Reaction of Iodonium Ylides of 1,3-Dicarbonyl Compounds with HF Reagents
Abstract
:1. Introduction

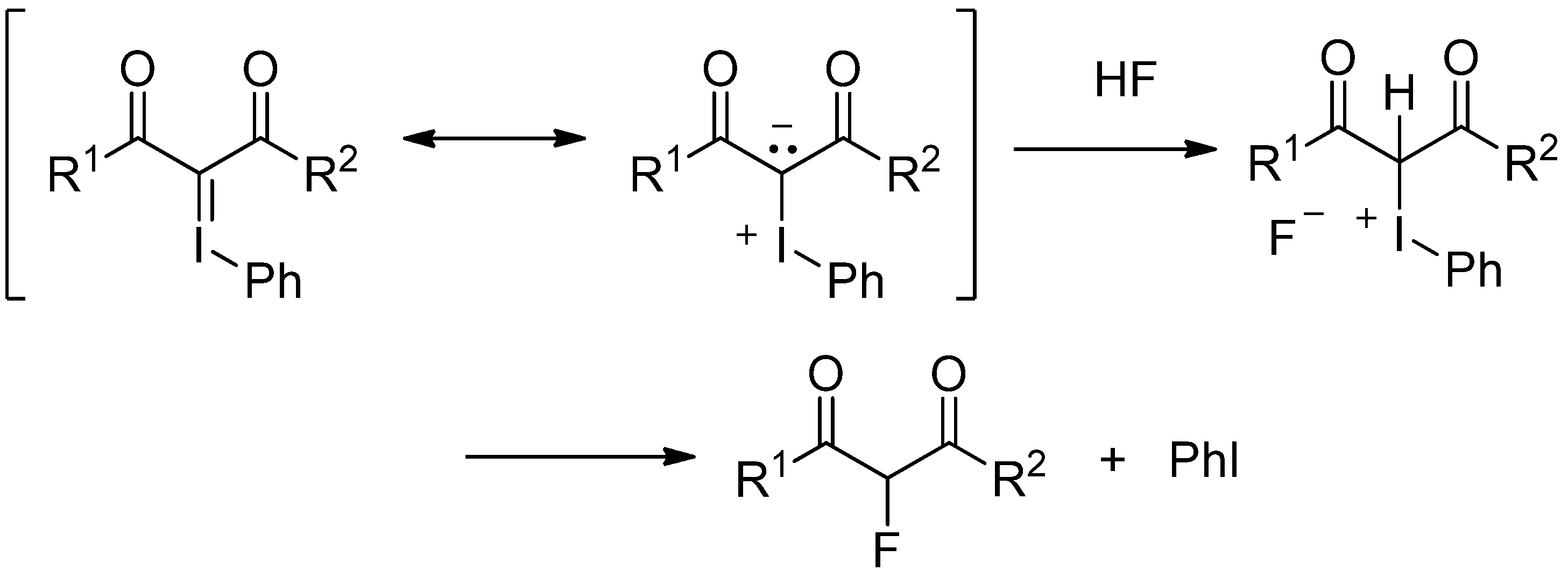
2. Results and Discussion
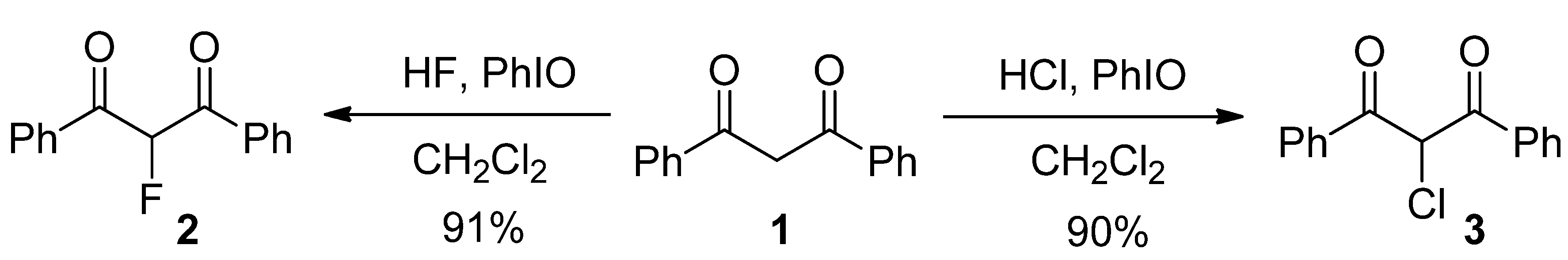
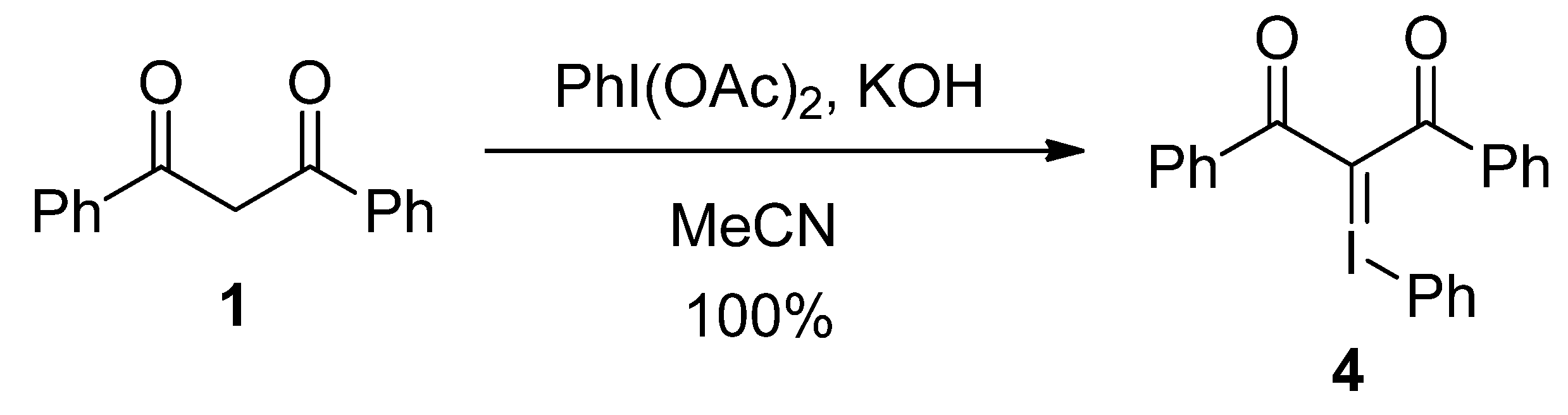
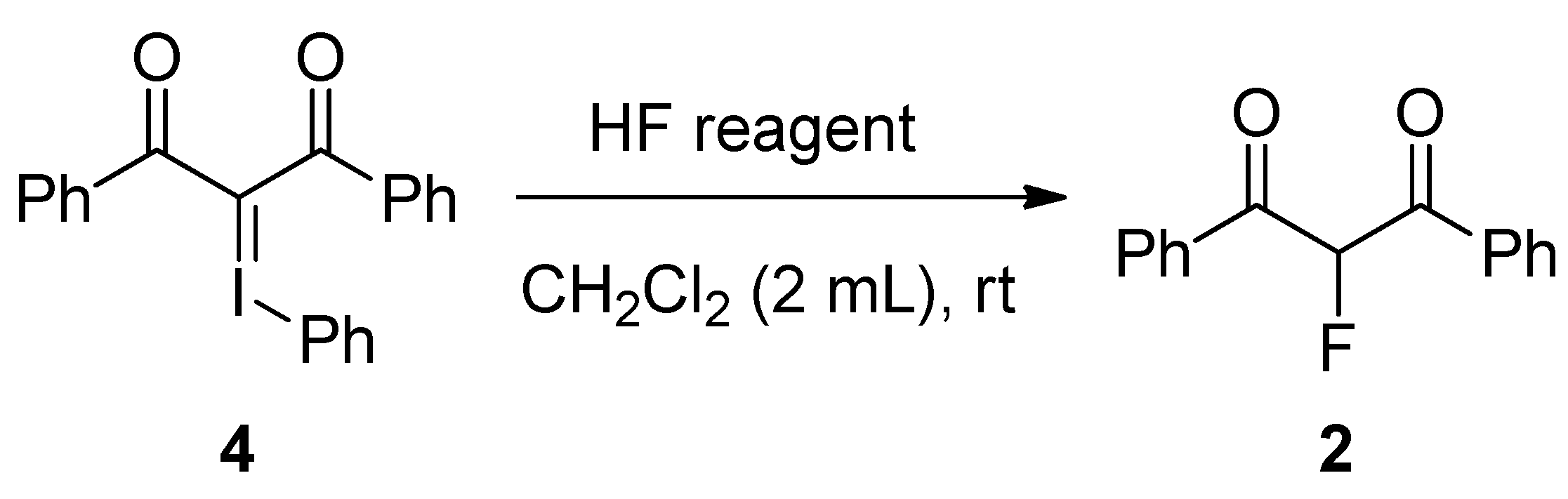
| Entry | HF reagent | Time (h) | Yield of 2 (%) b |
|---|---|---|---|
| 1 | 55% HF | 17 | 20 |
| 2 | TEA·3HF | 17 | 45 |
| 3 | TEA·5HF | 17 | 32 |
| 4 | TEA·3HF | 1.5 | 41 |
| 5 | TEA·3HF c | 1.5 | 50 |
| 6 | TEA·3HF c,d | 36 | 14 |



3. Experimental
3.1. General
3.2. General Procedure for the Reaction of Iodonium Ylides with HF or HCl Reagents
4. Conclusions
References and Notes
- Gudriniece, E.; Neiland, O.; Vanags, G. Iodonium derivatives of β-diketones. I. Reactions of dimedon with iodosobenzene. Zh. Obsch. Khim. 1957, 27, 2737–2740. [Google Scholar]
- Koser, G.F. Hypervalent Halogen Compounds. In The Chemistry of Functional Groups, Supplement D; Patai, S., Rappoport, Z., Eds.; John Wiley & Sons: New York, NY, USA, 1983; pp. 721–811, Chapter 18. [Google Scholar]
- Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Ochiai, M.; Kitagawa, Y. Reaction of λ3-vinyliodanes: Generation and alkylidene-transfer of monocarbonyl iodonium ylides. J. Syn. Org. Chem. Jpn. 2000, 58, 1048–1056. [Google Scholar] [CrossRef]
- Kirmse, W. Carbene complexes of nonmetals. Eur. J. Org. Chem. 2005, 237–260. [Google Scholar] [CrossRef]
- Muller, P. Asymmetric transfer of carbenes with phenyliodonium ylides. Acc. Chem. Res. 2004, 37, 243–251. [Google Scholar] [CrossRef]
- Moriarty, R.M. Organohypervalent iodine: Development, applications, and future directions. J. Org. Chem. 2005, 70, 2893–2903. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Stang, P.J. Chemistry of polyvalent iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef]
- Malamidou-Xenikaki, E.; Spyroudis, S. Zwitterionic iodonium compounds: Useful tools in organic synthesis. Synlett 2008, 2725–2740. [Google Scholar]
- Kitamura, T.; Kuriki, S.; Morshed, M.H.; Hori, Y. A Practical and convenient fluorination of 1,3-dicarbonyl compounds using aqueous HF in the presence of iodosylbenzene. Org. Lett. 2011, 13, 2392–2394. [Google Scholar] [CrossRef]
- Kitamura, T.; Tazawa, Y.; Morshed, M.H.; Kobayashi, S. Convenient chlorination with concentrated hydrochloric acid in the presence of iodosylbenzene. Synthesis 2012, 44, 1159–1162. [Google Scholar] [CrossRef]
- Neilands, O. Iodonium derivatives of β-diketones. VII. Reaction of phenyliodosoacetate with benzoylacetone and dibenzoylmethane. Zh. Org. Khim. 1965, 1, 1858–1862. [Google Scholar]
- Schank, K.; Lick, C. Ozonolytic fragmentation of phenyliodonium β-diketonates; a convenient synthesis of unsolvated vic-triketones. Synthesis 1983, 392–395. [Google Scholar] [CrossRef]
- Goudreau, S.R.; Marcoux, D.; Charette, A.B. General method for the synthesis of phenyliodonium ylides from malonate esters: Easy access to 1,1-cyclopropane diesters. J. Org. Chem. 2009, 74, 470–473. [Google Scholar] [CrossRef]
- Papadopoulou, M.; Varvoglis, A. The reaction of (disaccharinyliodo)benzene with ketones and active methylene compounds. J. Chem. Res. (S) 1984, 166–167. [Google Scholar]
- Yoshida, M.; Fujikawa, K.; Sato, S.; Hara, S. α-Fluorination of β-dicarbonyl compounds using p-iodotoluene difluoride under neutral conditions. ARKIVOC 2003, 36–42. [Google Scholar]
- Linderman, R.J.; Graves, D.M. Oxidation of fluoroalkyl-substituted carbinols by the Dess-Martin reagent. J. Org. Chem. 1989, 54, 661–668. [Google Scholar] [CrossRef]
- Zhang, J.; DesMarteau, D.D.; Zuberi, S.; Ma, J.-J.; Xue, L.; Gillette, S.M.; Blau, H.; Gerhardt, R. Synthesis of the first difunctional N-fluoro perfluoroalkylsulfonylimides, CF3SO2NFSO2(CF2)nSO2NFSO2CF3. J. Fluorine Chem. 2002, 116, 45–48. [Google Scholar] [CrossRef]
- Roshchupkina, G.I.; Gatilov, Y.V.; Rybalova, T.V.; Reznikov, V.A. Reactions of 1,3-diaryl-2-chloropropane-1,3-diones with nucleophiles-cyanide-induced retro-Claisen-Claisen condensation. Eur. J. Org. Chem. 2004, 1765–1773. [Google Scholar]
- Sample Availability: Samples of the compounds 2, 3, 6, 8, and 10 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gondo, K.; Kitamura, T. Reaction of Iodonium Ylides of 1,3-Dicarbonyl Compounds with HF Reagents. Molecules 2012, 17, 6625-6632. https://doi.org/10.3390/molecules17066625
Gondo K, Kitamura T. Reaction of Iodonium Ylides of 1,3-Dicarbonyl Compounds with HF Reagents. Molecules. 2012; 17(6):6625-6632. https://doi.org/10.3390/molecules17066625
Chicago/Turabian StyleGondo, Keisuke, and Tsugio Kitamura. 2012. "Reaction of Iodonium Ylides of 1,3-Dicarbonyl Compounds with HF Reagents" Molecules 17, no. 6: 6625-6632. https://doi.org/10.3390/molecules17066625
APA StyleGondo, K., & Kitamura, T. (2012). Reaction of Iodonium Ylides of 1,3-Dicarbonyl Compounds with HF Reagents. Molecules, 17(6), 6625-6632. https://doi.org/10.3390/molecules17066625




