Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule
Abstract
:1. Introduction

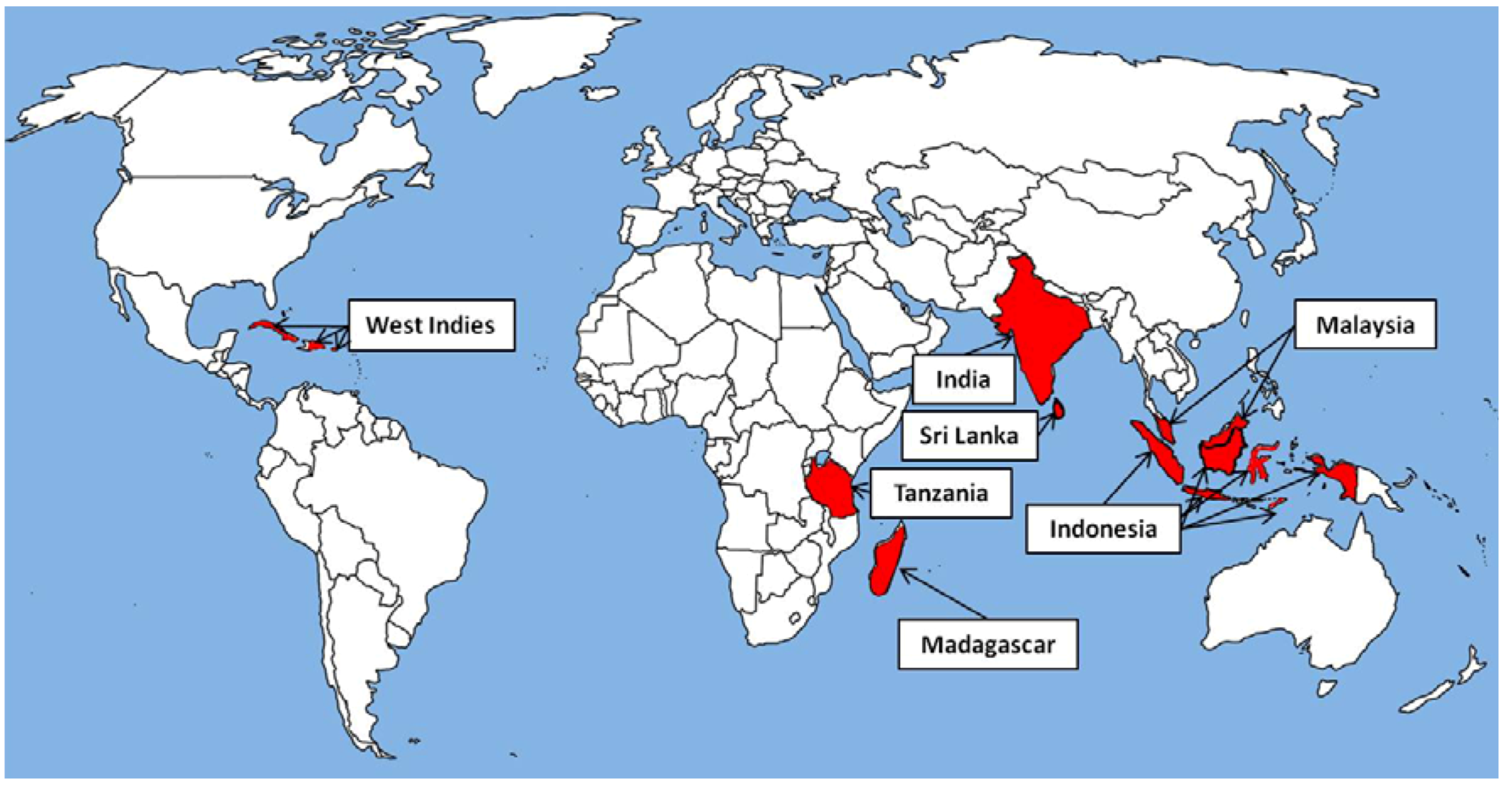
2. Natural Sources of Eugenol
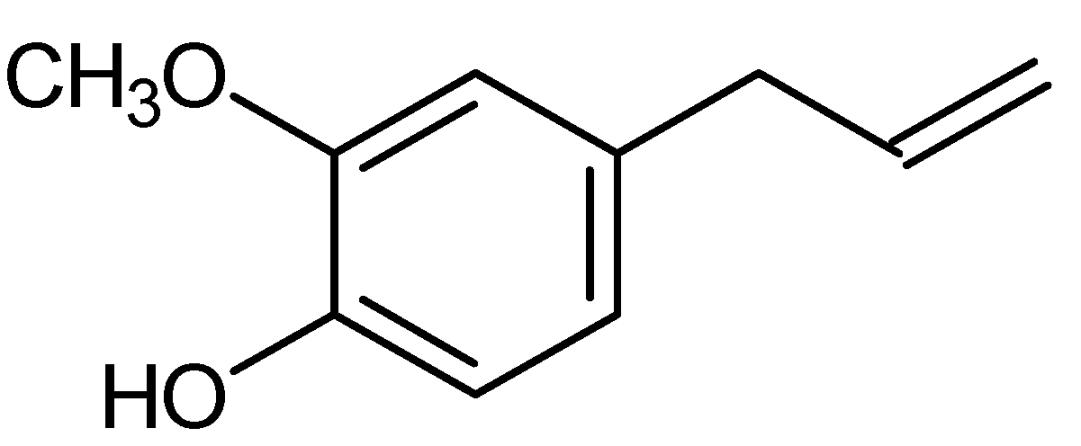
3. Chemical and Physical Properties
4. Pharmacological Properties of Eugenol and Possible Mechanisms of Action
4.1. Anti-Infective Activity
4.1.1. Antibacterial Activity
4.1.2. Antifungal Activity
4.1.3. Antiplasmodial Activity
4.1.5. Anthelmintic Activity
4.2. Anti-Inflammatory Activity
4.3. Analgesic Activity
4.4. Anti-Oxidant Activity
4.5. Anticancer Activity
4.6. Antimutagenic and Antigenotoxic Activities
4.7. Modulatory Effects
5. Other Pharmacological Properties
6. Agricultural Applications
7. Insecticidal and Fumigant Properties
8. Skin Permeation Enhancement
9. Toxicity and Allergenicity of Eugenol
10. Conclusions
References
- Zheng, G.Q.; Kenney, P.M.; Lam, L.K.T. Sesquiterpenes from clove (Eugenia caryophyllata). J. Nat. Prod. 1992, 55, 999–1003. [Google Scholar] [CrossRef]
- Bulbeck, D.; Reid, A.; Tan, L.C.; Wu, Y. Southeast Asian Exports Since the 14th Century: Cloves, Pepper, Coffee, and Sugar; Institute of Southeast Asian Studies: Singapore, 1998. [Google Scholar]
- Chomchalow, N. Spice production in Asia–An overview. Presented at IBC Asian Spice Markets Conference, Singapore; 27–28 May 1996. [Google Scholar]
- Hartnoll, G.; Moore, D.; Douek, D. Near fatal ingestion of oil of cloves. Arch. Dis. Child. 1993, 69, 392–393. [Google Scholar] [CrossRef]
- Barceloux, D.G. Medical Toxicology of Natural Substances. Foods, Fungi, Medicinal Herbs, Plants and Venomous Animals; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Harborne, J.B.; Baxter, H. Phytochemical Dictionary; Taylor and Francis: London, UK, 1993. [Google Scholar]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef]
- Ogendo, J.O.; Kostyukovsky, M.; Ravid, U.; Matasyoh, J.C.; Deng, A.L.; Omolo, E.O.; Kariuki, S.T.; Shaaya, E. Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J. Stored Prod. Res. 2008, 44, 328–334. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nguyen, H.R.; Lewis, N.G. Bioengineering and Molecular Biology of Plant Pathways; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Bedoukian, P.Z. Perfumery and Flavouring Synthetics, 3rd ed; Allured Publishing Corporation: Carol Stream, IL, USA, 1986. [Google Scholar]
- Good Guide Inc. Eugenol Guide. Available online: http://www.goodguide.com/ingredients/83549-eugenol (accessed on 25 March 2012).
- Lee, K.-Y.M.; Patterson, A.; Piggot, J.R.; Richardson, G.D. Origins of flavour in whiskies and a revised flavour wheel: A review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef]
- Chang, M.C.; Uang, B.J.; Wu, H.L.; Lee, J.J.; Hahn, L.J.; Jeng, J.H. Inducing the cell cycle arrest and apoptosis of oral KB carcinoma cells by hydroxychavicol: Roles of glutathione and reactive oxygen species. Br. J. Pharmacol. 2002, 135, 619–630. [Google Scholar] [CrossRef]
- Li, Y.-H.; Sun, Z.-H.; Zheng, P. Determination of vanillin, eugenol and isoeugenol by RP-HPLC. Chromatographia 2004, 60, 709–713. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of anti-oxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; deLampasona, M.P.; Catalan, C.A.N. A comparison of chemical, anti-oxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]
- Laekeman, G.M.; van Hoof, L.; Haemers, A.; Berghe, D.A.V.; Herman, A.G.; Vlietinck, A.J. Eugenol a valuable compound for in vitro experimental research and worthwhile for further in vivo investigation. Phytother. Res. 1990, 4, 90–96. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20–24. [Google Scholar] [CrossRef]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; de Souza, E.L.; Diniz, M.D.F.F.M.; Trajano, V.N.; de Medeiros, I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar]
- El-Abed, S.; Houari, A.; Latrache, H.; Remmal, A.; Koraichi, S.I. In vitro activity of four common essential oil components against biofilm-producing Pseudomonas aeruginosa. Res. J. Microbiol. 2011, 6, 394–401. [Google Scholar]
- Iwu, M.M. African Medicinal Plants in the Search for New Drugs Based on Ethnobotanical Leads. In Ethnobotany and the Search for New Drugs; Chadwick, D.J., Marsh, J., Eds.; Ciba Foundation: Chichester, UK, 2006; pp. 116–129. [Google Scholar]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram-negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef]
- Moon, S.-E.; Kim, H.-Y.; Cha, J.-D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef]
- Pei, R.-S.; Zhou, F.; Ji, B.-P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- Qiu, J.; Feng, H.; Lu, J.; Xiang, H.; Wang, D.; Dong, J.; Wang, J.; Wang, X.; Liu, J.; Deng, X. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microb. 2010, 76, 5846–5851. [Google Scholar] [CrossRef]
- Rico-Molina, D.; Aparicio-Ozores, G.; Dorantes-Alvarez, L.; Hernández-Sánchez, H. Antimicrobial activity of cinnamate-eugenol: Synergistic potential, evidence of efflux pumps and amino acid effects. Am. J. Food Technol. 2012, 7, 289–300. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar]
- Chami, F.; Chami, N.; Bennis, S.; Trouillas, J.; Remmal, A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004, 54, 909–914. [Google Scholar] [CrossRef]
- Garg, A.; Singh, S. Enhancement in antifungal activity of eugenol in immunosuppressed rats through lipid nanocarriers. Colloid Surface B 2011, 87, 280–288. [Google Scholar] [CrossRef]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Alfieri, M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 2007, 78, 396–400. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Marcos-Arias, C.; Eraso, E.; Madariaga, L.; Quindos, G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement. Altern. Med. 2011, 11, 119. [Google Scholar] [CrossRef]
- Lee, S.-J.; Han, J.-I.; Lee, G.-S.; Park, M.-J.; Choi, I.-G.; Na, K.-J.; Jeung, E.-B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007, 30, 184–188. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.C.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of thymus oils and their major compounds. J. Eur. Acad. Derm. Vener. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar]
- Gayoso, C.W.; Lima, E.O.; Oliveira, V.T.; Pereira, F.O.; Souza, E.L.; Lima, I.O.; Navarro, D.F. Sensitivity of fungi isolated from onychomycosis to Eugenia cariophyllata essential oil and eugenol. Fitoterapia 2005, 76, 247–249. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Wada, S.; Hatai, K. Antimycotic activity of eugenol against selected water molds. J. Aquat. Anim. Health 2000, 12, 224–229. [Google Scholar] [CrossRef]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Bertolone, E.; Minati, J.L.; Zanonp, B.; Ambrosoli, R. Biolog methodology for the antimicrobial activity evaluation of essential oils’ volatile compounds against foodborne microflora of fresh-cut salad. Ital. J. Food Sci. 2011, 23, 289–301. [Google Scholar]
- Kumar, A.; Shukla, R.; Singh, P.; Dubey, N.K. Biodeterioration of some herbal raw materials by storage fungi and aflatoxin and assessment of Cymbopogon flexuosus essential oil and its components as antifungal. Int. Biodeter. Biodegr. 2009, 63, 712–716. [Google Scholar] [CrossRef]
- Benencia, F.; Courrges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Tragoolpua, Y.; Jatisatienr, A. Anti-herpes simplex virus activities of Eugenia caryophyllus (Spreng.) Bullock & S. G. Harrison and essential oil, eugenol. Phytother. Res. 2007, 21, 1153–1158. [Google Scholar]
- Asha, M.K.; Prashanth, D.; Murali, B.; Padmaja, R.; Amit, A. Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia 2001, 72, 669–670. [Google Scholar] [CrossRef]
- Ueda-Nakamura, T.; Mendonça-Filho, R.R.; Morgado-Díaz, J.A.; Korehisa Maza, P.; Prado Dias Filho, B.; Aparício Garcia Cortez, D.; Alviano, D.S.; Rosa, M.D.S.S.; Lopes, A.H.C.S.; Alviano, C.S.; et al. Antileishmanial activity of eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006, 55, 99–105. [Google Scholar] [CrossRef]
- Leem, H.-H.; Kim, E.-O.; Seo, M.-J.; Choi, S.-W. Anti-oxidant and anti-inflammatory activities of eugenol and its derivatives from clove (Eugenia caryophyllata Thunb.). J. Korean Soc. Food Sci. Nutr. 2011, 40, 1361–1370. [Google Scholar]
- Daniel, A.N.; Sartoretto, S.M.; Schmidt, G.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Rev. Bras. Farmacogn. 2009, 19, 212–217. [Google Scholar] [CrossRef]
- Kim, S.S.; Oh, O.-J.; Min, H.-Y.; Park, E.-J.; Kim, Y.; Park, H.J.; Han, Y.N.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Bachiega, T.F.; de Sousa, J.P.B.; Bastos, J.K.; Sfrocin, J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012, 64, 610–616. [Google Scholar]
- Thompson, D.; Eling, T. Mechanism of inhibition of prostaglandin H synthase by eugenol and other phenolic peroxidase sunstrates. Mol. Pharmacol. 1989, 36, 809–817. [Google Scholar]
- Lasmann, G.; Curtis, J.; Liermann, B.; Mason, R.P.; Eling, T.E. ESR studies on reactivity of protein-derived tyrosyl radicals formed by prostaglandin H synthase and ribonucleotide reductase. Arch. Biochem. Biophys. 1993, 300, 132–136. [Google Scholar] [CrossRef]
- Raghavenra, H.; Diwakr, B.T.; Lokesh, B.R.; Naidu, K.A. Eugenol-The active principle from cloves inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 23–27. [Google Scholar] [CrossRef]
- Naidu, K.A. Eugenol-An inhibitor of lipoxygenase-dependent lipid peroxidation. Prostaglandins Leukot. Essent. Fatty Acids 1995, 53, 381–383. [Google Scholar] [CrossRef]
- Sharma, J.N.; Srivastava, K.C.; Gan, E.K. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology 1994, 49, 314–318. [Google Scholar] [CrossRef]
- Kurian, R.; Arulmozhi, D.K.; Veeranjaneyulu, A.; Bodhankar, S.L. Effect of eugenol on animal models of nociception. Indian J. Pharmacol. 2006, 38, 341–345. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B. The analgesic effects and mechanisms of orally administered eugenol. Arch. Pharm. Res. 2011, 34, 501–507. [Google Scholar] [CrossRef]
- Hellwig, N.; Plant, T.D.; Janson, W.; Schäfer, M.; Schultz, G.; Schaefer, M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 2004, 279, 34553–34561. [Google Scholar]
- Li, H.Y.; Park, C.-K.; Jung, S.J.; Choi, S.-Y.; Lee, S.J.; Park, K.; Kim, J.S.; Oh, S.B. Eugenol inhibits K+ currents in trigeminal ganglion neurons. J. Dent. Res. 2007, 86, 898–902. [Google Scholar] [CrossRef]
- Inoue, M.; Fujita, T.; Goto, M.; Kumamoto, E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 2012, in press.. [Google Scholar]
- Lee, M.H.; Yeon, K.-Y.; Park, C.-K.; Li, H.-Y.; Fang, Z.; Kim, M.S.; Choi, S.-Y.; Lee, S.J.; Lee, S.; Park, K.; et al. Eugenol inhibits calcium currents in dental afferent neurons. J. Dent. Res. 2005, 84, 848–851. [Google Scholar] [CrossRef]
- Ferland, C.E.; Beaudry, F.; Vachon, P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phytother. Res. 2012. [Google Scholar] [CrossRef]
- Vidal, L.V.O.; Furuya, W.M.; Graciano, T.S.; Schamber, C.R.; Dos Santos, L.D.; Soares, C.M. Eugenol concentrations for deep anesthesia and acute toxicity in piavuçu (Leporinus macrocephalus) juveniles. Acta Sci. 2007, 29, 357–362. [Google Scholar]
- Okamoto, M.H.; Tesser, M.B.; Louzada, L.R.; dos Santos, R.A.; Sampaio, L.A. Benzocaine and eugenol as anaesthetics for pompano juvenile Trachinotus marginatus. Cienc. Rural 2009, 39, 866–870. [Google Scholar] [CrossRef]
- Filiciotto, F.; Buscaino, G.; Buffa, G.; Bellante, A.; Maccarrone, V.; Mazzola, S. Anaesthetic qualities of eugenol and 2-phenoxyethanol and their effect on some haematological parameters in farmed European sea bass (Dicentrarchus labrax L.). J. Anim. Vet. Adv. 2012, 11, 494–502. [Google Scholar]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; de Souza, D.M.; Martins, A.C.; Garcia, L.D.O.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O.; et al. The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Phys. C 2012, 155, 462–468. [Google Scholar]
- Ito, M.; Murakami, K.; Yoshino, M. Anti-oxidant action of eugenol compounds: role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 2005, 43, 461–466. [Google Scholar] [CrossRef]
- Chogo, J.B.; Crank, G. Chemical composition and biological activity of the Tanzanian plant Ocimum suave. J. Nat. Prod. 1981, 42, 308–311. [Google Scholar] [CrossRef]
- Kabuto, H.; Yamanushi, T.T. Effects of zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] and eugenol [2-methoxy-4-(2-propenyl)phenol] on the pathological progress in the 6-hydroxydopamine-induced parkinson’s disease mouse model. Neurochem. Res. 2011, 36, 2244–2249. [Google Scholar] [CrossRef]
- Hussain, A.; Brahmbhatt, K.; Priyani, A.; Ahmed, M.; Rizvi, T.A.; Sharma, C. Eugenol enhances the chemotherapeutic potential of gemcitabine and induces anticarcinogenic and anti-inflammatory activity in human cervical cancer cells. Cancer Biother. Radiopharm. 2011, 26, 519–527. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Yoo, C.-B.; Han, K.-T.; Cho, K.-S.; Ha, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 18, 5812–5829. [Google Scholar]
- Kalmes, M.; Hennen, J.; Blömeke, B. Eugenol and isoeugenol as antiproliferative agents in skin cancer cells. Toxicol. Lett. 2009, 189, S100. [Google Scholar]
- Ghosh, R.; Ganapathy, M.; Alworth, W.L.; Chan, D.C.; Kumar, A.P. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J. Steroid. Biochem. Mol. Biol. 2009, 113, 25–35. [Google Scholar] [CrossRef]
- Carrasco, A.H.; Espinoza, C.L.; Cardile, V.; Gallardo, C.; Cardona, W.; Lombardo, L.; Catalán, M.K.; Cuellar, F.M.; Russo, A. Eugenol and its synthetic analogues inhibit cell growth of human cancer cells (Part I.). J. Braz. Chem. Soc. 2008, 19, 543–548. [Google Scholar]
- Rompelberg, C.J.M.; Steenwinkel, M.-J.S.T.; van Asten, J.G.; van Delft, J.H.M.; Baan, R.A.; Verhagen, H. Effect of eugenol on the mutagenicity of benzo[α]pyrene and the formation of benzo[α]pyrene-DNA adducts in the k-lacZ-transgenic mouse. Mutat. Res. 369, 87–96.
- Rompelberg, C.J.M.; Evertz, S.J.C.J.; Bruijntjesrozier, G.C.D.M.; van den Heuvel, P.D.; Verhagen, H. Effect of eugenol on the genotoxicity of established mutagens in the liver. Food Chem. Toxicol. 1996, 34, 33–42. [Google Scholar] [CrossRef]
- Ramos, A.; Visozo, A.; Piloto, J.; García, A.; Rodriguez, C.A.; Rivero, R. Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J. Ethnopharmacol. 2003, 87, 241–246. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of trans-anethole and eugenol in mice. Food Chem. Toxicol. 2001, 39, 493–498. [Google Scholar] [CrossRef]
- Sukumaran, K.; Kuttan, R. Inhibition of tobacco-induced mutagenesis by eugenol and plant extracts. Mutat. Res. 1995, 343, 25–30. [Google Scholar] [CrossRef]
- Rompelberg, C.J.M.; Vogels, J.T.W.E.; de Vogel, N.; Bruijntjes-Rozier, G.C.D.M.; Stenhuis, W.H.; Bogaards, J.J.P.; Verhagen, H. Effect of short-term dietary administration of eugenol in humans. Hum. Exp. Toxicol. 1996, 15, 129–135. [Google Scholar] [CrossRef]
- Aizenman, E.; Hartnett, K.A.; Reynolds, I.J. Oxygen free radicals regulates NMDA receptor function via a redox modulatory site. Neuron 1990, 5, 841–846. [Google Scholar] [CrossRef]
- Volterra, A.; Trotti, D.; Racagni, G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol. Pharmacol. 1994, 46, 986–992. [Google Scholar]
- Volterra, A.; Trotti, D.; Tromba, C.; Floridi, S.; Racagni, G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocyte. J. Neurosci. 1994, 14, 2924–2932. [Google Scholar]
- Wie, M.-B.; Won, M.-H.; Lee, K.-H.; Shin, J.-H.; Lee, J.-C.; Suh, H.-W.; Song, D.-K.; Kim, Y.-H. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci. Lett. 1997, 225, 93–96. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Chakraborty, S.P.; Majumdar, S.; Bag, B.; Roy, S. Eugenol protects nicotine-induced superoxide mediated oxidative damage in murine peritoneal macrophages in vitro. Eur. J. Pharmacol. 2009, 623, 132–140. [Google Scholar] [CrossRef]
- Capasso, R.; Pinto, L.; Vuotto, M.L.; Di Carlo, G. Preventive effect of eugenol on PAF and ethanol-induced gastric mucosal damage. Fitoterapia 2000, 71, S131–S137. [Google Scholar] [CrossRef]
- Morsy, M.A.; Fouad, A.A. Mechanisms of gastroprotective effect of eugenol in indomethacin-induced ulcer in rats. Phytother. Res. 2008, 22, 1361–1366. [Google Scholar] [CrossRef]
- Bennett, A.; Stamford, I.F.; Tavares, I.A.; Jacobs, S.; Capasso, F.; Mascolo, N.; Autore, G. The biological activity of eugenol, a major constituent of nutmeg [Myristica fragrans]: Studies on prostaglandins, the intestine and other tissues. Phytother. Res. 1988, 2, 124–130. [Google Scholar] [CrossRef]
- Trailovic, M.S.; Robertson, P.A.; Jelena, N.-T. Inhibitory effect of eugenol on rat ileal motility in vitro. Acta Vet. 2009, 59, 123–131. [Google Scholar] [CrossRef]
- Lima, F.C.; Peixoto-Neves, D.; Gomes, M.D.; Coelho-de-Souza, A.N.; Lima, C.C.; Araújo Zin, W.; Magalhães, P.J.C.; Saad, L.; Leal-Cardoso, J.H. Antispasmodic effects of eugenol on rat airway smooth muscle. Fundam. Clin. Pharm. 2011, 25, 690–699. [Google Scholar] [CrossRef]
- Feng, J.; Lipton, J.M. Eugenol: Antipyretic activity in rabbits. Neuropharmacology 1987, 26, 1775–1778. [Google Scholar] [CrossRef]
- Tao, G.; Irie, Y.; Li, D.-J.; Keung, W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorgan. Med. Chem. 2005, 13, 4777–4788. [Google Scholar] [CrossRef]
- Garabadu, D.; Shah, A.; Ahmad, A.; Joshi, V.B.; Saxena, B.; Palit, G.; Krishnamurthy, S. Eugenol as an anti-stress agent: Modulation of hypothalamic-pituitary-adrenal axis and brain monoaminergic systems in a rat model of stress. Stress 2011, 14, 145–155. [Google Scholar]
- Huang, C.-W.; Chow, J.C.; Taid, J.J.; Wu, S.-N. Characterizing the effects of eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacology 2012, 221, 575–587. [Google Scholar] [CrossRef]
- Karmakar, S.; Choudhury, M.; Sekhar Das, A.; Maiti, A.; Majumdar, S.; Mitra, C. Clove (Syzygium aromaticum Linn) extract rich in eugenol and eugenol derivatives shows bone-preserving efficacy. Nat. Prod. Res. 2012, 26, 500–509. [Google Scholar] [CrossRef]
- Amiri, A.; Dugas, R.; Pichot, A.L.; Bompeix, G. In vitro and in vitro activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food. Microbiol. 2008, 126, 13–19. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crop. Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Combining eugenol and cinnamaldehyde to control the growth of Alicyclobacillus acidoterrestris. Food Control 2010, 21, 172–177. [Google Scholar]
- Darabi, H.R.; Mohandessi, S.; Balavar, Y.; Moghaddam, M.M.; Aghapoor, K.; Mohsenzadeh, F.; Nourinia, A.A. Clove bud oil: an efficient, economical and widely available oil for the inhibition of wheat seed germination. Environ. Chem. Lett. 2011, 9, 519–524. [Google Scholar] [CrossRef]
- Komala, V.V.; Ratnavathi, C.V.; Vijay Kumar, B.S.; Das, I.K. Inhibition of aflatoxin B1 production by an antifungal component, eugenol in stored sorghum grains. Food Control 2012, 26, 139–146. [Google Scholar] [CrossRef]
- Yen, T.-B.; Chang, S.-T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresource Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C. Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild.) against four species of stored-product Coleoptera. Int. J. Pest Manag. 1997, 43, 89–94. [Google Scholar]
- Huang, H.; Ho, S.-H.; Lee, H.-C.; Yap, Y.-L. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored. Prod. Res. 2002, 38, 403–412. [Google Scholar]
- Krist, S.; Halwachs, L.; Sallaberger, G.; Buchbauer, G. Effects of scents on airborne microbes, part I: thymol, eugenol, trans-cinnamaldehyde and linalool. Flavour Frag. J. 2007, 22, 44–48. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.-I.; Choi, B.-R.; Lee, S.-G.; Ahn, Y.-J. Fumigant toxicity of lemon eucalyptus oil constituents to acaricide-susceptible and acaricide-resistant Tetranychus urticae. Pest Manag. Sci. 2011, 67, 1583–1588. [Google Scholar] [CrossRef]
- Kunta, J.R.; Goskonda, V.R.; Brotherton, H.O.; Khan, M.A.; Reddy, I.K. Effects of menthol and related terpenes on the percutaneous absorption of propanolol across excised hairless mouse skin. J. Pharm. Sci. 1997, 86, 1369–1373. [Google Scholar] [CrossRef]
- Lim, P.F.C.; Liu, X.Y.; Kang, L.; Ho, P.C.L.; Chan, Y.W.; Chan, S.Y. Limonene GP1/PG organogel as a vehicle in transdermal delivery of haloperidol. Int. J. Pharm. 2006, 311, 157–164. [Google Scholar] [CrossRef]
- Zhao, K.; Singh, J. Mechanisms of percutaneous absorption of tamoxifen by terpenes: Eugenol, D-limonene and menthone. J. Control. Release 1998, 55, 253–260. [Google Scholar] [CrossRef]
- Shen, Q.; Li, W.; Li, W. The effect of clove oil on the transdermal delivery of ibuprofen in the rabbit by in vitro and in vivo methods. Drug Dev. Ind. Pharm. 2007, 33, 1369–1374. [Google Scholar] [CrossRef]
- Expert Committee on Food Additives, Evaluation of Certain Food Additives and Contaminants; WHO Technical Report Series 683; WHO Press: Geneva, Switzerland, 1982; p. 20.
- Okada, N.; Hirata, A.; Murakami, Y.; Shoji, M.; Sakagami, H.; Fujisawa, S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression by eugenol-related compounds. Anticancer Res. 2005, 25, 3263–3269. [Google Scholar]
- Slameňová, D.; Horváthová, E.; Wsólová, L.; Šramková, M.; Navarová, J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat. Res. 2009, 677, 46–52. [Google Scholar]
- Munerato, M.C.; Sinigaglia, M.; Reguly, M.L.; de Andrade, H.L.R. Genotoxic effects of eugenol, isoeugenol and safrole in the wing spot test of Drosophila melanogaster. Mutat. Res. 2005, 582, 87–94. [Google Scholar] [CrossRef]
- Maralhas, A.; Monteiro, A.; Martins, C.; Kranendonk, M.; Laires, A.; Rueff, J.; Rodrigues, A.S. Genotoxicity and endoreduplication inducing activity of the food flavouring eugenol. Mutagenesis. 2006, 21, 199–204. [Google Scholar] [CrossRef]
- Clark, G.C. Acute inhalation toxicity of eugenol in rats. Arch. Toxicol. 1988, 62, 381–386. [Google Scholar] [CrossRef]
- Hilton, J.; Dearman, R.J.; Fielding, I.; Basketter, D.A.; Kimber, I. Evaluation of the sensitizing potential of eugenol and isoeugenol in mice and guinea pigs. J. Appl. Toxicol. 1996, 16, 459–464. [Google Scholar] [CrossRef]
- Wright, S.E.; Baron, D.A.; Heffner, J.E. Intravenous eugenol causes hemorrhagic lung edema in rats: proposed oxidant mechanisms. J. Lab. Clin. Med. 1995, 125, 257–264. [Google Scholar]
- Sarrami, N.; Pemberton, M.N.; Thornhill, M.H.; Theaker, E.D. Adverse reactions associated with the use of eugenol in dentistry. Brit. Dent. J. 2002, 193, 257–259. [Google Scholar]
- Fisher, I.U.; von Unruh, G.E.; Dangler, H.J. The metabolism of eugenol in man. Xenobiotica 1990, 20, 209–222. [Google Scholar] [CrossRef]
- Thompson, D.C.; Constantin, T.; Eodosiu, D.; Moldeus, P. Metabolism and cytoxicity of eugenol in isolated rat hepatocytes. Chem.-Biol. Interact. 1991, 77, 137–147. [Google Scholar]
- Buckley, D.A.; Rycroft, R.J.G.; White, I.R.; McFadden, J.P. Fragrance as an occupational allergen. Occup. Med. 2002, 52, 13–16. [Google Scholar] [CrossRef]
- Heisterberg, M.V.; Menné, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU cosmetics directive. Contact Derm. 2011, 65, 266–275. [Google Scholar] [CrossRef]
- Galbiati, V.; Mitjans, M.; Lucchi, L.; Viviani, B.; Galli, C.L.; Marinovich, M.; Corsini, E. Further development of the NCTC 2544 IL-18 assay to identify in vitro contact allergens. Toxicol. In Vitro 2011, 25, 724–732. [Google Scholar]
- Rothenstein, A.S.; Booman, K.A.; Dorsky, J. Eugenol and clove leaf oil: A survey of consumer patch-test sensitization. Food Chem. Toxicol. 1983, 21, 727–733. [Google Scholar] [CrossRef]
- Ohsumi, T.; Higashi, S.; Ozumi, K.; Kuroki, K. Study on allergic contact dermatitis of eugenol in guinea pig. Oral Ther. Pharmacol. 1996, 15, 63–68. [Google Scholar]
- Svedman, C.; Engfeldt, M.; Api, A.M.; Politano, V.T.; Belsito, D.V.; Isaksson, M.; Bruze, M. A pilot study aimed at finding a suitable eugenol concentration for a leave-on product for use in a repeated open application test. Contact Dermatitis 2012, 66, 137–139. [Google Scholar] [CrossRef]
- Bouhlel, C.; Dolhem, G.; Fernandez, X.; Antoniotti, S. Model study of the enzymatic modification of natural extracts: Peroxidase-based removal of eugenol from rose essential oil. J. Agric. Food Chem. 2012, 60, 1052–1058. [Google Scholar] [CrossRef]
- Robin, O.; Alaoui-Ismaïli, O.; Dittmar, A.; Vernet-Maury, E. Basic emotions evoked by eugenol odor differ according to the dental experience. A neurovegetative analysis. Chem. Senses 1999, 24, 327–335. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules 2012, 17, 6953-6981. https://doi.org/10.3390/molecules17066953
Kamatou GP, Vermaak I, Viljoen AM. Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules. 2012; 17(6):6953-6981. https://doi.org/10.3390/molecules17066953
Chicago/Turabian StyleKamatou, Guy P., Ilze Vermaak, and Alvaro M. Viljoen. 2012. "Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule" Molecules 17, no. 6: 6953-6981. https://doi.org/10.3390/molecules17066953
APA StyleKamatou, G. P., Vermaak, I., & Viljoen, A. M. (2012). Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules, 17(6), 6953-6981. https://doi.org/10.3390/molecules17066953




