Protective Effect of T. violacea Rhizome Extract Against Hypercholesterolemia-Induced Oxidative Stress in Wistar Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Protein and Albumin Contents
2.2. Lipid Peroxidation
2.3. Glutathione Content in Liver, Serum, Heart and Aorta
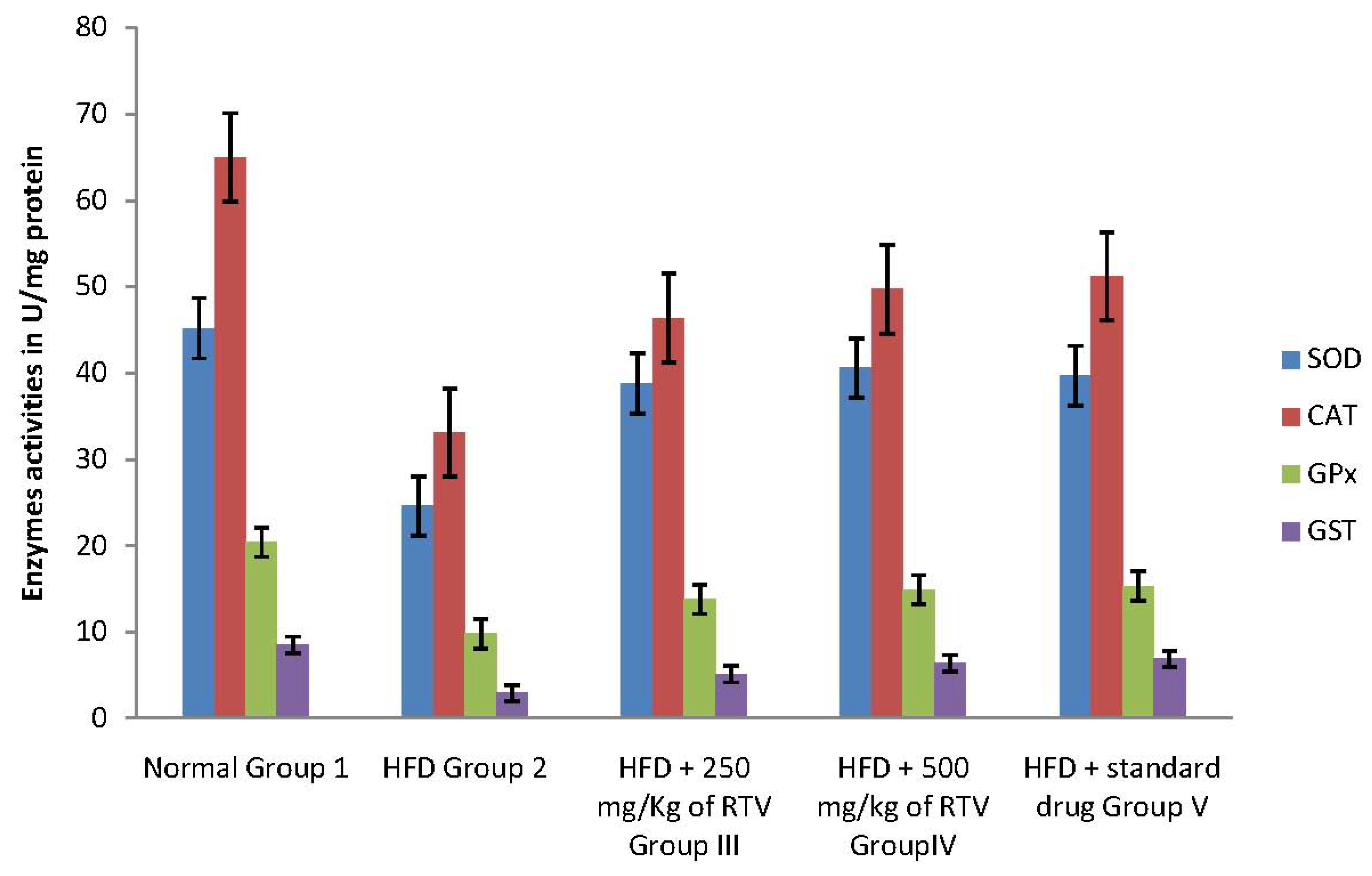
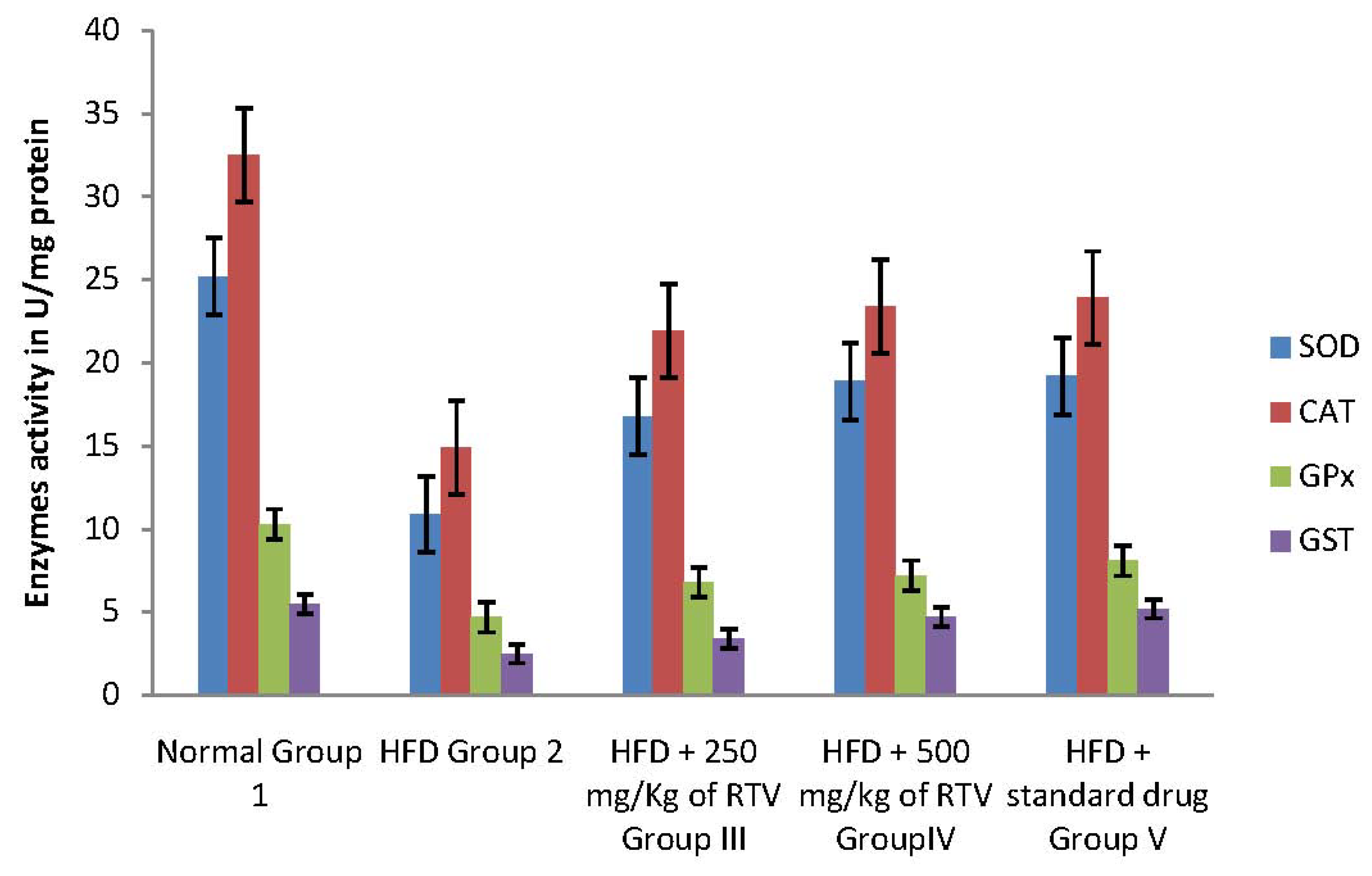
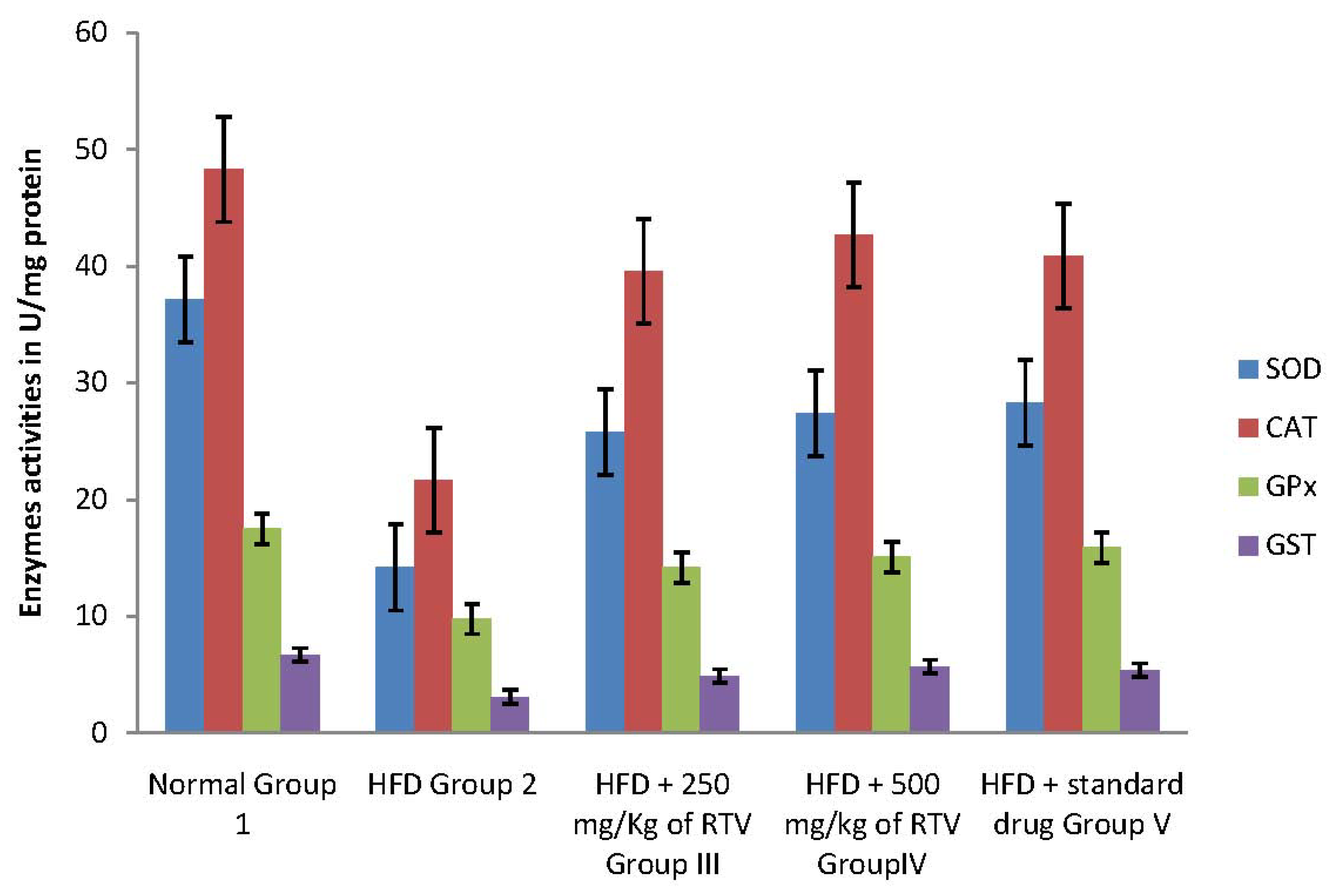
2.4. Tissue Antioxidant Enzyme Defense System
3. Experimental
3.1. Plant Collection and Extract Preparation
3.2. Animals
3.3. Experiment Design
3.4. Preparation of Liver Homogenate
3.5. Heart Homogenate Preparation
3.6. Aorta Homogenate Preparation
3.7. Biochemical Estimations
3.7.1. Estimation of Total Protein and Albumin
3.7.2. Estimation of Malondialdehyde (MDA)
3.7.3. Estimation of Glutathione Peroxidase
3.7.4. Determination of Superoxide Dismutase Activity
3.7.5. Estimation of Catalase
3.7.6. Estimation of GSH
3.7.7. Determination of GST Activity
3.8. Statistical Evaluation
4. Conclusions
Acknowledgements
References and Notes
- Attia, D.M.; Ni, Z.N.; Boer, P.; Attia, M.A.; Goldschmeding, R.; Koomans, H.A.; Vaziri, N.D.; Joles, J.A. Proteinuria is preceded by decreased nitric oxide synthesis and prevented by a NO donor in cholesterol-fed rats. Kidney Int. 2002, 61, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Mune, M.; Meydani, M.; Gong, J.; Fotouhi, N.; Ohtani, H.; Smith, D.; Blumberg, J.B. Effect of dietary fish oil, vitamin E, and probucol on renal injury in the rat. J. Nutr. Biochem. 1999, 10, 539–546. [Google Scholar] [CrossRef]
- Balakumar, P.; Jindal, S.; Shah, D.I.; Singh, M. Experimental models for vascular endothelial dysfunction. Trends Med. Res. 2007, 2, 12–20. [Google Scholar]
- Xu, Y.; Wei, Y.; Zhang, Y.; Gu, J.; Ma, J.; Zheng, L.; Hu, D. The characteristics of living and behaviour factors in Chinese patients metabolic syndrome. J. Health Sci. 2007, 53, 84–91. [Google Scholar] [CrossRef]
- Adult Treatment Panel (ATP) III. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Frederick, J.R. Hypercholesterolaemia in children and young adults—Current management. JEMDSA 2009, 14, 9–12. [Google Scholar]
- Chen, D.; Misra, A.; Garg, A. Lipodystrophy in human immunodeficiency virus infected patients. J. Clin. Endocrinol. Metab. 2002, 87, 4845–4856. [Google Scholar] [CrossRef] [PubMed]
- Furtado, J.; Zambrini, H.; Neto, D.; Scozzafave, G.; Brasileiro, R. Ambulatório de Lipodistrofia do Hospital Heliópolis-Uma experiência com as correções cirúrgicas em dois anos de atendimento. Prática hospitalar 2007, 58, 28–32. [Google Scholar]
- Ma, J.; Qiao, Z.; Xiang, X. Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J. Med. Plants Res. 2011, 5, 855–858. [Google Scholar]
- Yang, R.-L.; Shi, Y.-H.; Hao, G.; Li, W.; Le, G.-W. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Vandewoude, M.F.; Vandewoude, M.G. Vitamin E status in normal population: The influence of age. J. Am. Coll. Nutr. 1987, 6, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.N.; Megahed, H.A.; Eltoukhy, S.I.; Youness, E.R.; Habib, D.F.; Wafai, H.A. Antioxidant and radical scavenging properties of garlic oil in streptozotocin induced diabetic rats. Aust. J. Basic Appl. Sci. 2011, 5, 280–286. [Google Scholar]
- Gutteridge, J.M.C.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. NY Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.A.; Hamed, T.E.; Al-Okbi, S.Y. Reduction in hypercholesterolemia and risk of cardiovascular diseases by mixtures of plant food extracts: A study on plasma lipid profile, oxidative stress and testosterone in rats. Grasas y aceites 2010, 61, 378–389. [Google Scholar]
- Vasthi, K.E.; Devarajan, N. Evaluation of free radical scavenging activity and biological properties of Spinacia oleracea. IJEST 2011, 3, 25–30. [Google Scholar]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.F. Ethnobotanical information on plants used for the management of cardiovascular diseases in Nkonkobe Municipality, South Africa. J. Med. Plants Res. 2011, 5, 4256–4260. [Google Scholar]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.F. Antioxidant properties and cytotoxicity evaluation of methanolic extract of dried and fresh rhizomes of Tulbaghia violacea. Afr. J. Pharm. Pharmacol. 2011, 5, 2490–2497. [Google Scholar] [CrossRef]
- Bungu, L.; van de Venter, M.; Frost, C. Evidence for an in vitro anticoagulant and antithrombotic activity in Tulbaghia violacea. Afr. J. Biotechnol. 2006, 7, 681–688. [Google Scholar]
- McGaw, L.J.; Jager, A.K.; van Staden, J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Abu-Hijleh, G.; Said, S. Safety of traditional Arab herbal medicine. Evid. Based Complement. Alternat. Med. 2006, 3, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Akiibinu, O.M.; Ogunyemi, O.E.; Arinola, O.G.; Adenaike, A.F.; Adegoke, O.D. Assessment of antioxidants and nutritional status of pulmonary tuberculosis patients in Nigeria. Eur. J. Gen. Med. 2008, 5, 208–211. [Google Scholar]
- Rolls, B.J. The role of energy density in the over consumption of fat. J. Nutr. 2000, 130, S268–S271. [Google Scholar] [CrossRef] [PubMed]
- Woodman, D.D. Assessment of Hepatoxicity. In Animal Clinical Chemistry, A Primer for Toxicologist; Evans, G.O., Ed.; Taylor & Francis: London, UK, 1996; pp. 71–86. [Google Scholar]
- Lu, L.-S.; Wu, C.-C.; Hung, L.-M.; Chiang, M.-T.; Lin, C.-T.; Lin, C.-W.; Su, M.-J. Apocynin alleviated hepatic oxidative burden and reduced liver injury in hypercholesterolaemia. Liver Int. 2007, 27, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S. Hypolipidemic and antioxidant activities of avocado fruit pulp on high cholesterol fed diet in rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1475–1483. [Google Scholar] [CrossRef]
- Beltowski, J.; Wójcicka, G.; Górny, D.; Marciniak, A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000, 51, 883–896. [Google Scholar] [PubMed]
- Dutta, K.; Bishayi, B. Escherichia coli lipopolysaccharide administration alters antioxidant profile during hypercholesterolemia. Indian J. Clin. Biochem. 2009, 24, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, N.; Aw, T.Y.; Ookhtens, M. The regulation of hepatic glutathione. Ann. Rev. Pharmacol. Toxicol. 1985, 25, 715–744. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Parris, M.K. Glutathione systemic protectant against oxidative and free radical damage. Altern. Med. Rev. 1997, 2, 155–176. [Google Scholar]
- Cui, B.K.; Liu, S.; Lin, X.J.; Wang, J.; Li, S.H.; Wang, Q.B.; Li, S.P. Effects of Lycium barbarum aqueous and ethanol extracts on high fat-diet induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrica parameters and markers of obesity in rats. Lab. Anim. (NY) 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Diniz, Y.S.; Burneiko, R.M.; Seiva, F.R.F.; Almeida, F.Q.A.; Galhardi, C.M.; Novelli Filho, J.L.V.B.; Mani, F.; Novelli, E.L.B. Diet compounds, glycemic index and obesity related cardiac effects. Int. J. Cardiol. 2008, 124, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, J.-Y.; Hwang, Y.-J.; Hwang, K.-A.; Om, A.-S.; Kim, J.-H.; Cho, K.-J. The beneficial effects of aged black garlic extract on obesity and hyperlipidemia in rats fed a high-fat diet. J. Med. Plants Res. 2011, 5, 3159–3168. [Google Scholar]
- Wang, Q.; Zeng, T.; Yu, L.; Xie, K. Preventive effect of garlic oil on fatty liver in experimental rats. Dulixue Zazhi 2007, 21, 450–453. [Google Scholar]
- Oyedemi, O.S.; Bradley, G.; Afolayan, A.J. In-vitro and in-vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010, 4, 70–78. [Google Scholar]
- Fridovich, I. Superoxide dismutases. Adv. Enzymol. 1986, 58, 61–97. [Google Scholar] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Najman, K.; Bielecki, W.; Ham, K.-S.; Kang, S.-G.; Paredes-Lopez, O.; Martinez-Ayal, A.L.; Trakhtenberg, S. Aorta and liver changes in rats fed cholesterol-containing and raw vegetable-supplemented diets: Experiments in vitro and in vivo. Agric. Food Chem. 2011, 59, 7441–7451. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, O.; Akin, B.; Aktan, F.; Karasu, C. Short-term gemfibrozil treatment reverses lipid profile and peroxidation but does not alter blood glucose and tissue antioxidant enzymes in chronically diabetic rats. Mol. Cell. Biochem. 2001, 216, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, M.P.; Venukumar, M.R.; Latha, M.S. Antioxidant potential of the Syzygium aromaticum (Gaertn.) Linn. (Cloves) in rats fed with high fat diet. Indian J. Pharmacol. 2003, 35, 99–103. [Google Scholar]
- Mohammad, S.F.; Woodward, S.C. Characterisation of a potent inhibitor of platelet aggregation and release reaction isolated from Allium sativum (Garlic). Thrombosis Res. 1986, 44, 793–806. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T. Aphrodisiac potentials of aqueous extract of Fadogia agrestis (Schweinf. Ex Heirn) stem in male albino rats. Asian J. Androl. 2005, 7, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Akanji, M.A.; Yakubu, M.T. α-Tocopherol protects against metabisulphite-induced tissue damage in rats. Nigerian J. Biochem. Mol. Biol. 2000, 16, 147–148. [Google Scholar]
- Noori, S.; Azmat, M.; Mahboob, T. Study on antioxidant effects of cinnamon and garlic extract in liver, kidney and heart tissue of rat. Biosci. Res. 2012, 9, 17–22. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swason, A.B. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 17, 588–590. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Sinha, K.A. Colorimetric assay of catalase. Anal Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Meth. Enzymol. 1985, 113, 484–490. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.S.; Jekpoly, W.B. Glutathione transferase: A first enzymatic step in mercaptuiric acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
Sample Availability: Not available. |
| Treatment group | Total protein (mg/dL) | Albumin (mg/dL) | Globulin (mg/dL) | Albumin / globulin ratio |
|---|---|---|---|---|
| Normal | 7.41 ± 0.12 | 3.82 ± 0.38 | 3.01 ± 0.11 | 1.26 |
| HCD only | 6.62 ± 0.14 a | 3.31 ± 0.41 a | 3.31 ± 0.14 | 1.00 a |
| HCD + 250 mg/kg RTV | 6.92 ± 0.14 | 3.60 ± 0.43 | 3.32 ± 0.13 | 1.08 |
| HCD + 500 mg/kg RTV | 7.06 ± 0.19 | 3.79 ± 0.39 | 3.27 ± 0.12 | 1.16 |
| HCD + 50 mg/kg Gemfibrozil | 7.29 ± 0.13 | 3.84 ± 0.33 | 3.45 ± 0.19 | 1.11 |
| Group | NC | HCD | HCD + 250 mg/kg of RTV | HCD + 500 mg/kg of RTV | HCD + Gemfibrozil |
|---|---|---|---|---|---|
| Liver | |||||
| TBARS | 2.75 ± 0.12 | 4.65 ± 0.19 a | 3.89 ± 0.14 b | 3.01 ± 0.16 b | 2.95 ± 0.11 b |
| GSH | 5.32 ± 0.17 | 3.12 ± 0.13 a | 4.22 ± 0.10 b | 4.89 ± 0.14 b | 5.01 ± 0.18 b |
| Serum | |||||
| TBARS | 11.2 ± 0.13 | 17.45 ± 1.14 a | 13.65 ± 0.11 b | 12.50 ± 0.15 b | 12.41 ± 0.18 b |
| GSH | 1.23 ± 0.11 | 0.19 ± 0.12 a | 0.89 ± 0.17 b | 1.02 ± 0.13 b | 1.01 ± 0.31 b |
| Heart | |||||
| TBARS | 8.52 ± 1.12 | 14.25 ± 0.17 a | 9.85 ± 0.21 b | 9.01 ± 1.10 b | 8.95 ± 0.12 b |
| GSH | 4.85 ± 0.20 | 2.15 ± 0.28 a | 3.19 ± 0.15 b | 3.39 ± 0.24 b | 3.97 ± 0.13 b |
| Aorta | |||||
| TBARS | 10.5 ± 1.32 | 15.23 ±0.52 a | 12.35 ± 0.15 b | 11.51 ± 0.16 b | 10.99 ± 0.20 b |
| GSH | 8.25 ± 1.70 | 4.82 ± 0.19 a | 6.98 ± 0.17 b | 7.21 ± 0.13 b | 7.37 ± 0.10 b |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Protective Effect of T. violacea Rhizome Extract Against Hypercholesterolemia-Induced Oxidative Stress in Wistar Rats. Molecules 2012, 17, 6033-6045. https://doi.org/10.3390/molecules17056033
Olorunnisola OS, Bradley G, Afolayan AJ. Protective Effect of T. violacea Rhizome Extract Against Hypercholesterolemia-Induced Oxidative Stress in Wistar Rats. Molecules. 2012; 17(5):6033-6045. https://doi.org/10.3390/molecules17056033
Chicago/Turabian StyleOlorunnisola, Olorunnisola Sinbad, Graeme Bradley, and Anthony Jide Afolayan. 2012. "Protective Effect of T. violacea Rhizome Extract Against Hypercholesterolemia-Induced Oxidative Stress in Wistar Rats" Molecules 17, no. 5: 6033-6045. https://doi.org/10.3390/molecules17056033
APA StyleOlorunnisola, O. S., Bradley, G., & Afolayan, A. J. (2012). Protective Effect of T. violacea Rhizome Extract Against Hypercholesterolemia-Induced Oxidative Stress in Wistar Rats. Molecules, 17(5), 6033-6045. https://doi.org/10.3390/molecules17056033




