Stability of Carotenoid Diets During Feed Processing and Under Different Storage Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stability of Carotenoid Diets during Feed Processing
| Experimental Diets | Total carotenoid (mg kg−1) | |
|---|---|---|
| Without antioxidant | With antioxidant | |
| Dry mix | ||
| Diet 1 | 5.72 ± 0.12 | 5.99 ± 0.55 |
| Diet 2 | 27.44 ± 4.89 | 27.64 ± 0.18 |
| Diet 3 | 27.78 ± 1.84 | 28.49 ± 1.12 |
| Diet 4 | 26.74 ± 4.65 | 28.57 ± 3.55 |
| Diet 5 | 47.89 ± 2.93 | 48.51 ± 3.93 |
| Diet 6 | 88.49 ± 7.95 | 87.27 ± 2.99 |
| After being processed | ||
| Diet 1 | 5.60 ± 0.10 | 5.86 ± 0.19 |
| Diet 2 | 26.60 ± 0.54 | 27.00 ± 2.63 |
| Diet 3 | 25.26 ± 1.87 | 27.08 ± 1.59 |
| Diet 4 | 25.34 ± 3.39 | 27.98 ± 1.50 |
| Diet 5 | 46.68 ± 1.88 | 47.16 ± 2.01 |
| Diet 6 | 86.13 ± 2.25 | 86.17 ± 3.76 |
| Drying | ||
| Diet 1 | 5.54 ± 0.19 | 5.70 ± 0.27 |
| Diet 2 | 25.56 ± 0.98 | 26.91 ± 0.84 |
| Diet 3 | 23.67 ± 2.45 | 26.08 ± 0.75 |
| Diet 4 | 25.04 ± 2.97 | 26.25 ± 2.90 |
| Diet 5 | 45.85 ± 4.72 | 46.75 ± 3.08 |
| Diet 6 | 84.97 ± 4.28 | 86.01 ± 3.00 |
| P-value | 0.0001 | 0.0001 |
| Diet | 0.0001 | 0.0001 |
| Unit operations | 0.1417 | 0.1770 |
| Diet*Unit operations | 0.9989 | 0.9991 |
| Experimental diets | Total carotenoid (mg kg−1) | ||
|---|---|---|---|
| Without antioxidant | With antioxidant | P-value | |
| Diet 1 | 5.62 ± 0.14 | 5.85 ± 0.34 | 0.57 |
| Diet 2 | 26.54 ± 2.14 | 27.18 ± 1.21 | 0.30 |
| Diet 3 | 25.57 ± 2.06 | 27.21 ± 1.15 | 0.51 |
| Diet 4 | 25.71 ± 3.68 | 27.60 ± 2.65 | 0.72 |
| Diet 5 | 46.80 ± 3.18 | 47.78 ± 3.01 | 0.89 |
| Diet 6 | 86.53 ± 4.83 | 86.48 ± 3.25 | 0.26 |
2.2. Stability of Carotenoid Diets under Different Storage Temperatures
| Experimental Diets | Total carotenoid (mg kg−1) | |
|---|---|---|
| Without antioxidant | With antioxidant | |
| Week 0 | ||
| Diet 1 | 5.54 ± 0.19 | 5.54 ± 0.19 |
| Diet 2 | 25.56 ± 0.98 | 25.56 ± 0.98 |
| Diet 3 | 23.67 ± 2.45 | 23.67 ± 2.45 |
| Diet 4 | 25.04 ± 2.97 | 25.04 ± 2.97 |
| Diet 5 | 45.85 ± 4.72 | 45.85 ± 4.72 |
| Diet 6 | 84.97 ± 4.28 | 84.97 ± 4.28 |
| Week 4 | ||
| Diet 1 | 5.46 ± 0.38 | 5.65 ± 0.42 |
| Diet 2 | 24.04 ± 3.39 | 26.22 ± 5.01 |
| Diet 3 | 22.88 ± 0.67 | 24.64 ± 3.33 |
| Diet 4 | 23.83 ± 1.59 | 25.36 ± 1.55 |
| Diet 5 | 43.70 ± 0.75 | 45.40 ± 0.81 |
| Diet 6 | 82.85 ± 2.39 | 84.50 ± 2.24 |
| Week 8 | ||
| Diet 1 | 5.28 ± 0.73 | 5.55 ± 0.10 |
| Diet 2 | 23.27 ± 3.93 | 25.55 ± 2.36 |
| Diet 3 | 21.83 ± 1.72 | 25.07 ± 1.07 |
| Diet 4 | 23.17 ± 2.16 | 25.03 ± 0.72 |
| Diet 5 | 42.45 ± 4.85 | 45.02 ± 2.33 |
| Diet 6 | 80.74 ± 4.33 | 83.75 ± 1.25 |
| P-value | 0.0001 | 0.0001 |
| Diet | 0.0001 | 0.0001 |
| Storage time | 0.0597 | 0.9372 |
| Diet*Storage time | 0.9962 | 0.9997 |
| Experimental Diets | Total carotenoid (mg kg−1) | |
|---|---|---|
| Without antioxidant | With antioxidant | |
| Week 0 | ||
| Diet 1 | 5.54 ± 0.19 | 5.54 ± 0.19 |
| Diet 2 | 25.56 ± 0.98 | 25.56 ± 0.98 |
| Diet 3 | 23.67 ± 2.45 | 23.67 ± 2.45 |
| Diet 4 | 25.04 ± 2.97 | 25.04 ± 2.97 |
| Diet 5 | 45.85 ± 4.72 | 45.85 ± 4.72 |
| Diet 6 | 84.97 ± 4.28 | 84.97 ± 4.28 |
| Week 4 | ||
| Diet 1 | 5.46 ± 0.38 | 5.65 ± 0.42 |
| Diet 2 | 24.04 ± 3.39 | 26.22 ± 5.01 |
| Diet 3 | 22.88 ± 0.67 | 24.64 ± 3.33 |
| Diet 4 | 23.83 ± 1.59 | 25.36 ± 1.55 |
| Diet 5 | 43.70 ± 0.75 | 45.40 ± 0.81 |
| Diet 6 | 82.85 ± 2.39 | 84.50 ± 2.24 |
| Week 8 | ||
| Diet 1 | 5.28 ± 0.73 | 5.55 ± 0.10 |
| Diet 2 | 23.27 ± 3.93 | 25.55 ± 2.36 |
| Diet 3 | 21.83 ± 1.72 | 25.07 ± 1.07 |
| Diet 4 | 23.17 ± 2.16 | 25.03 ± 0.72 |
| Diet 5 | 42.45 ± 4.85 | 45.02 ± 2.33 |
| Diet 6 | 80.74 ± 4.33 | 83.75 ± 1.25 |
| P-value | 0.0001 | 0.0001 |
| Diet | 0.0001 | 0.0001 |
| Storage time | 0.0597 | 0.9372 |
| Diet*Storage time | 0.9962 | 0.9997 |
| Experimental diets | Total carotenoid (mg kg−1) | ||
|---|---|---|---|
| Without antioxidant | Antioxidant | P-value | |
| Room temperature | |||
| Diet 1 | 5.43 ± 0.43 | 5.58 ± 0.24 | 0.35 |
| Diet 2 | 24.29 ± 2.76 | 25.78 ± 2.78 | 0.19 |
| Diet 3 | 22.79 ± 1.61 | 24.46 ± 2.28 | 0.75 |
| Diet 4 | 24.01 ± 2.24 | 25.14 ± 1.74 | 0.08 |
| Diet 5 | 43.99 ± 3.43 | 45.43 ± 2.51 | 0.11 |
| Diet 6 | 82.85 ± 3.67 | 84.41 ± 2.59 | 0.16 |
| Cool temperature (4 °C) | |||
| Diet 1 | 5.47 ± 0.16 | 5.66 ± 0.14 | 0.42 |
| Diet 2 | 25.25 ± 2.69 | 26.45 ± 2.21 | 0.55 |
| Diet 3 | 23.36 ± 1.92 | 25.60 ± 0.94 | 0.47 |
| Diet 4 | 24.56 ± 1.62 | 25.67 ± 2.18 | 0.80 |
| Diet 5 | 44.81 ± 3.39 | 46.41 ± 2.34 | 0.19 |
| Diet 6 | 83.74 ± 2.31 | 85.42 ± 1.77 | 0.35 |
3. Experimental
3.1. Diets
| Ingredient (Kg) | Experimental diets | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Fish meal | 30 | 30 | 30 | 30 | 30 | 30 |
| Soybean meal | 24 | 24 | 24 | 24 | 24 | 24 |
| Rice bran | 24 | 24 | 24 | 24 | 24 | 24 |
| Tapioca starch | 5 | 5 | 5 | 5 | 5 | 5 |
| Wheat Flour | 5 | 5 | 5 | 5 | 5 | 5 |
| Fish oil | 2 | 2 | 2 | 2 | 2 | 2 |
| Alpha-starch | 5 | 5 | 5 | 5 | 5 | 5 |
| Dicalcuimphosphate | 1 | 1 | 1 | 1 | 1 | 1 |
| Premix | 2 | 2 | 2 | 2 | 2 | 2 |
| Astaxanthin | - | ✓ | - | - | - | - |
| Lutein | - | - | ✓ | - | ✓ | ✓ |
| β-carotene | - | - | - | ✓ | ✓ | ✓ |
| Lecithin | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Proximate composition by analysis (% dry weight on basis) | ||||||
| Protein | 29.54 ± 2.05 | |||||
| Fat | 5.05 ± 0.29 | |||||
| Fiber | 4.81 ± 0.06 | |||||
| Moist | 6.40 ± 0.70 | |||||
| Ash | 9.36 ± 0.96 | |||||
| Carotenoids compositions by analysis (mgkg−1 dry weight on basis) | ||||||
| Total carotenoids | 4.45 ± 0.46 | 29.51 ± 2.12 | 27.73 ± 2.73 | 27.01 ± 3.65 | 57.43 ± 3.36 | 109.39 ± 7.84 |
| Astaxanthin | ND. | 25.79 ± 0.81 | ND. | ND. | ND. | ND. |
| Lutein | ND. | ND. | 23.35 ± 2.92 | ND. | 25.53 ± 5.71 | 46.31 ± 5.71 |
| β-carotene | ND. | ND. | ND. | 24.14 ± 1.51 | 28.57 ± 0.57 | 59.54 ± 1.14 |
3.2. Sampling and Analyses
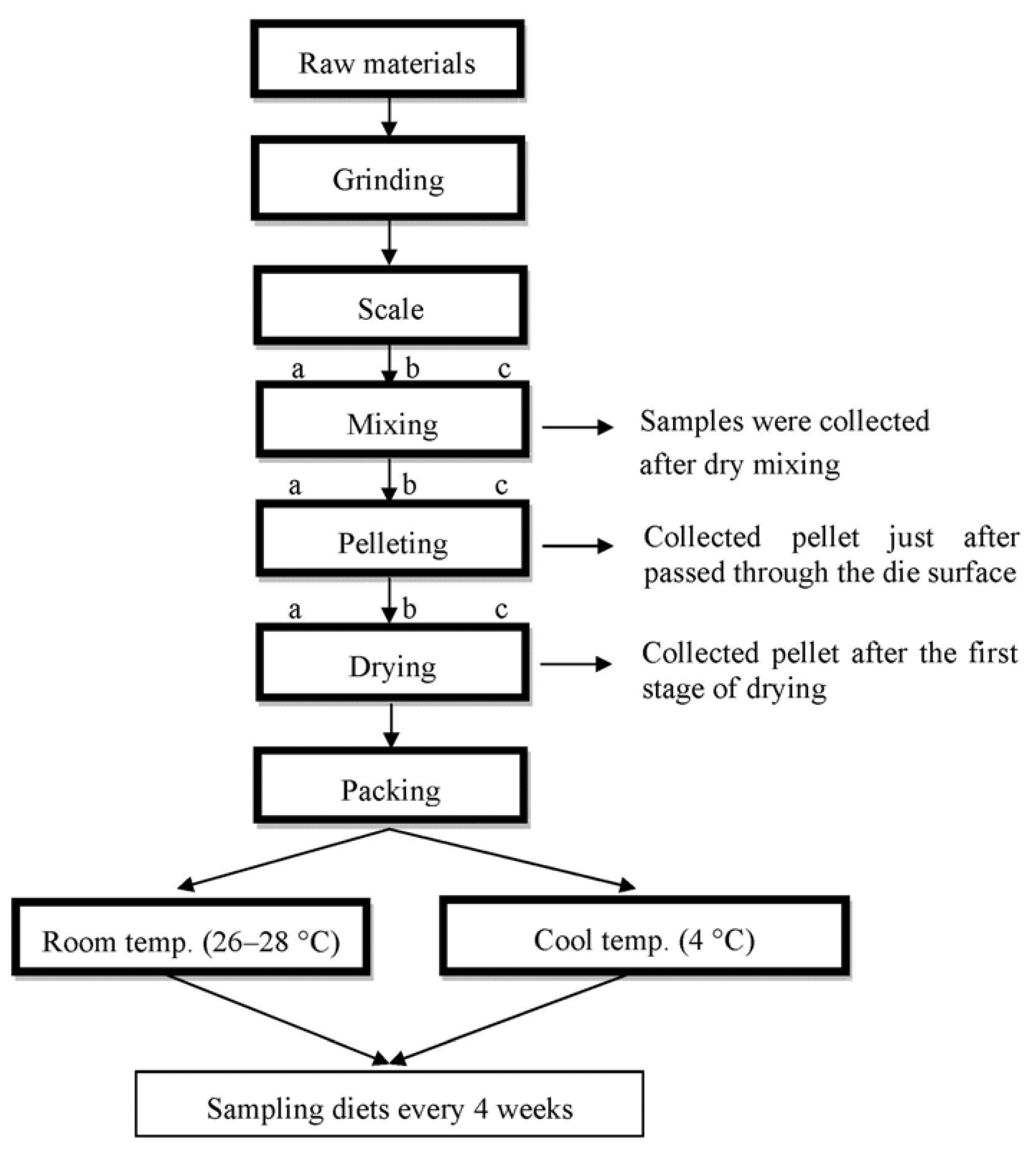
3.3. Total Carotenoids Determination
3.4. Statistical Analysis
4. Conclusions
- Samples Availability: Not available
References
- Simpson, K.L.; Katayama, T.; Chichester, C.O. Carotenoids in Fish Feeds. In Carotenoids as Colorants and Vitamin A Precursors; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 463–538. [Google Scholar]
- Goodwin, T.W. The Biochemistry of the Carotenoids. Volume II Animals; Chapman and Hall: New York, NY, USA, 1984. [Google Scholar]
- Torrissen, O.J.; Hardy, R.W.; Shearer, K.D. Pigmentation of salmonids: Carotenoid deposition and metabolism. Crit. Rev. Aquat. Sci. 1989, 1, 209–225. [Google Scholar]
- Storebakken, T.; No, H.K. Pigmentation in rainbow trout. Aquaculture 1992, 100, 209–229. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Changes in carotenoids during processing and storage of foods. Arch. Latinoam. Nutr. 1999, 49, 38–47. [Google Scholar]
- Rickman, J.C.; Bruhn, C.M.; Barrett, D.M. Nutritional composition of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Food Agric. 2007, 87, 1185–1196. [Google Scholar]
- McGinnis, C.H. Vitamin Stability, and Activity of Water-Soluble Vitamins as Influenced by Manufacturing Processes and Recommendations for the Water-Soluble Vitamins. In Proceedings of the NFIA Nutrition Institute; NFIA: West Des Moines, IA, USA, 1986; pp. 1–44. [Google Scholar]
- Coelho, M.B. Fate of vitamins in premixes and feeds: Vitamin stability influencing factors during pelleting, extrusion, storage, trace minerals. Feed Manag. 1991, 42, 30–33. [Google Scholar]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef]
- Davis, A.R.; Collins, J.; Fish, W.W.; Tadmor, Y.; Webber, C.L.; Perkins-Veazie, P. Rapid method for total carotenoid detection in canary yellow-fleshed watermelon. J. Food Sci. 2007, 72, 319–323. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, 55–61. [Google Scholar]
- Hwang, D.F.; Lin, J.H.; Cheng, H.M. Level of synthetic antioxidant in cultured fish and fish feed. J. Food Drug Anal. 1995, 3, 27–32. [Google Scholar]
- Yuangsoi, B.; Jintasataporn, O.; Tabthipwon, P.; Mahasawas, S. Optimal Level of Supplemental BHT in Tilapia Diet by Using Tuna Oil. In Proceedings of 41st Kasetsart University Annual Conference, Bangkok, Thailand, 3ߝ7 February 2003; Subject; pp. 78–85.
- Dutta, D.; Chaudhuri, U.R.; Chakraborty, R. Structure, health benefits, antioxidant property and processing and storage of carotenoids. Afr. J. Biotechnol. 2005, 4, 1510–1520. [Google Scholar]
- Salunkhe, D.K.; Bolin, H.R.; Reddy, N.R. Chemical Composition and Nutritional Quality in Storage, Processing and Nutritional Quality of Fruit and Vegetables; Volume 2: Processed Fruit and Vegetables; CRC Press: Boca Raton, FL, USA, 1991; pp. 115–145. [Google Scholar]
- Anderson, J.S.; Sunderland, R. Effect of extruder moisture and dryer processing temperature on vitamin C and E and astaxanthin stability. Aquaculture 2002, 207, 137–149. [Google Scholar] [CrossRef]
- Haaland, H.; Ladstein, K.G.; Oliveira, M.A. Stability of Vitamins and Pigment in Extruded Fish Feed. In Fish Farming Technology; Reinertsen, H., Dahle, L.A., Jorgensen, L., Tvinnereim, K., Eds.; Balkema: Rotterdam, The Netherlands, 1993; pp. 477–479. [Google Scholar]
- Oliveira, R.G.A.; Carvalho, M.J.L.; Nutti, R.M.; Carvalho, L.V.J.; Fukuda, W.G. Assessment and degradation study of total carotenoid and β-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. Afr. J. Food Sci. 2010, 4, 148–155. [Google Scholar]
- Nghia, P.T.; Liem, D.T.; Hai, T.V.; Hoa, T.T.C. Effect of storage conditions on total carotenoid content in golden rice grains. Omonrice 2006, 14, 18–27. [Google Scholar]
- Biacs, P.A.; Czinkotai, B.; Hoschke, A. Factors affecting stability of colored substances in paprika powders. J. Agric. Food Chem. 1992, 40, 363–367. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, B.H. Stability of carotenoids in tomato juice during storage. Food Chem. 2005, 90, 837–846. [Google Scholar] [CrossRef]
- Tang, Y.C.; Chen, B.H. Pigment change of freeze dried carotenoid powder during storage. Food Chem. 2002, 69, 11–17. [Google Scholar]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids. Volume 1B: Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; pp. 13–63. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jintasataporn, O.; Yuangsoi, B. Stability of Carotenoid Diets During Feed Processing and Under Different Storage Conditions. Molecules 2012, 17, 5651-5660. https://doi.org/10.3390/molecules17055651
Jintasataporn O, Yuangsoi B. Stability of Carotenoid Diets During Feed Processing and Under Different Storage Conditions. Molecules. 2012; 17(5):5651-5660. https://doi.org/10.3390/molecules17055651
Chicago/Turabian StyleJintasataporn, Orapint, and Bundit Yuangsoi. 2012. "Stability of Carotenoid Diets During Feed Processing and Under Different Storage Conditions" Molecules 17, no. 5: 5651-5660. https://doi.org/10.3390/molecules17055651
APA StyleJintasataporn, O., & Yuangsoi, B. (2012). Stability of Carotenoid Diets During Feed Processing and Under Different Storage Conditions. Molecules, 17(5), 5651-5660. https://doi.org/10.3390/molecules17055651




