Synthesis of Chalcones with Anticancer Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Chalcones
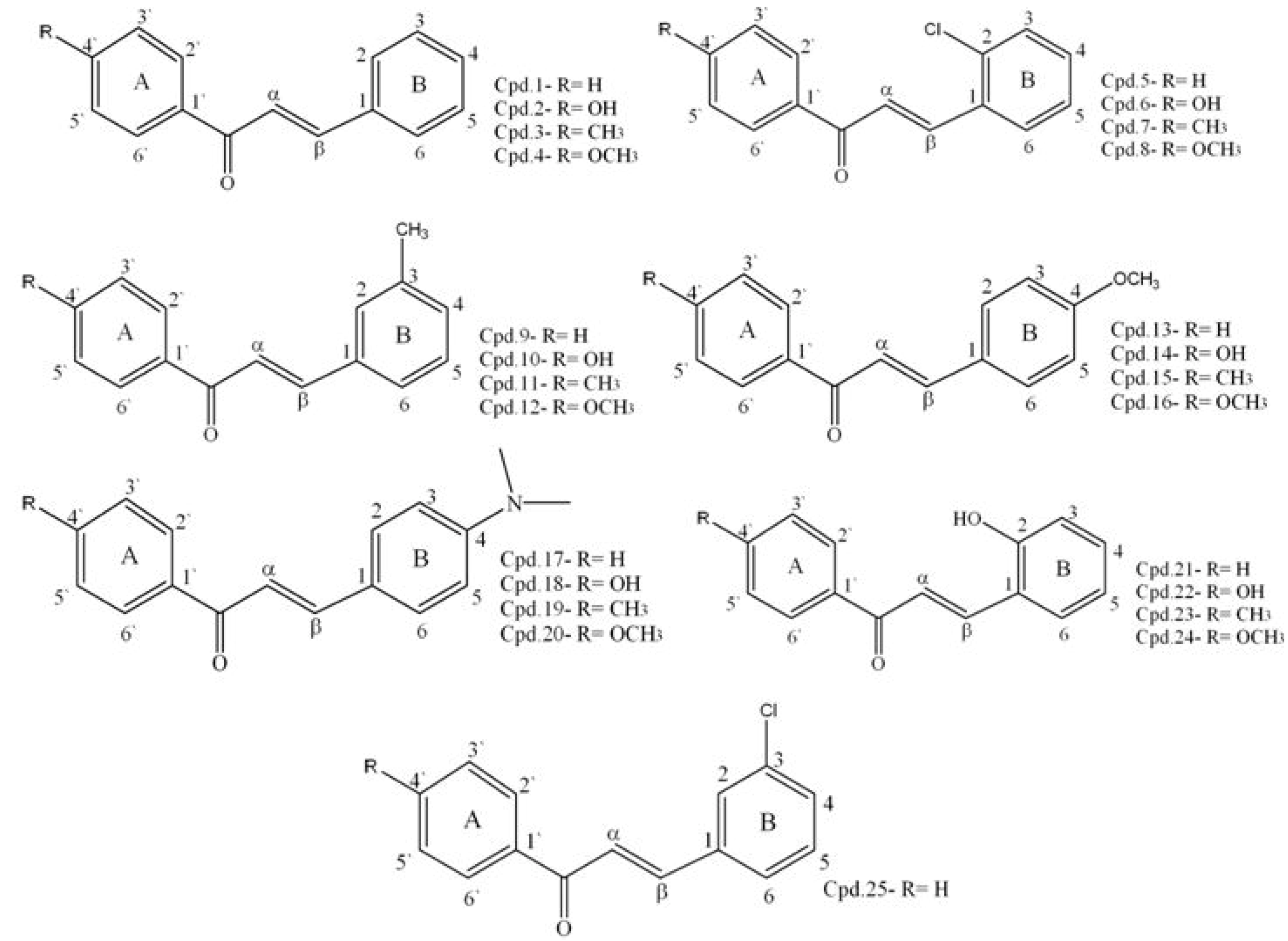
| No | Reactants | Chalcone (Chemical name) | Formula |
|---|---|---|---|
| 1 | Acetophenone + Benzaldehyde | 1,3-Diphenylpropenone | C15H12O |
| 2 | 4H Acetophenone + Benzaldehyde | 1-(4-Hydroxyphenyl)-3-phenylpropenone | C15H12O2 |
| 3 | 4-Methylacetophenone + Benzaldehyde | 3-Phenyl-1- p-tolylpropenone | C16H14O |
| 4 | 4-Methoxyacetophenone + Benzaldehyde | 1-(4-Methoxyphenyl)-3-phenylpropenone | C16H14O2 |
| 5 | Acetophenone + 2-Chloro-benzaldehyde | 3-(2-Chlorophenyl)-1-phenylpropenone | C15H11OCl |
| 6 | 4H Acetophenone + 2-Chloro-benzaldehyde | 3-(2-Chlorophenyl)-(4-hydroxyphenyl)-propenone | C15H11O2Cl |
| 7 | 4-Methylacetophenone + 2-Chloro-benzaldehyde | 3-(2-Chlorophenyl)-1-P-tolylpropenone | C16H13OCl |
| 8 | 4-Methoxyacetophenone + 2-Chloro-benzaldehyde | 3-(2-Chlorophenyl)-1-(4-methoxy-phenyl)propenone | C16H13O2Cl |
| 9 | Acetophenone + 3-Methyl-benzaldehyde | 1-Phenyl-3- m-tolylpropenone | C16H14O |
| 10 | 4H Acetophenone + 3-Methyl-benzaldehyde | 1-(4-Hydroxyphenyl)-3- m-tolylpropenone | C16H14O2 |
| 11 | 4-Methylacetophenone + 3-Methyl-benzaldehyde | 3- m-Tolyl-1-p-tolylpropenone | C17H16O |
| 12 | 4-Methoxyacetophenone + 3-Methyl-benzaldehyde | 1-(4-Methoxyphenyl)-3- m-tolylpropenone | C17H16O2 |
| 13 | Acetophenone + Anisaldehyde | 3-(4-Methoxyphenyl)-1-phenylpropenone | C16H14O2 |
| 14 | 4H Acetophenone + Anisaldehyde | 1-(4-Hydroxyphenyl)-3-(4-methoxyphenyl)-propenone | C16H15O3 |
| 15 | 4-Methylacetophenone + Anisaldehyde | 3-(4-Methoxyphenyl)-1- p-tolylpropenone | C17H16O2 |
| 16 | 4-Methoxyacetophenone + Anisaldehyde | 1,3-Bis-(4-Methoxyphenyl)-propenone | C17H16O3 |
| 17 | Acetophenone + 4-Dimethylamino- benzaldehyde | 3-(4-Dimethylaminophenyl)-1-phenyl-propenone | C17H17NO |
| 18 | 4H Acetophenone + 4-Dimethylamino-benzaldehyde | 3-(4-Dimethylaminophenyl)-1-(4-hydroxy-phenyl)-propenone | C17H17NO2 |
| 19 | 4-Methylacetophenone + 4-Dimethylamino- benzaldehyde | 3-(4-Dimethylaminophenyl)-1- p-tolyl-propenone | C18H19NO |
| 20 | 4-Methoxyacetophenone + 4-Dimethylamino-benzaldehyde | 3-(4-Dimethylaminophenyl)-1-(4-methoxy-phenyl)-propenone | C18H19NO2 |
| 21 | Acetophenone + Salicylaldehyde | 3-(2-Hydroxyphenyl)-1-phenylpropenone | C15H12O2 |
| 22 | 4H Acetophenone + Salicylaldehyde | 3-(2-Hydroxyphenyl)-1-(4-hydroxyphenyl)-propenone | C15H12O3 |
| 23 | 4-Methylacetophenone + Salicylaldehyde | 3-(2-Hydroxyphenyl)-1- p-tolylpropenone | C16H14O2 |
| 24 | 4-Methoxyacetophenone + Salicylaldehyde | 3-(2-Hydroxyphenyl)-1-(4-methoxyphenyl)-propenone | C16H14O3 |
| 25 | Acetophenone + 3-Chlorobenzaldehyde | 3-(3-Chlorophenyl)-1-phenylpropenone | C15H11OCl |
| Cpd. | IR-Spectrum (KBr), сm−1 | 1H-NMR spectrum (δ, ppm) | Yield (%) | m.p. (°C) |
|---|---|---|---|---|
| 1 | 3030 (C-H aromatic), 1664 (C=O), 1598 (C=C) | 7.77 (d, 1Hα), 8.08 (d, 1Hβ), 7.4–8 (m, 10H, Ar-H) | 70–80 | 56–57 |
| 2 | 3083 (C-H aromatic), 1680 (C=O), 1580 (C=C), 3380 (-OH) | 7.56 (d, 1Hα), 7.99 (d, 1Hβ), 7.04–7.9 (m, 9H, Ar-H), 12.9 (s, 1H, -OH) | 35–45 | 120–121 |
| 3 | 3030 (C-H aromatic), 1649 (C=O), 1598 (C=C) | 7.38 (d, 1Hα), 8.1 (d, 1Hβ), 7.5–7.9 (m, 9H, Ar-H), 2.29 (s, 3H, -CH3) | 70–73 | 70–72 |
| 4 | 3047 (C-H aromatic), 1649 (C=O), 1603 (C=C), 1128 (-OCH3) | 7.19 (d, 1Hα), 8.2 (d, 1Hβ), 7.29–7.9 (m, 9H, Ar-H), 3.79 (s, 3H, -OCH3) | 70–74 | 108–110 |
| 5 | 3047 (C-H aromatic), 1664 (C=O), 1608(C=C), 829 (Ar-Cl) | 7.9 (d,1Hα), 8.2 (d, 1Hβ), 7.39–8 (m, 9H, Ar-H) | 75–80 | 53–54 |
| 6 | 3452 (-OH), 1690 (C=O), 1613 (C=C), 829 (Ar-Cl) | 7.5 (d, 1Hα), 8.19 (d, 1Hβ), 6.4–8 (m, 8H, Ar-H), 12.7 (s, 1H, -OH) | 40–43 | 191–193 |
| 7 | 3052 (C-H aromatic), 1664 (C=O), 1598 (C=C), 829 (Ar-Cl) | 7.9 (d, 1Hα), 8.19 (d, 1Hβ), 7.3–8.1 (m, 8H, Ar-H), 2.3 (s, 3H, -CH3) | 70–75 | 50–51 |
| 8 | 3034 (C-H aromatic), 1664 (C=O), 1590 (C=C), 1130 (-OCH3), 828 (Ar-Cl) | 7 (d, 1Hα), 7.7 (d, 1Hβ), 6.9–8.1 (m, 8H, Ar-H), 3.79 (s, 3H, -OCH3) | 60–65 | 124–126 |
| 9 | 3057 (C-H aromatic), 1664 (C=O), 1590 (C=C) | 7.19 (d,1Hα), 8.2 (d, 1Hβ), 7.2–8 (m, 9H, Ar-H), 2.3 (s, 3H, -CH3) | 68–70 | 68–70 |
| 10 | 3457 (-OH), 3011 (C-H aromatic), 1685 (C=O), 1591 (C=C) | 7.2 (d, 1Hα), 7.7 (d, 1Hβ), 7.1–8 (m, 8H, Ar-H), 12.8 (s, 1H, -OH), 2.3 (s, 3H, CH3) | 30 | 114–116 |
| 11 | 3027 (C-H aromatic), 1654 (C=O), 1593 (C=C) | 7.8 (d, 1Hα), 8.2 (d, 1Hβ), 7.1–8 (m, 8H, Ar-H), 2.3 (s, 3H, -CH3) | 84 | 89–91 |
| 12 | 3011 (C-H aromatic), 1659 (C=O), 1588 (C=C), 1126 (-OCH3) | 7.19 (d, 1Hα), 8.21 (d, 1Hβ), 6.9–8 (m, 8H, Ar-H), 3.8 (s, 3H, -OCH3), 2.96 (s, 3H, CH3) | 80 | 74–75 |
| 13 | 3057 (C-H aromatic), 1659 (C=O), 1588 (C=C), 1168 (-OCH3) | 7 (d, 1Hα), 8.2 (d, 1Hβ), 6.9–8.2 (m, 9H, Ar-H), 3.8 (s, 3H, -OCH3) | 80 | 77–78 |
| 14 | 3380 (-OH), 3035 (C-H aromatic) 1660 (C=O), 1590 (C=C), 1166 (-OCH3) | 6.9 (d, 1Hα), 7.9 (d, 1Hβ), 6.7–8.1 (m, 8H, Ar-H), 12.8 (s, 1H, -OH), 3.8 (s, 3H, -OCH3) | 35 | 179–181 |
| 15 | 3080 (C-H aromatic), 1649 (C=O), 1588 (C=C), 1170 (-OCH3) | 7.7 (d, 1Hα), 8.2 (d, 1Hβ), 6.9–8.2 (m, 8H, Ar-H), 2.29 (s, 3H, -CH3), 3.8 (s, 3H, -OCH3) | 85–90 | 99–100 |
| 16 | 3033 (C-H aromatic), 1659 (C=O), 1588 (C=C), 1168 (-OCH3) | 7.7 (d, 1Hα), 8.2 (d, 1Hβ), 6.9–8.2 (m, 8H, Ar-H), 3.8(s, 3H, -OCH3), 3.8 (s, 3H, OCH3) | 80–88 | 102–103 |
| 17 | 3010 (C-H aromatic), 1654(C=O), 1562(C=C), 1340 (C-N) | 6.7 (d, 1Hα), 8.21 (d, 1Hβ), 7.3–7.8 (m, 9H, Ar-H), 2.9 (s, 6H, N-(CH3)2) | 80 | 111–113 |
| 18 | 3380 (-OH), 3012 (C-H aromatic), 1664 (C=O), 1598 (C=C), 1372 (C-N) | 6.8 (d, 1Hα), 8.7 (d, 1Hβ), 6.8–8.8 (m, 8H, Ar-H), 9.7 (s, 1H, -OH), 2.99 (s, 6H, N-(CH3)2) | 47 | 76–78 |
| 19 | 3083 (C-H aromatic), 1649 (C=O), 1603 (C=C), 1360 (C-N) | 6.7 (d, 1Hα), 8.21 (d, 1Hβ), 7.3–8 (m, 8H, Ar-H), 2.98 (s, 6H, N-(CH3)2), 2.3 (s, 3H, -CH3) | 70 | 124–125 |
| 20 | 3010 (C-H aromatic), 1650 (C=O), 1593 (C=C), 1161 (-OCH3), 1330 (C-N) | 6.7 (d, 1Hα), 8.2 (d, 1Hβ), 6.7–8.1 (m, 8H, Ar-H), 2.9 (s, 6H, N-(CH3)2), 3.79 (s, 3H, -OCH3) | 86–90 | 129–131 |
| 21 | 1639 (C=O), 1557 (C=C), 3206 (-OH), 3010 (C-H aromatic) | 7.6 (d, 1Hα), 8.2 (d, 1Hβ), 6.7–8.2 (m, 9H, Ar-H), 10.3 (s, 1H, -OH) | 50 | 154–155 |
| 22 | 1669 (C=O), 1590 (C=C), 3467 (-OH), 3005 (C-H aromatic) | 7.8 (d, 1Hα), 7.5 (d, 1Hβ), 6.3–8.3 (m, 8H, Ar-H), 10.2 (s, 1H, -OH), 10.2 (s, 1H, -OH) | 8–10 | 287–289 |
| 23 | 1644 (C=O), 1577 (C=C) , 3216 (-OH), 3030 (C-H aromatic) | 6.8 (d, 1Hα), 7.8 (d, 1Hβ), 6.7–8.2 (m, 8H, Ar-H), 10.2 (s, 1H, -OH), 2.28 (s, 3H, -CH3) | 37–40 | 165–167 |
| 24 | 3252 (-OH), 3000 (C-H aromatic), 1640 (C=O), 1603 (C=C), 1165 (-OCH3) | 7.96 (d, 1Hα), 8.19 (d, 1Hβ), 6.7–8.2 (m, 8H, Ar-H), 10.19 (s, 1H, -OH), 3.69 (s, 3H, OCH3) | 45 | 151–153 |
| 25 | 1654 (C=O), 1603 (C=C), 3052 (C-H aromatic), 823 (Ar-Cl) | 7.9 (d, 1Hα), 8.2 (d, 1Hβ), 7.2–8 (m, 9H, Ar-H) | 80 | 78–80 |
2.2. Cytotoxic Screening
| Compound | Cell lines/IC50 values (µg/mL) | ||||
|---|---|---|---|---|---|
| A549 | PC3 | MCF-7 | HT-29 | WRL68 | |
| 1 | 16.76 ± 1.08 | 9.108 ± 0.9 | 6.875 ± 0.219 | 10.1 ± 1.01 | 10.55 ± 0.89 |
| 2 | >100 | >100 | >100 | >100 | >100 |
| 3 | 36.58 ± 1.76 | 17.30 ± 1.1 | 13.62 ± 1.01 | 19.10 ± 1.00 | 21.34 ± 3.01 |
| 4 | 77.04 ± 2.1 | 21.13 ± 1.24 | 19.15 ± 1.0 | 37.28 ± 2.81 | 57.29 ± 3.8 |
| 5 | 19.94 ± 1.66 | 13.84 ± 1.2 | 7.992 ± 0.81 | 13.24 ± 1.2 | 11.46 ± 1.1 |
| 6 | 25.22 ± 1.21 | 10.99 ± 1.8 | 10.01 ± 1.4 | 15.52 ± | 13.22 ± 1.3 |
| 7 | >100 | >100 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 |
| 9 | 24.74 ± 2.0 | 9.40 ± 1.9 | 9.34 ± 0.56 | 18.96 ± 1.5 | 9.78 ± 1.01 |
| 10 | 19.68 ± 1.22 | 13.71 ±1.1 | 8.343 ± 0.472 | 12.1 ± 3.0 | 13.45 ± 2.09 |
| 11 | 24.89 ± 1.9 | 8.73 ± 0.21 | 10.16 ± 1.89 | 16.30 ± 1.9 | 13.95 ± 1.3 |
| 12 | 31.76 ± 1.5 | 9.87 ± 0.71 | 9.53 ± 1.99 | 15.76 ± 2.1 | 11.13 ± 1.8 |
| 13 | 32.57 ± 2.5 | 12.09 ± 0.99 | 11.62 ± 0.101 | 22.79 ± 2.3 | 21.46 ± 1.7 |
| 14 | >100 | >100 | >100 | >100 | >100 |
| 15 | >100 | 43.27 ± 4.01 | 32.37 ± 1.88 | 29.55 ± 2.8 | 50.94 ± 3.51 |
| 16 | >100 | >100 | 41.44 ± 1.91 | 67.07 ± 4.1 | 60.79 ± 4.22 |
| 17 | >100 | >100 | 99.29 ± 6.16 | 64.26 ± 5.02 | >100 |
| 18 | >100 | >100 | >100 | >100 | >100 |
| 19 | 58.54 ± 3.2 | >100 | 57.28 ± 3.1 | 69.54 ± 4.23 | 39.78 ± 3.9 |
| 20 | 78.85 ± 4.4 | >100 | >100 | >100 | >100 |
| 21 | 22.61 ± 1.1 | 11.07 ± 0.4 | 9.353 ± 1.2 | 19.84 ± 2.01 | 17.02 ± 1.7 |
| 22 | >100 | >100 | >100 | >100 | >100 |
| 23 | 16.79 ± 1.76 | 9.492 ± 0.7 | 6.873 ± 1.2 | 12.98 ± 0.54 | 9.533 ± 1.5 |
| 24 | 14.16 ± 1.0 | 5.584 ± 0.2 | 7.149 ± 0.4 | 11.43 ± 1.0 | 8.722 ± 1.0 |
| 25 | 14.49 ± 0.2 | 6.936 ± 0.61 | 5.251 ± 0.67 | 7.772 ± 1.1 | 7.72 ± 1.6 |
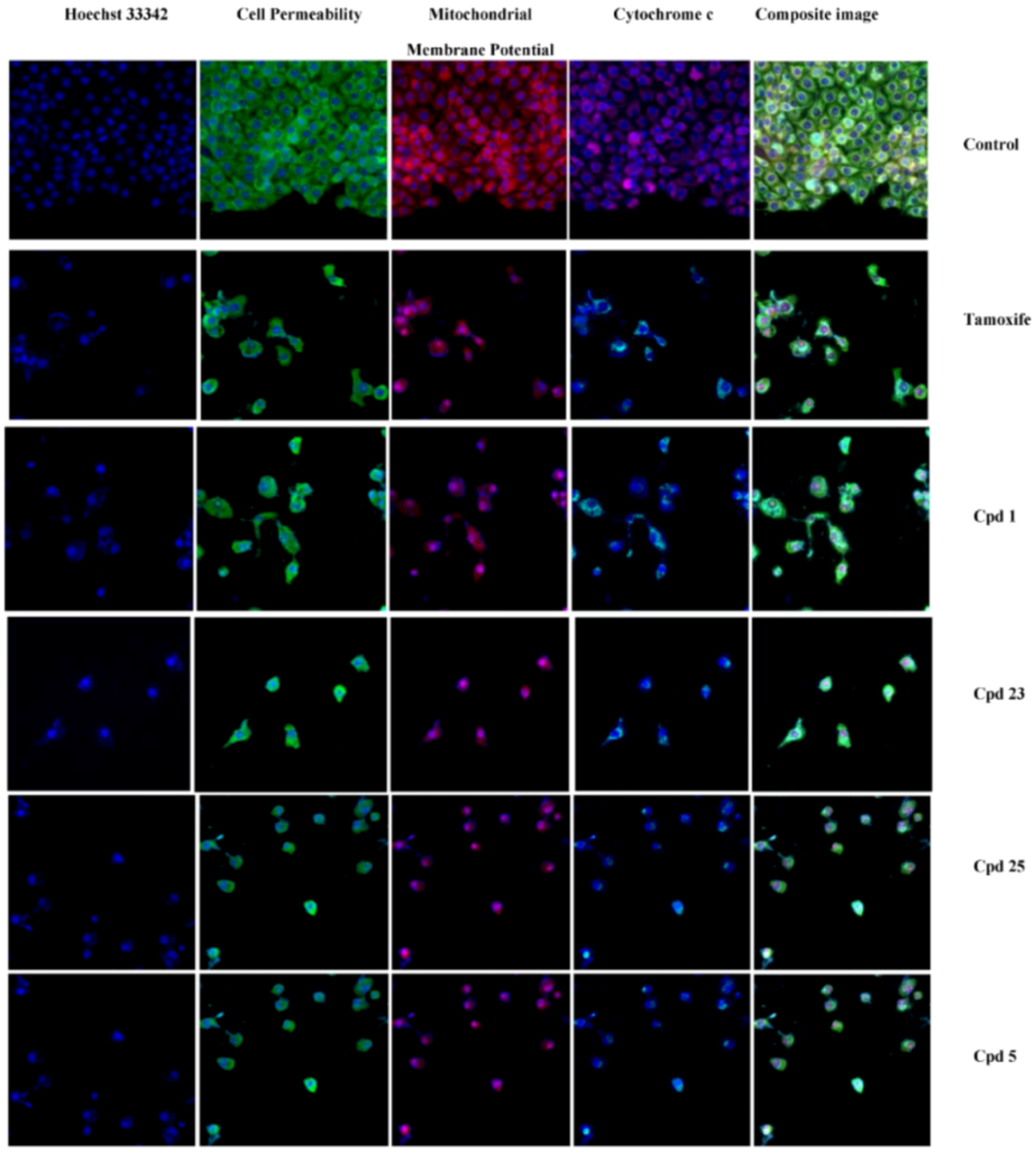
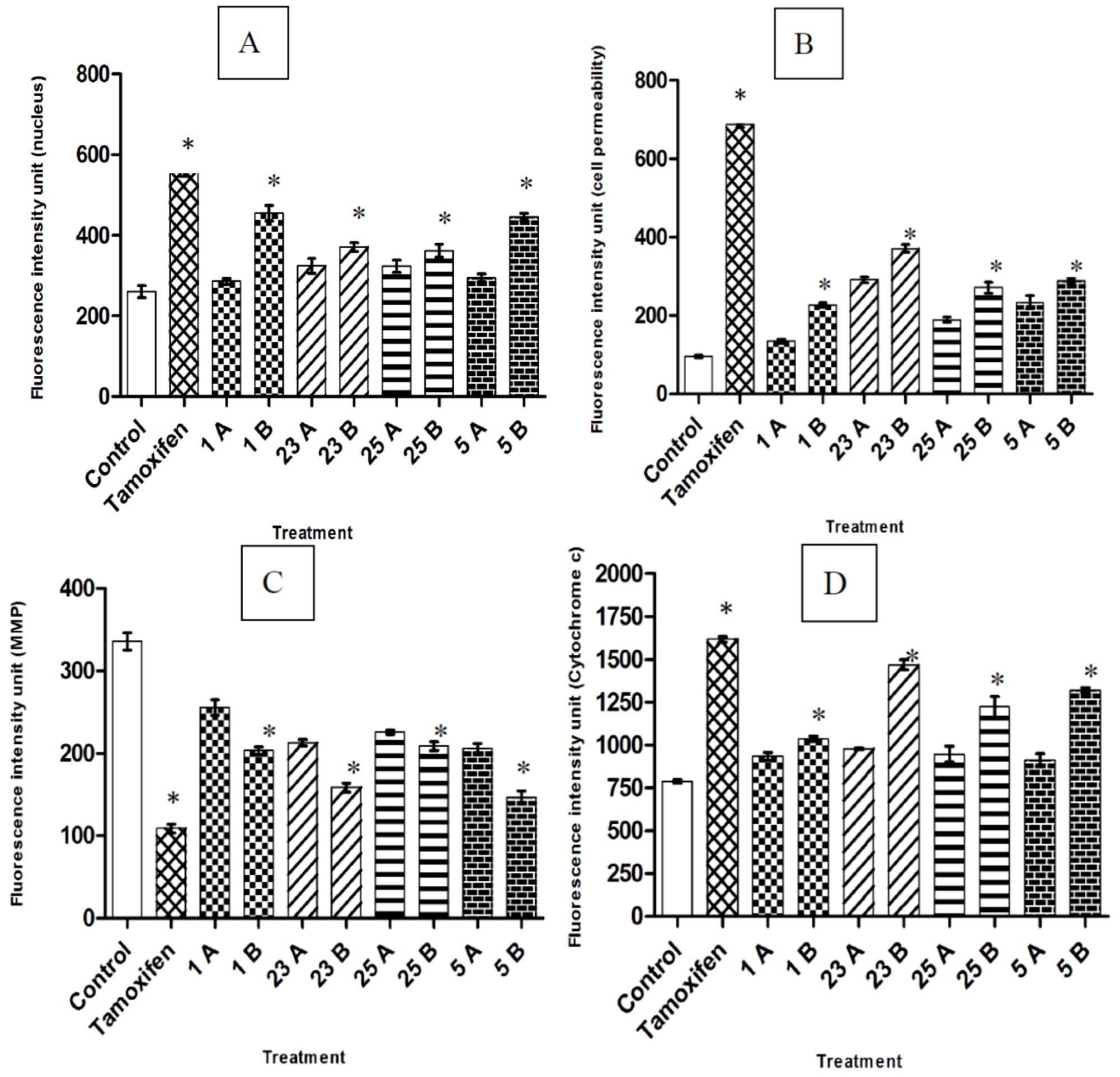
2.3. Multiparameter Cytotoxic Analysis
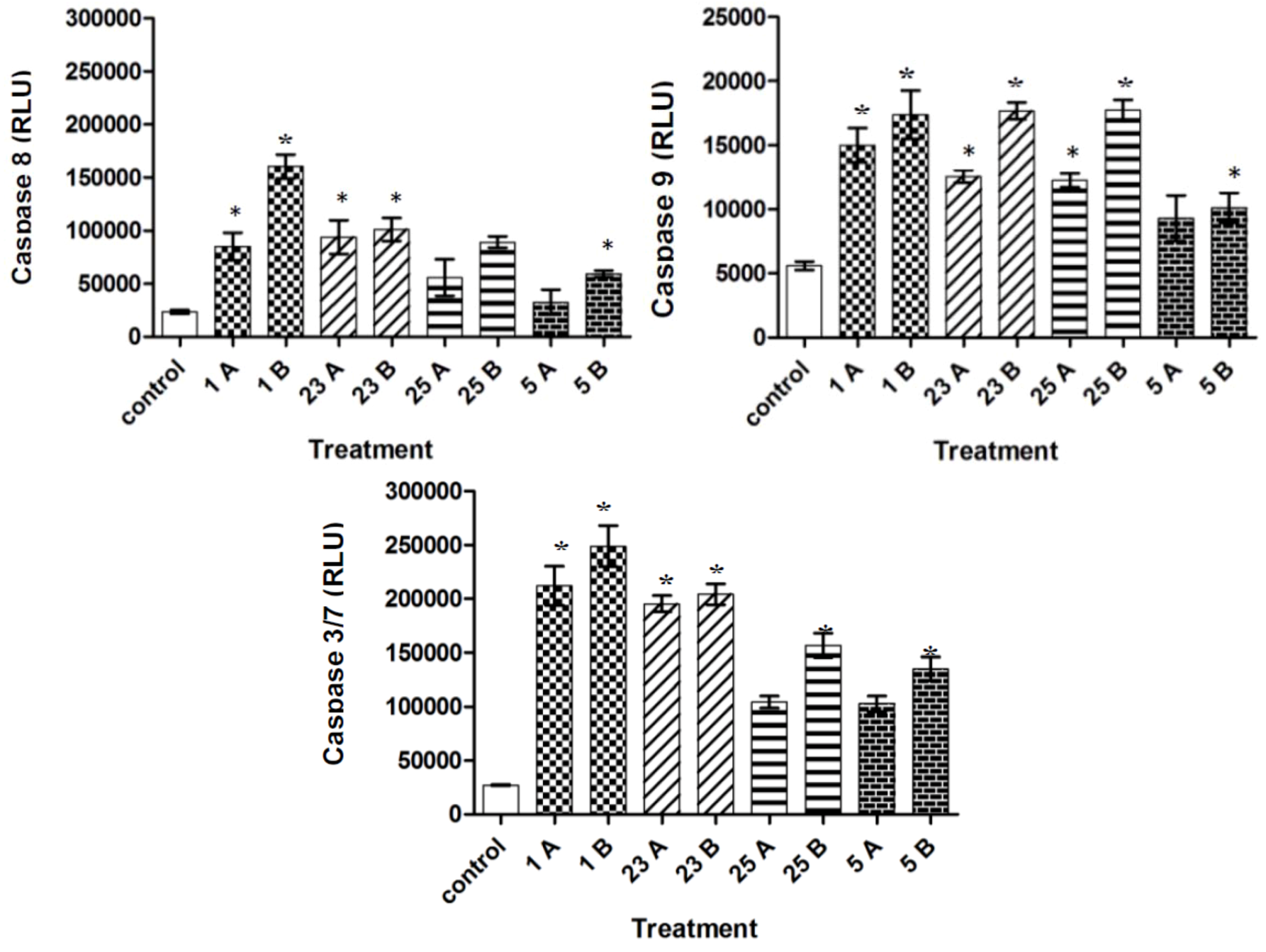
2.4. Caspase-3/7, -8, and -9 Activity
2.5. Intracellular ROS Level

3. Experimental
3.1. Synthesis of Chalcones
3.2. Identification of Chalcones
3.3. Cell Culture and Cell Viability Assay
3.4. Cell Treatment
3.5. Multiparametric High Content Screening (HCS) Assays
3.6. Caspase Activity
3.7. Intracellular ROS Level
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Hiss, D.C.; Gabriels, G.A. Implications of endoplasmic reticulum stress, the unfolded protein response and apoptosis for molecular cancer therapy. Part I: targeting p53, Mdm2, GADD153/CHOP, GRP78/BiP and heat shock proteins. Expert Opin. Drug Discov. 2009, 4, 799–821. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis-A Link between Cancer Genetics and Chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Trans. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Wang, W.; Rayburn, E.R.; Velu, S.E.; Nadkarni, D.H.; Murugesan, S.; Zhang, R. In vitro and in vivo anticancer activity of novel synthetic makaluvamine analogues. Clin. Cancer Res. 2009, 15, 3511–3518. [Google Scholar] [CrossRef]
- Nagy, A.; Schally, A.V.; Armatis, P.; Szepeshazi, K.; Halmos, G.; Kovacs, M.; Zarandi, M.; Groot, K.; Miyazaki, M.; Jungwirth, A. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500–1000 times more potent. Proc. Natl. Acad. Sci. USA 1996, 93, 7269–7273. [Google Scholar]
- Sirion, U.; Kasemsook, S.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. New substituted C-19-andrographolide analogues with potent cytotoxic activities. Bioorg. Med. Chem. Lett. 2011, 22, 49–52. [Google Scholar]
- Lin, C.M.; Jiang, Y.Q.; Chaudhary, A.G.; Rimoldi, J.M.; Kingston, D.G.I.; Hamel, E. A convenient tubulin-based quantitative assay for paclitaxel (Taxol) derivatives more effective in inducing assembly than the parent compound. Cancer Chemother. Pharmacol. 1996, 38, 136–140. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar]
- Kumar, D.; Kumar, N.M.; Akamatsu, K.; Kusaka, E.; Harada, H.; Ito, T. Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorg. Med. Chem. Lett. 2011, 20, 3916–3919. [Google Scholar]
- Sivakumar, P.M.; Ganesan, S.; Veluchamy, P.; Doble, M. Novel chalcones and 1, 3, 5-triphenyl-2-pyrazoline derivatives as antibacterial agents. Chem. Biol. Drug. Des. 2010, 76, 407–411. [Google Scholar] [CrossRef]
- Sakai, T.; Eskander, R.N.; Guo, Y.; Kim, K.J.; Mefford, J.; Hopkins, J.; Bhatia, N.N.; Zi, X.; Hoang, B.H. Flavokawain B, a kava chalcone, induces apoptosis in synovial sarcoma cell lines. J. Orthop. Res. 2011, 30, 1045–1050. [Google Scholar]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef]
- Hijova, E. Bioavailability of chalcones. Bratisl Lek Listy 2006, 107, 80–84. [Google Scholar]
- Zhang, H.J.; Qian, Y.; Zhu, D.D.; Yang, X.G.; Zhu, H.L. Synthesis, molecular modeling and biological evaluation of chalcone thiosemicarbazide derivatives as novel anticancer agents. Eur. J. Med. Chem. 2011, 46, 4702–4708. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorgan. Med. Chem. 2010, 18, 1364–1370. [Google Scholar]
- Bhat, B.A.; Dhar, K.L.; Puri, S.C.; Saxena, A.K.; Shanmugavel, M.; Qazi, G.N. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorgan. Med. Chem. Lett. 2005, 15, 3177–3180. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef]
- Ahmad, A.; Wang, Z.; Ali, R.; Kong, D.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Apoptosis-inducing effect of garcinol is mediated by NF-κB signaling in breast cancer cells. J. Cell. Biochem. 2010, 109, 1134–1141. [Google Scholar]
- Syam, S.; Abdul, A.B.; Sukari, M.A.; Mohan, S.; Abdelwahab, S.I.; Wah, T.S. The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest. Molecules 2011, 16, 7155–7170. [Google Scholar] [CrossRef]
- Ye, N.; Qin, J.; Shi, W.; Liu, X.; Lin, B. Cell-based high content screening using an integrated microfluidic device. Lab Chip 2007, 7, 1696–1704. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki–Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PAR. Carcinogenesis 2006, 27, 2018–2027. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Tzeng, W.S.; Lin, C.C. Chalcone inhibits the proliferation of human breast cancer cell by blocking cell cycle progression and inducing apoptosis. Food Chem. Toxicol. 2006, 44, 704–713. [Google Scholar] [CrossRef]
- He, Y.Y.; Huang, J.L.; Chignell, C.F. Cleavage of epidermal growth factor receptor by caspase during apoptosis is independent of its internalization. Oncogene 2005, 25, 1521–1531. [Google Scholar]
- Carter, B.Z.; Gronda, M.; Wang, Z.; Welsh, K.; Pinilla, C.; Andreeff, M.; Schober, W.D.; Nefzi, A.; Pond, G.R.; Mawji, I.A. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood 2005, 105, 4043–4050. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S. Selenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int. J. Biochem. Cell Biol. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Kuo, Y.F.; Su, Y.Z.; Tseng, Y.H.; Wang, S.Y.; Wang, H.M.; Chueh, P.J. Flavokawain B, a novel chalcone from from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar]
- Hseu, Y.C.; Lee, M.S.; Wu, C.R.; Cho, H.J.; Lin, K.Y.; Lai, G.H.; Wang, S.Y.; Kuo, Y.H.; Senthil Kumar, K.J.; Yang, H.L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ros generation and down-regulation of the Akt/p38 MAPK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef]
- Kim, T.H.; Seo, W.D.; Ryu, H.W.; Seo, H.R.; Jin, Y.B.; Lee, M.; Ji, Y.H.; Park, K.H.; Lee, Y.S. Anti-tumor effects by a synthetic chalcone compound is mediated by c-Myc-mediated reactive oxygen species production. Chem.-Biol. Interact. 2010, 188, 111–118. [Google Scholar] [CrossRef]
- Janicke, R.U.; Ng, P.; Sprengart, M.L.; Porter, A.G. Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 1998, 273, 15540–15545. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179-6195. https://doi.org/10.3390/molecules17066179
Syam S, Abdelwahab SI, Al-Mamary MA, Mohan S. Synthesis of Chalcones with Anticancer Activities. Molecules. 2012; 17(6):6179-6195. https://doi.org/10.3390/molecules17066179
Chicago/Turabian StyleSyam, Suvitha, Siddig Ibrahim Abdelwahab, Mohammed Ali Al-Mamary, and Syam Mohan. 2012. "Synthesis of Chalcones with Anticancer Activities" Molecules 17, no. 6: 6179-6195. https://doi.org/10.3390/molecules17066179
APA StyleSyam, S., Abdelwahab, S. I., Al-Mamary, M. A., & Mohan, S. (2012). Synthesis of Chalcones with Anticancer Activities. Molecules, 17(6), 6179-6195. https://doi.org/10.3390/molecules17066179






