; Ding, J.; Yue, J.M. Structural modification of an angiogenesis inhibitor discovered from traditional Chinese medicine and a structure-activity relationship study. J. Med. Chem. 2008, 51, 77–85. [Google Scholar] [CrossRef]
- St. Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes expressed in human tumor endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef]
- Kerbel, R.S. Inhibition of tumor angiogenesis as a strategy to circumvent acquited resistance to anti-cancer therapeutic agents. Bioessays 1991, 13, 31–36. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Madhusudan, S.; Harris, A.L. Drug inhibition of angiogenesis. Curr. Opin. Pharmacol. 2002, 2, 403–414. [Google Scholar] [CrossRef]
- Cook, K.M.; Figg, W.D. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J. Clin. 2010, 60, 222–243. [Google Scholar] [CrossRef]
- Staton, C.A.; Brown, N.J.; Reed, M.W. Current status and future prospects for anti-angiogenic therapies in cancer. Exp. Opin. Drug Discov. 2009, 4, 961–979. [Google Scholar] [CrossRef]
- Cepanec, I. Synthesis of Biaryls; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 1–235. [Google Scholar]
- Rao, C.V.; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of colon cancer by dietary curcumin. Ann. NY Acad. Sci. 1995, 768, 201–204. [Google Scholar]
- Yihai, C.; Renhai, C. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar]
- Arbiser, J.L.; Klauber, N.; Rohan, R.; van Leeuwen, R.; Huang, M.T.; Fisher, C.; Flynn, E.; Byers, H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998, 4, 376–383. [Google Scholar]
- Taferner, B.; Schuehly, W.; Huefner, A.; Baburin, I.; Wiesner, K.; Ecker, G.F.; Hering, S. Modulation of GABAA-receptors by honokiol and derivatives: Subtype selectivity and structure-activity relationship. J. Med. Chem. 2011, 54, 5349–5361. [Google Scholar]
- Amblard, F.; Delinsky, D.; Arbiser, J.L.; Schinazi, R.F. Facile purification of honokiol and its antiviral and cytotoxic properties. J. Med. Chem. 2006, 49, 3426–3427. [Google Scholar]
- Liang, M.; Jin, Y.C.; Xue, W.W.; Xiao, L.L.; You, F.L.; Wei, Z.; Tian, E.W.; Ming, P.; Shu, C.L.; Shi, J.; et al. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: The evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship. J. Med. Chem. 2011, 54, 6469–6481. [Google Scholar]
- Palmer, B.D.; Thompson, A.M.; Sutherland, H.S.; Blaser, A.; Kmentova, I.; Franzblau, S.G.; Wan, B.; Wang, Y.; Ma, Z.; Denny, W.A. Synthesis and Structure−Activity Studies of Biphenyl Analogues of the Tuberculosis Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 282–294. [Google Scholar]
- Pisco, L.; Kordian, M.; Peseke, K.; Feist, H.; Michalik, D.; Estrada, E.; Carvalho, J.; Hamilton, G.; Rando, D.; Quincoces, J. Synthesis of compounds with antiproliferative activity as analogues of prenylated natural products existing in Brazilian propolis. Eur. J. Med. Chem. 2006, 41, 401–407. [Google Scholar]
- Kromann, H.; Larsen, M.; Boesen, T.; Schønning, K.; Nielsen, S.F. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2004, 39, 993–1000. [Google Scholar]
- Abdollahi, A.; Folkman, J. Evading tumor evasion: Current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist. Update. 2010, 13, 16–28. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a-7d are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ran, Y.; Ma, L.; Wang, X.; Chen, J.; Wang, G.; Peng, A.; Chen, L. Design, Synthesis, and Anti-Proliferative Evaluation of [1,1′-biphenyl]-4-ols as Inhibitor of HUVEC Migration and Tube Formation. Molecules 2012, 17, 8091-8104. https://doi.org/10.3390/molecules17078091
Ran Y, Ma L, Wang X, Chen J, Wang G, Peng A, Chen L. Design, Synthesis, and Anti-Proliferative Evaluation of [1,1′-biphenyl]-4-ols as Inhibitor of HUVEC Migration and Tube Formation. Molecules. 2012; 17(7):8091-8104. https://doi.org/10.3390/molecules17078091
Chicago/Turabian StyleRan, Yan, Liang Ma, Xuewei Wang, Jinying Chen, Guangcheng Wang, Aihua Peng, and Lijuan Chen. 2012. "Design, Synthesis, and Anti-Proliferative Evaluation of [1,1′-biphenyl]-4-ols as Inhibitor of HUVEC Migration and Tube Formation" Molecules 17, no. 7: 8091-8104. https://doi.org/10.3390/molecules17078091
APA StyleRan, Y., Ma, L., Wang, X., Chen, J., Wang, G., Peng, A., & Chen, L. (2012). Design, Synthesis, and Anti-Proliferative Evaluation of [1,1′-biphenyl]-4-ols as Inhibitor of HUVEC Migration and Tube Formation. Molecules, 17(7), 8091-8104. https://doi.org/10.3390/molecules17078091




