Bio-Functional Constituents from the Stems of Liriodendron tulipifera
Abstract
:1. Introduction

2. Results and Discussion
2.1. Antioxidant Activities and Mushroom Tyrosinase Inhibition of Compounds 1 to 4 from L. tulipifera
| Compounds | DPPH (%) | Chelating (%) | Reducing power (OD700) | Mushroom tyrosinase inhibition (%) |
|---|---|---|---|---|
| Vitamin Ca | 83.6 ± 1.8 | - | - | - |
| EDTAb | - | 92.8 ± 4.5 | - | - |
| BHAc | - | - | 0.98 ± 0.1 | - |
| Kojic acidd | - | - | - | 69.2 ± 0.1 |
| Liriodenine (1) | ns | ns | 0.10 ± 0.0 | 20.5 ± 0.4 |
| (-)-Anonaine (2) | ns | ns | 0.16 ± 0.0 | ns |
| (-)-Glaucine (3) | ns | ns | 0.24 ± 0.0 | ns |
| (-)-Norglaucine (4) | 14.6 ± 3.7 | 14.7 ± 0.8 | 0.22 ± 0.0 | ns |
2.2. Anti-Proliferative Properties of Compounds 1 to 4 from L. tulipifera on A375.S2 Cells
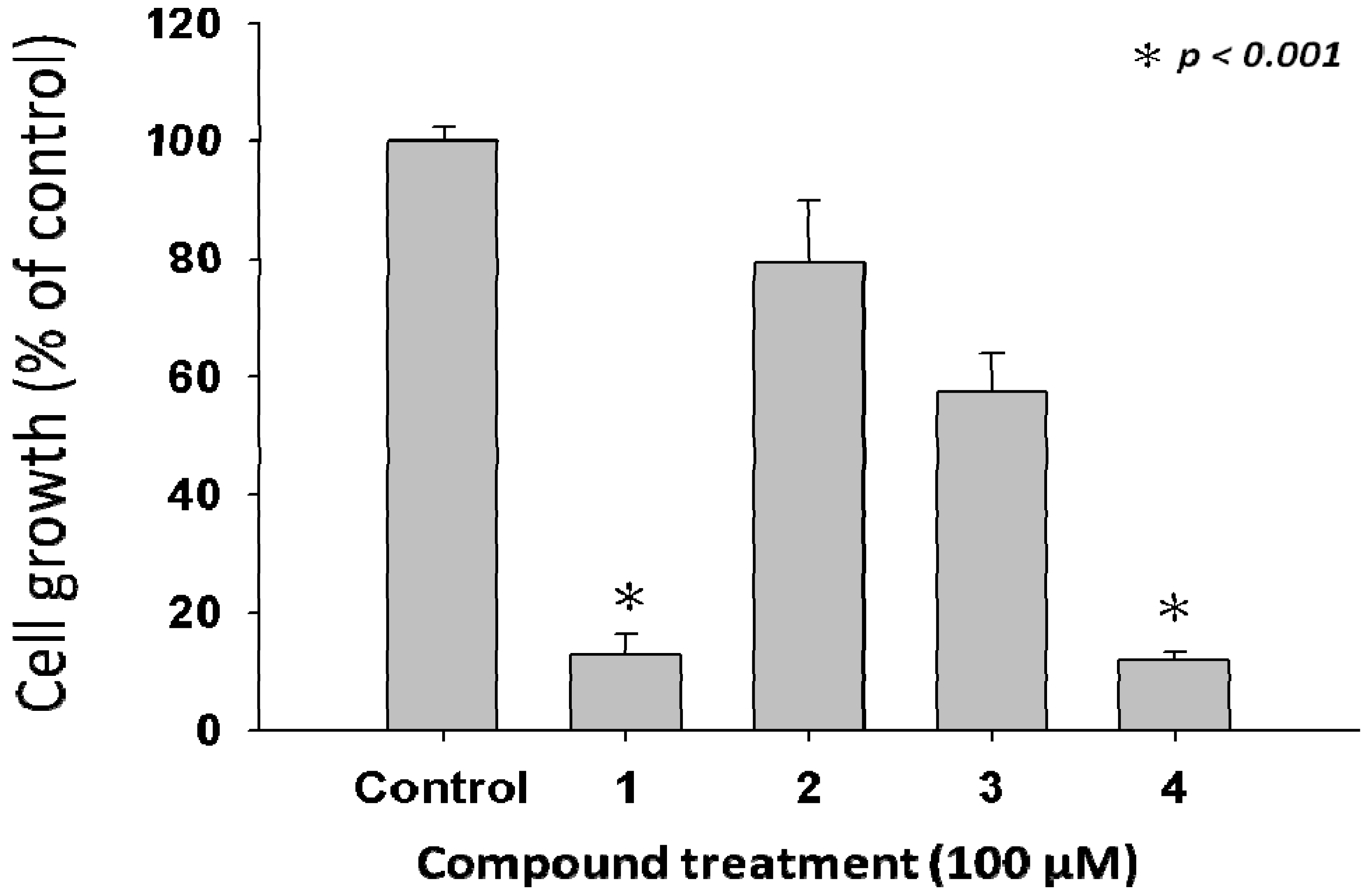
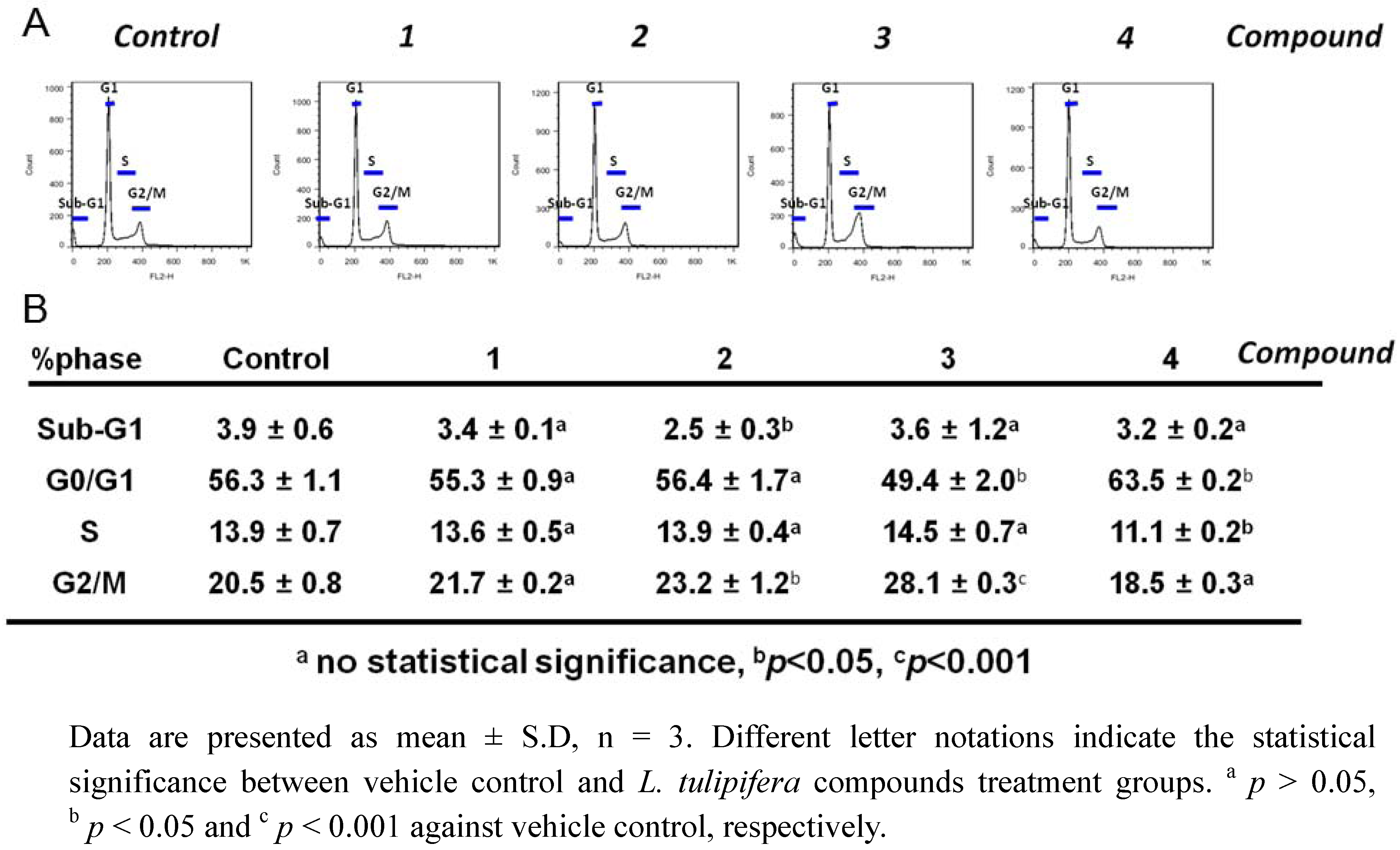
2.3. Compound 3 Causes a Moderate Accumulation of G2/M Phase Population
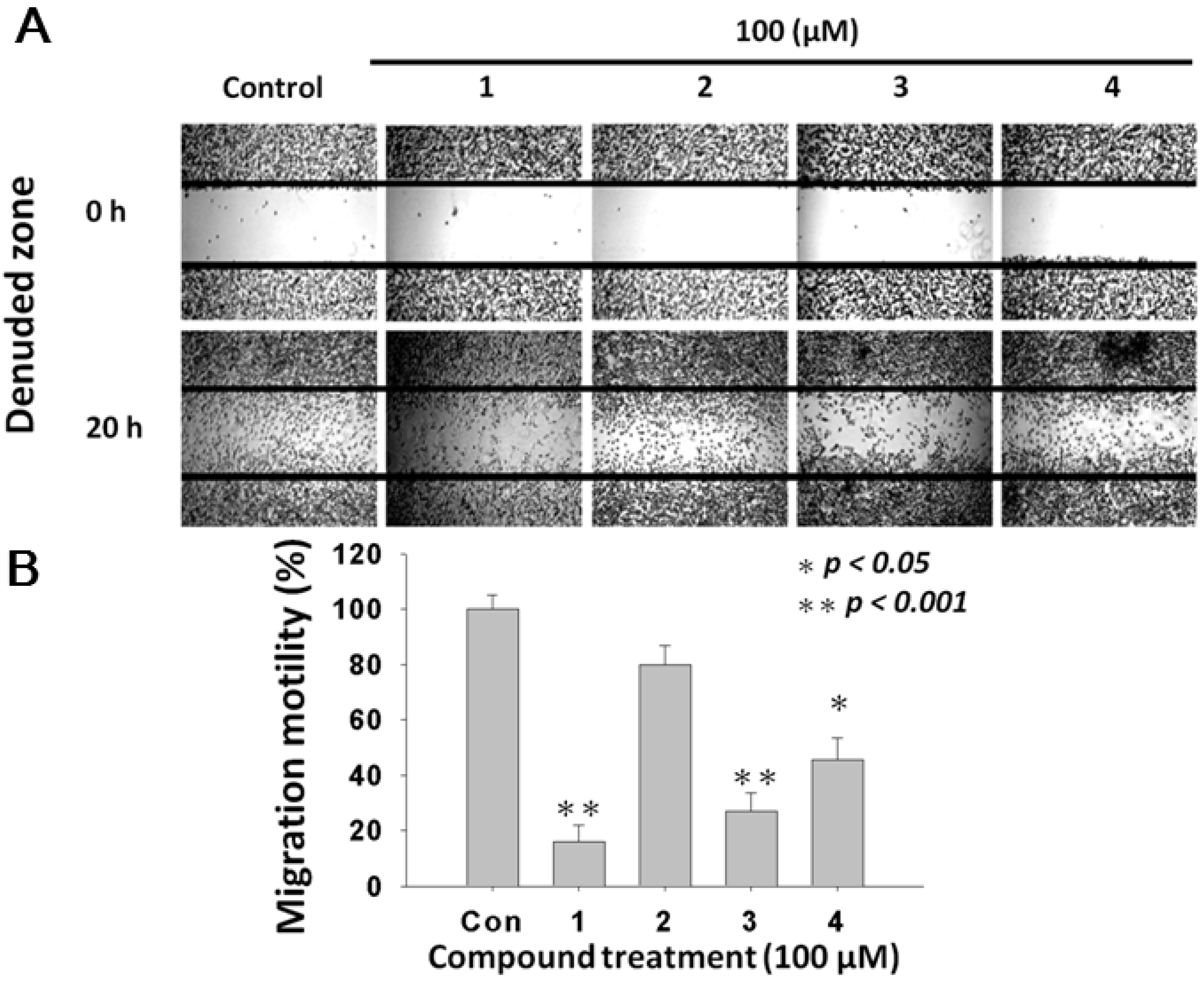
2.4. L. tulipifera Compounds 1–4 Attenuated Migration of A375.S2 Melanoma Cancer Cells
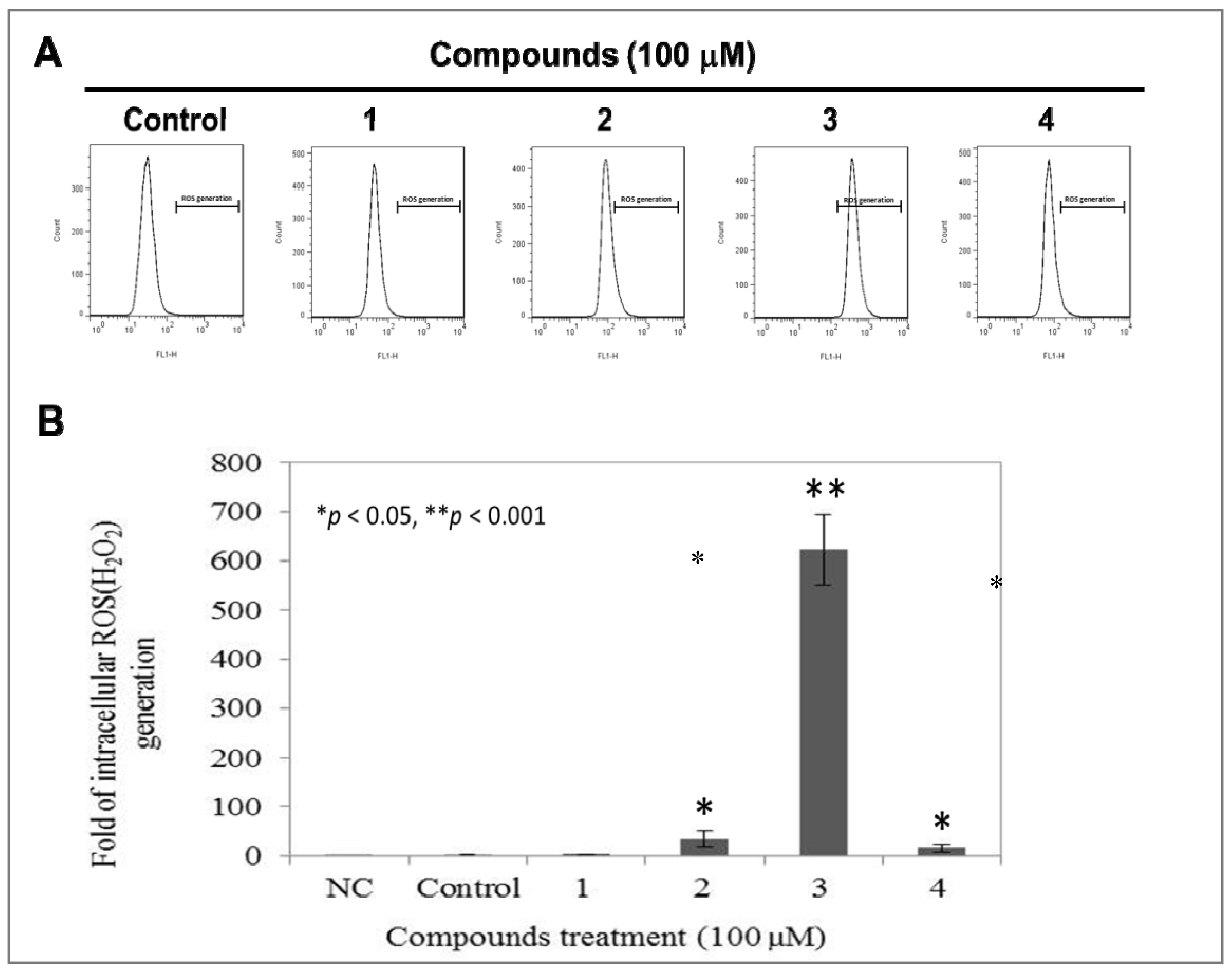
2.5. Compound 3 Causes a Dramatically Increased Level of Intracellular ROS
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction Isolation and Identification
3.4. Determination of DPPH·Radical Scavenging Capacity
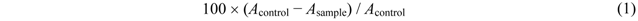
3.5. Metal Chelating Activity
3.6. Reducing Power
3.7. Assay on Mushroom Tyrosinase Activity
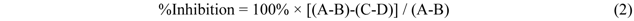
3.8. Cell Culture
3.9. Cell Viability Assay–MTT Assay
3.10. Assessment of Cell Cycle Distribution
3.11. Wound Healing Assay
3.12. Determination of Intracellular ROS
3.13. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Secombe, C.J.; Lester, G.D. The role of diet in the prevention and management of several equine diseases. Anim. Feed Sci. Technol. 2012, in press. [Google Scholar]
- Gil, A.; Ortega, R.M.; Maldonado, J. Wholegrain cereals and bread: A duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr. 2011, 14, 2316–2322. [Google Scholar]
- De Lorgeril, M.; Salen, P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006, 9, 118–123. [Google Scholar]
- Philippe, B.A.; Karine, N.; Barthelemy, A.K.; Noel, Z.G.; David, N.J.; Joseph, D.A.; Hosttetmann, K. Bio-guided isolation of antioxidant compounds from Chrysophyllum perpulchrum, a plant used in the Ivory Coast pharmacopeia. Molecules 2010, 15, 6386–6398. [Google Scholar]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar]
- Hossain, K.M.Z.; Chowdhury, A.M.S.; Haque, M.; Dafader, N.; Akhtar, F. Effect of natural antioxidant (Diospyros peregrina) on the aging properties of radiation vulcanized (γ-radiation) natural rubber latex film. Polym-Plast. Technol. 2010, 49, 136–140. [Google Scholar] [CrossRef]
- Aviram, M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radical Res. 2000, 33, S85. [Google Scholar]
- Calabrese, V.; Scapagnini, G.; Catalano, C.; Dinotta, F.; Geraci, D.; Morganti, P. Biochemical studies of a natural antioxidant isolated from rosemary and its application in cosmetic dermatology. Int. J. Tissue Reactions 2000, 22, 5–13. [Google Scholar]
- Mendiola, J.A.; Martín-Álvarez, P.J.; Señoráns, F.J.; Reglero, G.; Capodicasa, A.; Nazzaro, F.; Sada, A.; Cifuentes, A.; Ibáñez, E. Design of natural food antioxidant ingredients through a chemometric approach. J. Agric. Food Chem. 2009, 58, 787–792. [Google Scholar]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar]
- Qian, Q.; Qian, S.; Fan, P.; Huo, D.; Wang, S. Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: A randomized controlled trial. Phytother. Res. 2012, 26, 60–66. [Google Scholar]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar]
- Nirmala, B.; Hanchinal, R.; Basarkar, P. Antioxidant contents of whole grain cereals, millets and their milled fractions. J. Dairying Foods Home Sci. 2011, 30, 191–196. [Google Scholar]
- Zaknun, D.; Schroecksnadel, S.; Kurz, K.; Fuchs, D. Potential role of antioxidant food supplements, preservatives and colorants in the pathogenesis of allergy and asthma. Int. Arch. Allergy Immunol. 2012, 157, 113–124. [Google Scholar]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Zinkevich, N.S.; Gutterman, D.D. ROS-induced ROS release in vascular biology: Redox-redox signaling. Am. J. Physiol. 2011, 301, H647–H653. [Google Scholar]
- Lin, B.; Chen, Z.; Xu, Y.; Zhang, H.; Liu, J.; Qian, X. 7-b, a novel amonafide analogue, cause growth inhibition and apoptosis in Raji cells via a ROS-mediated mitochondrial pathway. Leukemia Res. 2011, 35, 646–656. [Google Scholar] [CrossRef]
- Wang, H.M.; Chiu, C.C.; Wu, P.F.; Chen, C.Y. Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells. J. Agric. Food Chem. 2011, 59, 8187–8192. [Google Scholar]
- Choi, Y.K.; Seo, H.S.; Choi, H.S.; Kim, S.R.; Shin, Y.C.; Ko, S.G. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol. Cell. Biochem. 2011, 363, 119–128. [Google Scholar]
- Ding, H.; Han, C.; Guo, D.; Chin, Y.W.; Ding, Y.; Kinghorn, A.D.; D’Ambrosio, S.M. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr. Cancer 2009, 61, 348–356. [Google Scholar]
- Adhikary, A.; Mohanty, S.; Lahiry, L.; Hossain, D.M.; Chakraborty, S.; Das, T. Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett. 2010, 584, 7–14. [Google Scholar]
- Chen, W.Y.; Wu, C.C.; Lan, Y.H.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Goniothalamin induces cell cycle-specific apoptosis by modulating the redox status in MDA-MB-231 cells. Eur. J. Pharmacol. 2005, 522, 20–29. [Google Scholar]
- Ortonne, J.P.; Bissett, D.L. Latest insights into skin hyperpigmentation. J. Invest. Dermatol. 2008, 13, 10–14. [Google Scholar]
- Yap, W.; Zaiden, N.; Xu, C.; Chen, A.; Ong, S.; Teo, V.; Yap, Y. Gamma‐and delta‐tocotrienols inhibit skin melanin synthesis by suppressing constitutive and UV‐induced tyrosinase activation. Pigment Cell Melanoma Res. 2010, 23, 688–692. [Google Scholar]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L‐tyrosine and L‐dihydroxyphenylalanine as hormone‐like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2011, 25, 14–27. [Google Scholar]
- Mapunya, M.B.; Hussein, A.A.; Rodriguez, B.; Lall, N. Tyrosinase activity of Greyia flanaganii (Bolus) constituents. Phytomedicine 2011, 18, 1006–1012. [Google Scholar] [CrossRef]
- Kozubek, J.; Altaf, F.; Dadras, S.S. MicroRNA Biomarkers in Melanoma. Diagn. Progn. Biomark. Ther. Targets Melanoma 2012, 113–126. [Google Scholar]
- Ascierto, P.A.; Ascierto, M.L.; Capone, M.; Elaba, Z.; Murphy, M.J.; Palmieri, G. Molecular Pathogenesis of Melanoma: Established and Novel Pathways. In Diagnostic and Prognostic Biomarkers and Therapeutic Targets in Melanoma; Springer: New York, NY, USA, 2012; pp. 19–37. [Google Scholar]
- Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Cutaneous melanoma. Lancet 2005, 365, 687–701. [Google Scholar]
- Boyle, G.M.; Pedley, J.; Martyn, A.C.; Banducci, K.J.; Strutton, G.M.; Brown, D.A.; Breit, S.N.; Parsons, P.G. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J. Invest. Dermatol. 2008, 129, 383–391. [Google Scholar]
- Ivanov, V.N.; Hei, T.K. Regulation of apoptosis in human melanoma and neuroblastoma cells by statins, sodium arsenite and TRAIL: A role of combined treatment versus monotherapy. Apoptosis 2011, 16, 1268–1284. [Google Scholar]
- Engesaeter, B.O.; Sathermugathevan, M.; Hellenes, T.; Engebraten, O.; Holm, R.; Florenes, V.A.; Maelandsmo, G.M. Targeting inhibitor of apoptosis proteins in combination with dacarbazine or TRAIL in melanoma cells. Cancer Biol. Ther. 2011, 12, 47–58. [Google Scholar]
- Wang, H.M.; Chen, C.Y.; Wen, Z.H. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp. Dermatol. 2011, 20, 242–248. [Google Scholar] [CrossRef]
- Wang, H.M.; Cheng, K.C.; Lin, C.J.; Hsu, S.W.; Fang, W.C.; Hsu, T.F.; Chiu, C.C.; Chang, H.W.; Hsu, C.H.; Lee, A.Y.L. Obtusilactone A and (−)‐sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar]
- Kubo, I.; Nitoda, T.; Nihei, K. Effects of quercetin on mushroom tyrosinase and B16-F10 melanoma cells. Molecules 2007, 12, 1045–1056. [Google Scholar]
- Chen, C.Y.; Wang, Y.D.; Juan, S.W.; Huang, J.C. Chemical constituents from the stems of Liriodendron tulipifera. Chem. Nat. Comp. 2011, 47, 1035–1037. [Google Scholar]
- Ryang, S.; Woo, S.; Kwon, S.; Kim, S.; Lee, S.; Kim, K.; Lee, D. Changes of net photosynthesis, antioxidant enzyme activities, and antioxidant contents of Liriodendron tulipifera under elevated ozone. Photosynthetica 2009, 47, 19–25. [Google Scholar] [CrossRef]
- Khamis, S.; Bibby, M.C.; Brown, J.E.; Cooper, P.A.; Scowen, I.; Wright, C.W. Phytochemistry and preliminary biological evaluation of cyathostemma argenteum, a malaysian plant used traditionally for the treatment of breast cancer. Phytother. Res. 2004, 18, 507–510. [Google Scholar]
- Hsieh, T.J.; Liu, T.Z.; Chern, C.L.; Tsao, D.A.; Lu, F.J.; Syu, Y.H.; Hsieh, P.Y.; Hu, H.S.; Chang, T.T.; Chen, C.H. Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food Chem. Toxicol. 2005, 43, 1117–1126. [Google Scholar]
- Graziose, R.; Rathinasabapathy, T.; Lategan, C.; Poulev, A.; Smith, P.J.; Grace, M.; Lila, M.A.; Raskin, I. Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L. J. Ethnopharmacol. 2011, 133, 26–30. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, F.R.; Wu, Y.C.; Lai, Y.H. Anti-cancer effect of liriodenine on human lung cancer cells. Kaohsiung J. Med. Sci. 2004, 20, 365–371. [Google Scholar]
- Wang, H.M.; Chen, C.Y.; Ho, M.L.; Chou, Y.T.; Chang, H.C.; Lee, C.H.; Wang, C.Z.; Chu, I.M. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. 2010, 18, 5241–5247. [Google Scholar]
- Chen, C.Y.; Kuo, P.L.; Chen, Y.H.; Huang, J.C.; Ho, M.L.; Lin, R.J.; Chang, J.S.; Wang, H.M. Tyrosinase inhibition, free radical scavenging, antimicroorganism and anticancer proliferation activities of Sapindus mukorossi extracts. J. Taiwan Inst. Chem. Eng. 2010, 41, 129–135. [Google Scholar] [CrossRef]
- Chiu, C.C.; Liu, P.L.; Huang, K.J.; Wang, H.M.; Chang, K.F.; Chou, C.K.; Chang, F.R.; Chong, I.W.; Fang, K.; Chen, J.S.; Chang, H.W.; Wu, Y.C. Goniothalamin inhibits growth of human lung cancer cells through DNA damage, apoptosis, and reduced migration ability. J. Agric. Food Chem. 2011, 59, 4288–4293. [Google Scholar]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chiu, C.-C.; Chou, H.-L.; Wu, P.-F.; Chen, H.-L.; Wang, H.-M.; Chen, C.-Y. Bio-Functional Constituents from the Stems of Liriodendron tulipifera. Molecules 2012, 17, 4357-4372. https://doi.org/10.3390/molecules17044357
Chiu C-C, Chou H-L, Wu P-F, Chen H-L, Wang H-M, Chen C-Y. Bio-Functional Constituents from the Stems of Liriodendron tulipifera. Molecules. 2012; 17(4):4357-4372. https://doi.org/10.3390/molecules17044357
Chicago/Turabian StyleChiu, Chien-Chih, Han-Lin Chou, Pei-Fang Wu, Hsin-Liang Chen, Hui-Min Wang, and Chung-Yi Chen. 2012. "Bio-Functional Constituents from the Stems of Liriodendron tulipifera" Molecules 17, no. 4: 4357-4372. https://doi.org/10.3390/molecules17044357
APA StyleChiu, C.-C., Chou, H.-L., Wu, P.-F., Chen, H.-L., Wang, H.-M., & Chen, C.-Y. (2012). Bio-Functional Constituents from the Stems of Liriodendron tulipifera. Molecules, 17(4), 4357-4372. https://doi.org/10.3390/molecules17044357





