Chemical Constituents of the Ethyl Acetate Extract of Belamcanda chinensis (L.) DC Roots and Their Antitumor Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification
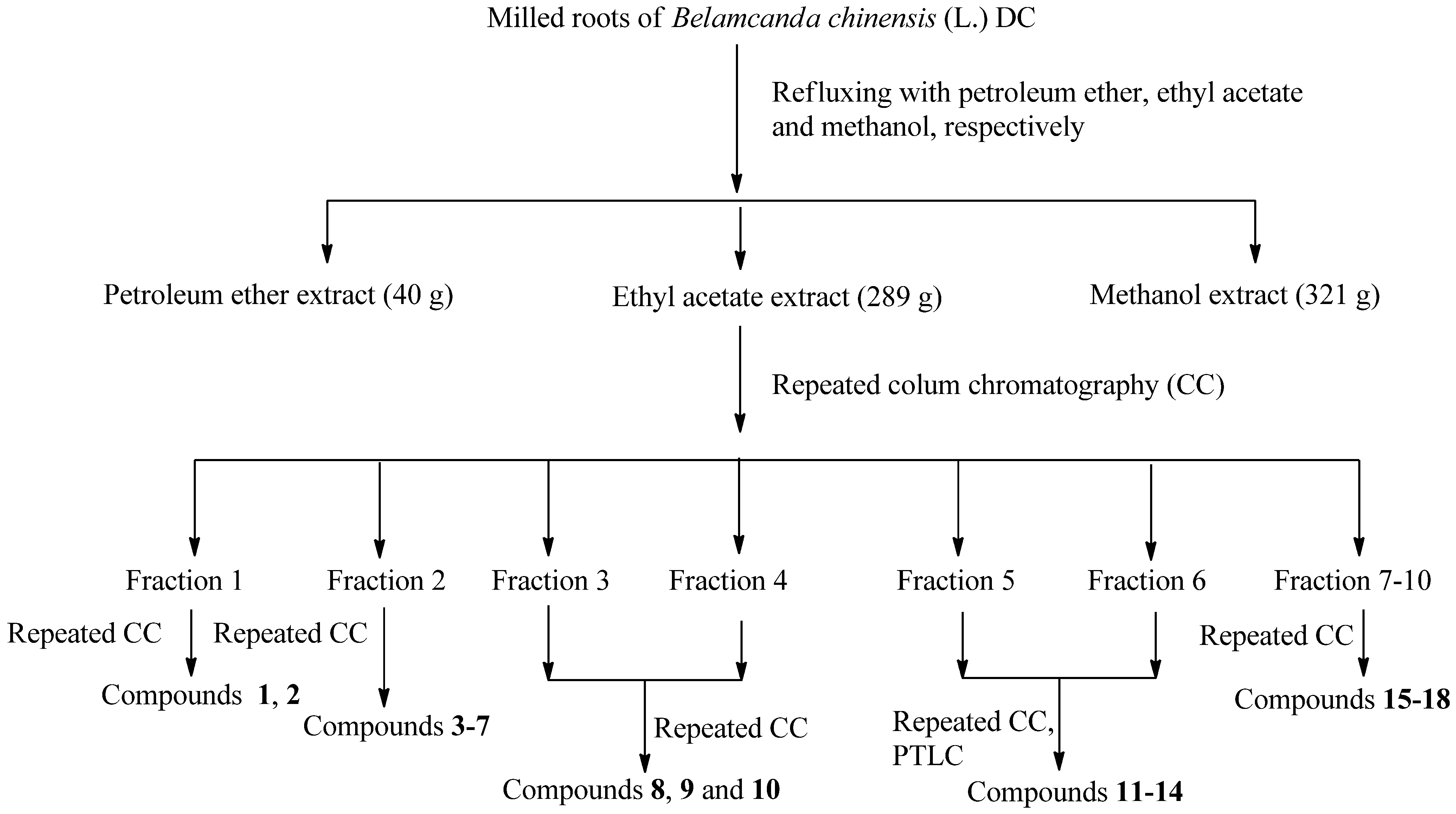
2.2. Antitumor Activities
| Test Extracts and Compounds | Inhibitory Rate for Different Cancer Cell Lines (%, mean ± SD) a | ||||
|---|---|---|---|---|---|
| MGC-803 b | Bcap-37 c | MCF-7 c | PC3 d | NIH3T3 e | |
| Petroleum ether extract f | 14.3 ± 9.1 | 43.1 ± 6.1 | 33.2 ± 1.1 | 27.1 ± 5.2 | 2.6 ± 12.4 |
| Ethyl acetate extract f | 94.1 ± 2.8 | 76.4 ± 4.6 | 80.4 ± 2.9 | 86.2 ± 2.1 | 36.5 ± 7.3 |
| Methanol extract f | 51.2 ± 2.1 | 51.6 ± 5.1 | 50.0 ± 2.1 | 65.7 ± 7.8 | 69.9 ± 4.8 |
| ADM g | 92.1 ± 1.3 | 92.1 ± 1.1 | 91.1 ± 2.2 | 93.4 ± 2.6 | 99.4 ± 0.4 |
| Quercetin (3) h | 19.2 ± 2.8 | 41.3 ± 2.9 | 43.5 ± 6.3 | 21.8 ± 8.9 | 5.8 ± 7.7 |
| Kampferol (4) | 58.2 ± 3.0 | 51.2 ± 8.1 | 39.2 ± 6.8 | 46.1 ± 5.9 | 11.1 ± 6.7 |
| Ursolic acid (7) | 51.7 ± 5.6 | 48.4 ± 5.9 | 49.4 ± 4.1 | 57.7 ±1.9 | 21.7 ± 4.9 |
| Betulin (8) | 43.7 ± 6.7 | 53.2 ± 3.2 | 53.2 ± 5.4 | 17.3 ± 5.2 | 33.5 ± 7.1 |
| Betulonic acid (9) | 68.1 ± 2.6 | 44.9 ± 2.9 | 56.1 ± 4.4 | 52.4 ± 4.2 | 22.1 ± 6.2 |
| Betulone (10) | 52.2 ± 5.3 | 54.2 ± 2.2 | 64.7 ± 7.3 | 52.3 ± 3.3 | 36.3 ± 7.1 |
| Tectoridin (11) | 12.6 ± 2.6 | 17.3 ± 4.2 | 10.0 ± 7.9 | 18.2 ± 6.2 | 5.9 ± 5.2 |
| Irisflorentin (12) | 29.4 ± 4.9 | 17.0 ± 9.8 | 39.1 ± 5.0 | 34.0 ± 4.8 | 25.0 ± 8.1 |
| 4′,5,6-Trihydroxy-7-methoxyisoflavone (13) | 13.4 ± 7.5 | 12.9 ± 4.8 | 20.9 ± 8.8 | 14.9 ± 2.0 | 1.7 ± 1.7 |
| Tectorigenin (14) | 22.6 ± 3.3 | 18.7 ± 5.4 | 23.7 ± 4.9 | 28.1 ± 4.8 | 18.4 ± 6.1 |
| Irilins A (15) | 13.4 ± 7.5 | 12.7 ± 5.1 | 10.4 ± 3.5 | 19.3 ± 4.3 | 9.0 ± 4.8 |
| Iridin (16) | 12.4 ± 2.7 | 21.8 ± 3.6 | 11.8 ± 5.2 | 22.7 ± 5.2 | 12.4 ± 7.3 |
| Irigenin (17) | 25.6 ± 5.3 | 20.1 ± 7.2 | 25.1 ± 1.2 | 25.7 ± 2.2 | 15.3 ± 1.8 |
| Iristectongenin A (18) | 26.6 ± 4.9 | 22.1 ± 8.2 | 19.1 ± 7.2 | 25.7 ± 2.2 | 5.2 ± 6.9 |
2.3. Investigation of Cell Apoptosis
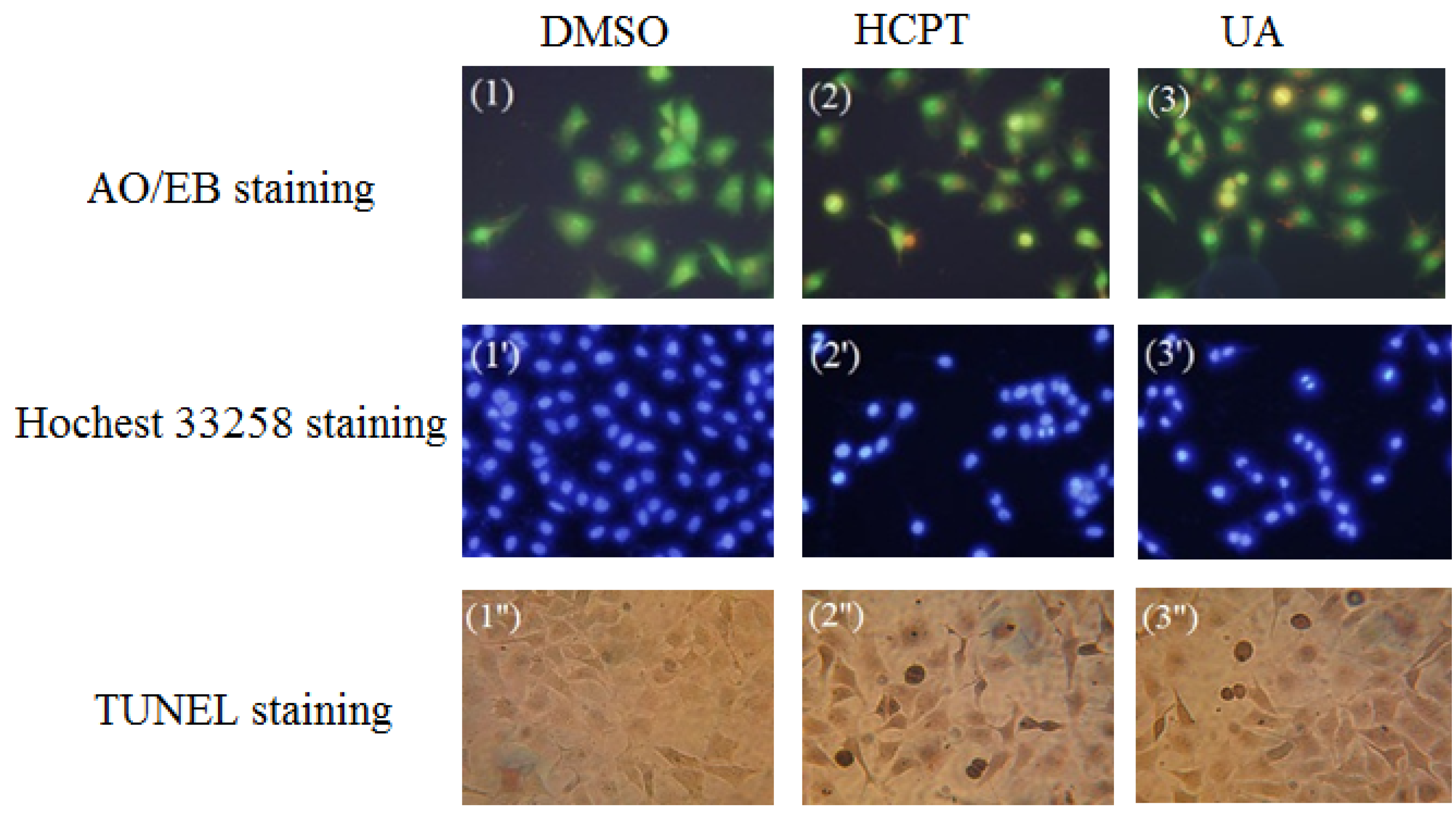
2.3.1. Acridine Orange/Ethidium Bromide (AO/EB) Staining
2.3.2. Hoechst 33258 Staining
2.3.3. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
3. Experimental Section
3.1. General Procedures and Reagents
3.2. Plant Materials
3.3. Extraction and Isolation of Antitumor Compounds
3.4. Spectroscopic Data
3.5. Cell Lines and Culture
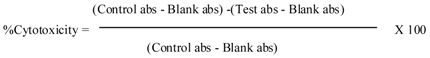
3.6. MTT Assays
3.7. AO/EB Staining
3.8. Hoechst 33258 Staining
3.9. TUNEL Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Wang, J.; Liu, X.J.; Zhong, Y. General Research of Blackberrylily Rhizome. Qilu Pharm. Aff. 2007, 26, 168–172. [Google Scholar]
- Ji, W.L.; Qin, M.J.; Wang, Z.T. Studies on the constituents of Bealmacada chinensis (I). J. Chin. Pharm. Univ. 2001, 32, 197–200. [Google Scholar]
- Jiangsu New Medical College, Dictionary of Chinese Materia Medica; Shanghai Scientific and Technical Publishing House: Shanghai, China, 2001.
- Ha, S.C.; Won, S.W. Flavonoids from the Rhizomes of Belamcanda chinesis. Arch. Pharm. Res. 1991, 14, 357–358. [Google Scholar] [CrossRef]
- Woo, W.S.; Woo, E.H. An Isoflavonenoririsfiorentin from Belamcanda chinensis. Phytochemistry 1993, 33, 939–940. [Google Scholar]
- Hideyuki, I.; Satomi, O.; Taksahi, Y. Isoflavonoids from Belamcanda chinesis. Chem. Pharm. Bull. 2001, 49, 1229–1231. [Google Scholar] [CrossRef]
- Masataka, M.; Yukari, I.; Momoyo, I.; Kinuko, I.; Junko, K. New isoflavones from Belamcandae rhizome. J. Nat. Med. 2007, 61, 329. [Google Scholar] [CrossRef]
- Takahashi, K.; Hoshino, Y.; Suzuki, S.; Hano, Y.; Nomura, T. Iridals from Iristectorum and Belamcandachinensis. Phytochemistry 2000, 53, 925–929. [Google Scholar]
- Ito, H.; Onoue, S.; Miyake, Y.; Yamada, T. Iridal-Type Triterpenoids with Ichthyotoxic Activity from Belamcanda chinensis. J. Nat. Prod. 1999, 62, 89–93. [Google Scholar] [CrossRef]
- Abe, F.; Chen, R.; Yamauchi, T. Iridals from Belamcanda chinensis and Iris japonica. Phytochemistry 1991, 30, 3379–3382. [Google Scholar] [CrossRef]
- Mao, Y.W.; Tseng, H.W.; Liang, W.L.; Chen, I.S.; Chen, S.T. Anti-Inflammatory and Free Radial Scavenging Activities of the Constituents Isolated from Machliuszuihoensis. Molecules 2011, 16, 9451–9466. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhu, H.; Liu, C.R.; Tu, P.F. Study on the chemical constituents of the flowers of Impatiens balsamina L. Chin. Tradit. Pat. Med. 2003, 10, 833–834. [Google Scholar]
- Bi, Y.F.; Zheng, X.K.; Liu, H.M.; Feng, W.S.; Ji, C.R. Studies on the chemical constituents from pineneedles of Pinus Massoniana Lamb. Acta Pharm. Sin. 2001, 36, 832–835. [Google Scholar]
- Sliva, M.G.V.; Vieira, I.G.P.; Mendes, F.N.P.; Albuquerque, I.L.; Santos, R.N.D. Variation of Ursolic acid Content in Eight Ocimum Species from Northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar] [CrossRef]
- Winston, F.T.; Lynn, C.B.; Azzam, A. Lupanetriterpenoids of Salacia Cordata. J. Nat. Prod. 1992, 3, 395–398. [Google Scholar]
- Zhang, H.J.; Tan, G.T.; Hoang, V.D.; Hung, N.V.; Cuong, N.M. Natural Anti-HIV Agents. Part IV. Anti-HIV Constituents from Vaticacinerea. J. Nat. Prod. 2003, 2, 263–268. [Google Scholar]
- Marvin, J.N.; Carolina, P.R.; Ignacio, A.J.; Laila, M. Lupane Triterpenoids from Maytenus Species. J. Nat. Prod. 2005, 68, 1018–1021. [Google Scholar] [CrossRef]
- Monthakantirat, O.; Ekanamkul, D.W.D.; Umehara, K.; Yoshinaga, Y. Phenolic Constituents of the Rhizomes of the Thai Medicinal Plant Belamcanda chinensis with Proliferative Activity for Two Breast Cancer Cell Lines. J. Nat. Prod. 2005, 68, 361–364. [Google Scholar] [CrossRef]
- Werner, H.; Kimberly, D.P.; Daniel, R. Isoflavones, a sesquiterpene lactone-monoterpene adduct and other constituents of Gaillardia species. Phytochemistry 1991, 30, 1273–1279. [Google Scholar]
- Miyazawa, M.; Sakano, K.; Nakamura, S.I.; Kosaka, H. Antimutagenic Acitivity of Isoflavone from Pueraria Lobata. J. Agric. Food Chem. 2001, 49, 336–341. [Google Scholar] [CrossRef]
- Moein, M.R.; Khan, S.I.; Ali, Z.; Ayatollahi, S.A.M.; Kobarfard, F. Flavonoids from Iris sogarica and their Antioxidant and Estrogenic Acitvity. Plant. Med. 2008, 74, 1492–1497. [Google Scholar] [CrossRef]
- Hanawa, F.; Tahara, S.; Mizutani, J. Isoflavonoids produced by Iris pseudacorus leaves treated with cupric chloride. Phytochemistry 1991, 30, 157–163. [Google Scholar]
- Guo, L.; Wu, J.Z.; Han, T.; Cao, T. Chemical Composition, Antifugal and Antitumor Properties of Ether Extracts of Scapania verrucosa Heeg. and its Endophytic Fungus Chaetomium fusiforme. Molecules 2008, 13, 2114–2125. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7–10 are available from the authors
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, M.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical Constituents of the Ethyl Acetate Extract of Belamcanda chinensis (L.) DC Roots and Their Antitumor Activities. Molecules 2012, 17, 6156-6169. https://doi.org/10.3390/molecules17056156
Liu M, Yang S, Jin L, Hu D, Wu Z, Yang S. Chemical Constituents of the Ethyl Acetate Extract of Belamcanda chinensis (L.) DC Roots and Their Antitumor Activities. Molecules. 2012; 17(5):6156-6169. https://doi.org/10.3390/molecules17056156
Chicago/Turabian StyleLiu, Mingchuan, Shengjie Yang, Linhong Jin, Deyu Hu, Zhibing Wu, and Song Yang. 2012. "Chemical Constituents of the Ethyl Acetate Extract of Belamcanda chinensis (L.) DC Roots and Their Antitumor Activities" Molecules 17, no. 5: 6156-6169. https://doi.org/10.3390/molecules17056156
APA StyleLiu, M., Yang, S., Jin, L., Hu, D., Wu, Z., & Yang, S. (2012). Chemical Constituents of the Ethyl Acetate Extract of Belamcanda chinensis (L.) DC Roots and Their Antitumor Activities. Molecules, 17(5), 6156-6169. https://doi.org/10.3390/molecules17056156





