Facile Synthesis and Herbicidal Evaluation of 4H-3,1-Benzoxazin-4-ones and 3H-Quinazolin-4-ones with 2-Phenoxymethyl Substituents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
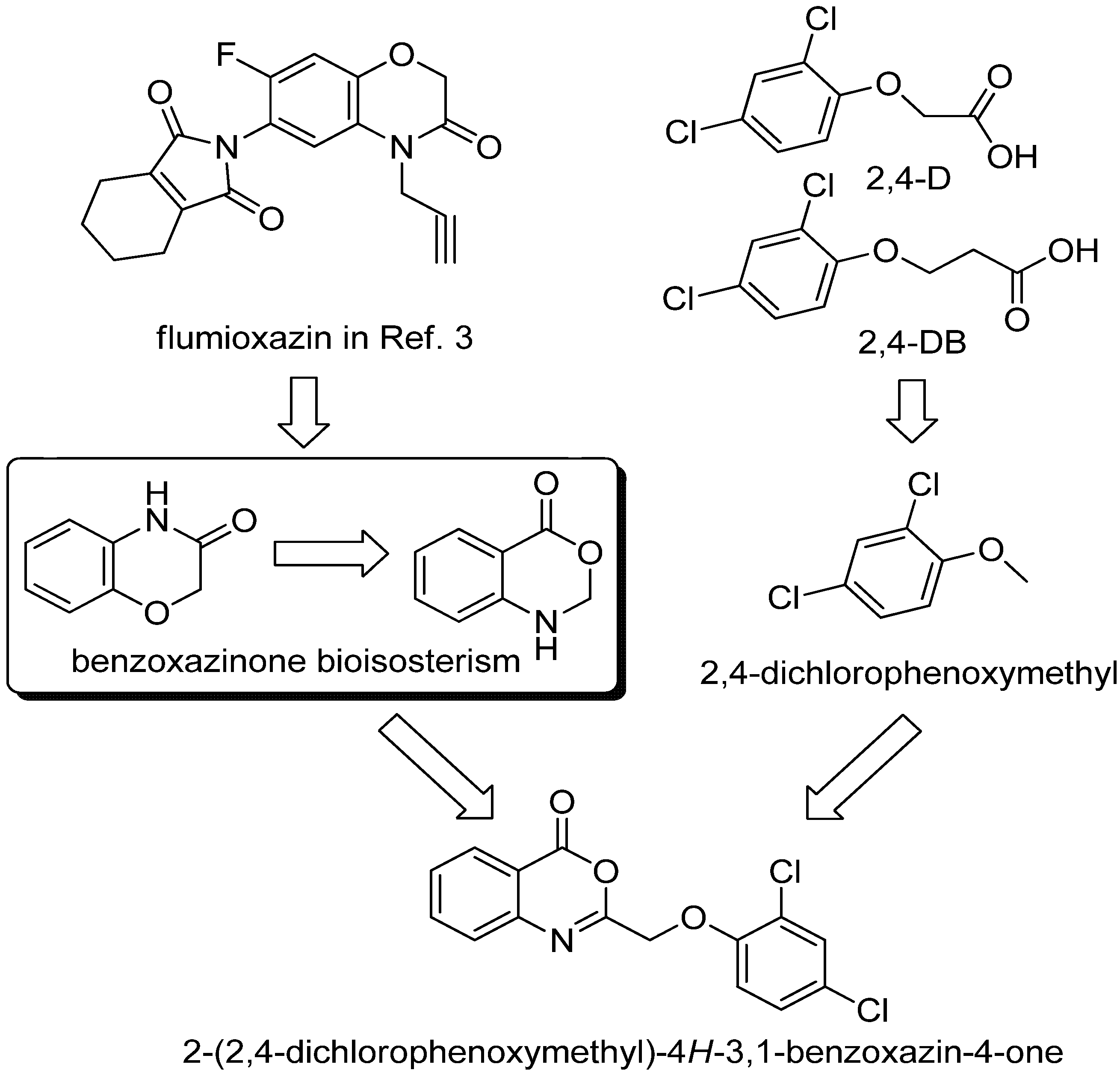
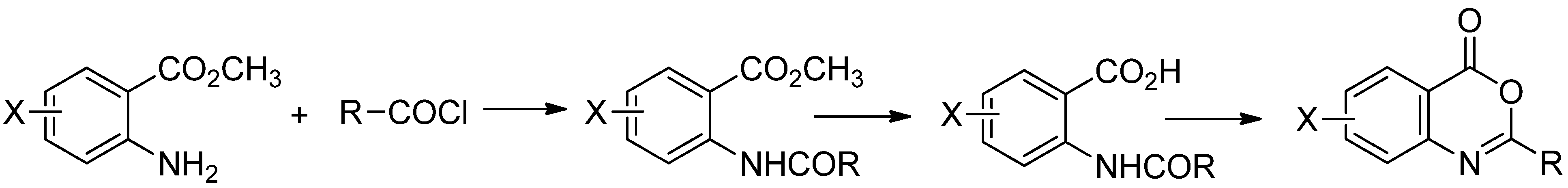
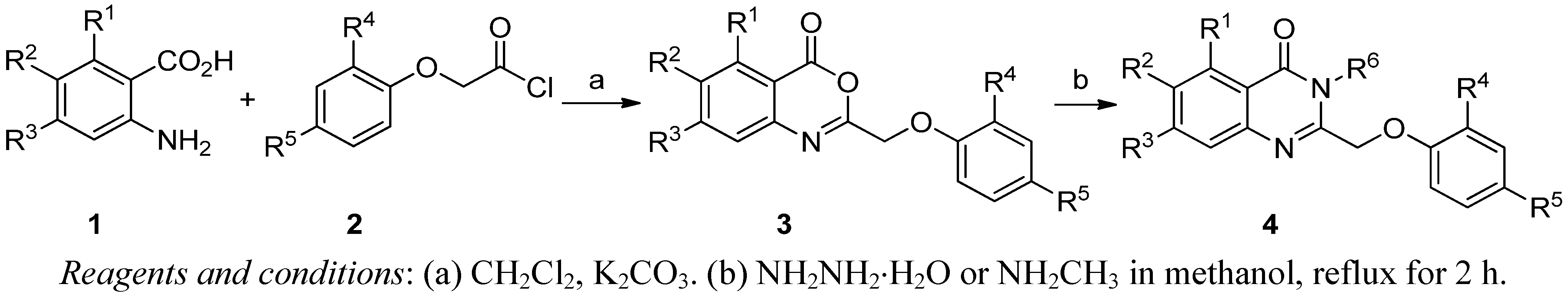
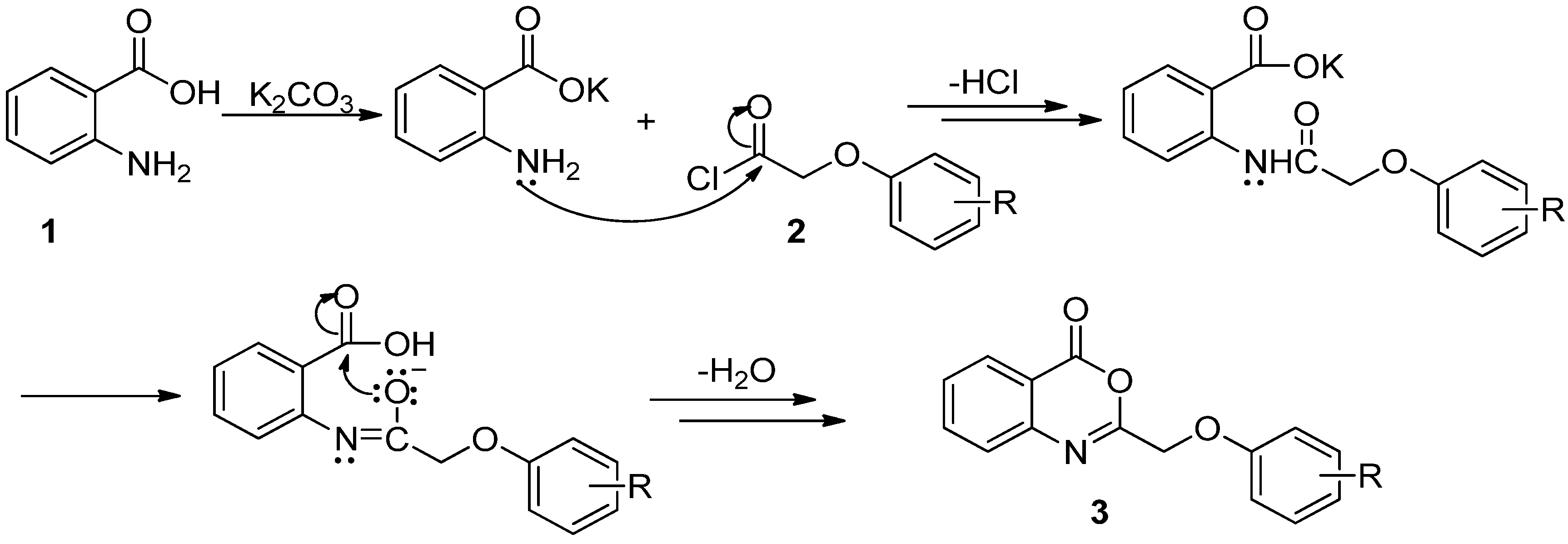
2.2. Herbicidal Activity and Inhibition Phenotype
| No. | R1 | R2 | R3 | R4 | R5 | Relative inhibition (root/stalk%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Barnyard grass | Rape | ||||||||
| 10 mg/L | 1 mg/L | 10 mg/L | 1 mg/L | ||||||
| 3a | H | H | H | H | H | 64.8/26.9 | 19.3/−29.6 | 89.1/79.4 | 71.2/27 |
| 3b | Cl | H | H | H | H | 56.3/24.1 | 47.2/0.5 | 83.3/51.9 | 59.6/39.2 |
| 3c | H | Cl | H | H | H | 62.6/46.7 | 23.7/0.3 | 83.9/64.6 | 45.4/26.7 |
| 3d | H | H | Cl | H | H | 62.3/28.3 | 41.9/−23.6 | 93.5/71.6 | 75.5/44.9 |
| 3e | H | CH3 | H | H | H | 71.4/31.1 | 26.1/−17.2 | 92.3/81.6 | 55.3/11.6 |
| 3f | H | OCH3 | OCH3 | H | H | 63.0/46.7 | 35.5/32.0 | 71.1/63.3 | 94.9/32.9 |
| 3g | H | H | H | H | Cl | 79.5/−1.8 | 53.7/−25.6 | 100.0/68.0 | 98.0/56.8 |
| 3h | Cl | H | H | H | Cl | 88.7/41.9 | 59.8/14.7 | 100.0/91.7 | 96.3/62.0 |
| 3i | H | Cl | H | H | Cl | 87.8/13.2 | 55.3/−7.3 | 100.0/82.1 | 91.4/40.2 |
| 3j | H | H | Cl | H | Cl | 92.3/31.1 | 43.6/12.8 | 100.0/91.1 | 91.4/59.5 |
| 3k | H | CH3 | H | H | Cl | 77.1/−8.8 | 48.4/18.2 | 100.0/82.1 | 84.7/29.3 |
| 3l | H | OCH3 | OCH3 | H | Cl | 78.7/22.0 | 65.5/12.8 | 100.0/83.4 | 96.3/74.5 |
| 3m | H | H | H | Cl | Cl | 100.0/−0.6 | 38.3/−22.7 | 100.0/93.8 | 96.3/77.7 |
| 3n | Cl | H | H | Cl | Cl | 57.5/−1.9 | 44.4/12.8 | 79.2/45.9 | 80.5/38.7 |
| 3o | H | Cl | H | Cl | Cl | 89.4/14.5 | 73.6/−29.1 | 100.0/91.4 | 93.7/66.2 |
| 3p | H | H | Cl | Cl | Cl | 100.0/−14.4 | 64.3/5.9 | 100.0/91.4 | 98.0/82.3 |
| 3q | H | OCH3 | OCH3 | Cl | Cl | 51.9/−52.8 | 35.5/−7.4 | 100.0/85.2 | 58.3/14.8 |
| 3r | H | H | H | H | F | 96.8/57.8 | 62.6/42.8 | 99.6/87.0 | 81.4/55.3 |
| 3s | Cl | H | H | H | F | 91.0/46.3 | 61.1/11.8 | 98.6/94.8 | 72.8/25.7 |
| 3t | H | Cl | H | H | F | 93.2/49.1 | 66.3/36.4 | 99.3/91.7 | 85.5/60.0 |
| 3u | H | H | Cl | H | F | 96.7/39.0 | 61.8/−7.3 | 99.0/92.2 | 92.7/65.2 |
| 3v | H | CH3 | H | H | F | 92.2/31.7 | 69.9/24.1 | 99.3/93.7 | 87.7/61.0 |
| 3w | H | OCH3 | OCH3 | H | F | 64.5/16.4 | 37.5/12.8 | 79.7/41.4 | 35.2/12.8 |
| 2,4-D | 99.1/76.2 | 67.3/45.5 | 100.0/93.6 | 91.0/89.1 | |||||
| No. | R1 | R2 | R3 | R4 | R5 | R6 | Relative inhibition (root/stalk%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Barnyard grass | Rape | |||||||||
| 10 mg/L | 1 mg/L | 10 mg/L | 1 mg/L | |||||||
| 4a | Cl | H | H | H | Cl | NH2 | 43.1/14.9 | 57.9/8.9 | 41.5/−5.9 | 28.5/13.2 |
| 4b | H | Cl | H | H | Cl | NH2 | 9.9/11.9 | 33.2/20.9 | 46.8/13.2 | 27.7/14.7 |
| 4c | H | H | Cl | H | Cl | NH2 | 42.1/17.9 | 10.4/1.4 | 69.7/41.6 | 27.7/−9.3 |
| 4d | H | CH3 | H | H | Cl | NH2 | 35.6/7.4 | 16.3/11.9 | 31.3/-3.4 | 26.4/−14.7 |
| 4e | H | OCH3 | OCH3 | H | Cl | NH2 | 23.3/11.9 | 13.8/2.9 | 30.8/6.4 | 21.9/1.5 |
| 4f | H | H | H | Cl | Cl | NH2 | 22.2/−6.6 | 35.0/−5.0 | 73.4/48.1 | 65.6/48.1 |
| 4g | Cl | H | H | Cl | Cl | NH2 | 25.8/−1.8 | 11.8/−5.9 | 17.5/44.9 | 5.7/10.0 |
| 4h | H | Cl | H | Cl | Cl | NH2 | 37.5/−12.7 | 20.0/−42.0 | 22.0/19.9 | 7.5/10.7 |
| 4i | H | H | Cl | Cl | Cl | NH2 | 68.5/38.1 | 24.3/−7.2 | 98.2/95.7 | 93.6/92.3 |
| 4j | H | OCH3 | OCH3 | Cl | Cl | NH2 | 60.0/5.0 | 7.7/8.3 | 84.9/67.2 | 58.4/40.2 |
| 4k | Cl | H | H | H | Cl | CH3 | 50.9/1.5 | 32.2/7.5 | 81.9/52.9 | 48.8/21.6 |
| 4l | H | Cl | H | H | Cl | CH3 | 33.7/11.9 | 24.7/8.9 | 42.2/10.8 | 28.5/1.5 |
| 4m | H | H | Cl | H | Cl | CH3 | 29.2/14.9 | 14.8/7.5 | 27.9/−0.5 | 26.2/1.5 |
| 4n | H | CH3 | H | H | Cl | CH3 | 43.5/14.9 | 26.7/14.9 | 45.0/16.6 | 39.4/7.8 |
| 4o | H | OCH3 | OCH3 | H | Cl | CH3 | 17.3/5.9 | 13.3/8.9 | 34.8/−6.8 | 25.1/1.9 |
| 4p | H | H | H | Cl | Cl | CH3 | 29.4/−47.3 | 2.5/−10.9 | 21.2/29.2 | 4.5/27.1 |
| 4q | H | Cl | H | Cl | Cl | CH3 | 31.9/−3.6 | 24.3/5.4 | 19.3/38.9 | 8.1/14.4 |
| 4r | H | H | Cl | Cl | Cl | CH3 | 34.6/0.0 | 12.3/1.4 | 44.5/−2.4 | 32.3/−8.8 |
| 4s | H | OCH3 | OCH3 | Cl | Cl | CH3 | 52.2/−54.5 | 30.0/−20.3 | 20.7/20.3 | 7.1/8.1 |
| 2,4-d | 99.1/76.2 | 67.3/45.5 | 100.0/93.6 | 91.0/89.1 | ||||||
| No. | R1 | R2 | R3 | R4 | R5 | R6 | IC50 (μmol) |
|---|---|---|---|---|---|---|---|
| 3g | H | H | H | H | Cl | 30.37 | |
| 3h | Cl | H | H | H | Cl | 138.24 | |
| 3i | H | Cl | H | H | Cl | 22.32 | |
| 3l | H | OCH3 | OCH3 | H | Cl | 80.14 | |
| 3m | H | H | H | Cl | Cl | 10.34 | |
| 3n | Cl | H | H | Cl | Cl | 43.54 | |
| 3o | H | Cl | H | Cl | Cl | 10.73 | |
| 3p | H | H | Cl | Cl | Cl | 11.05 | |
| 3q | H | OCH3 | OCH3 | Cl | Cl | 142.22 | |
| 4f | H | H | H | Cl | Cl | NH2 | 224.37 |
| 4i | H | H | Cl | Cl | Cl | NH2 | 27.23 |
| 4j | H | OCH3 | OCH3 | Cl | Cl | NH2 | 167.59 |
| 4k | Cl | H | H | H | Cl | CH3 | 201.13 |
| 2,4-D | 6.06 |

2.3. Docking Study
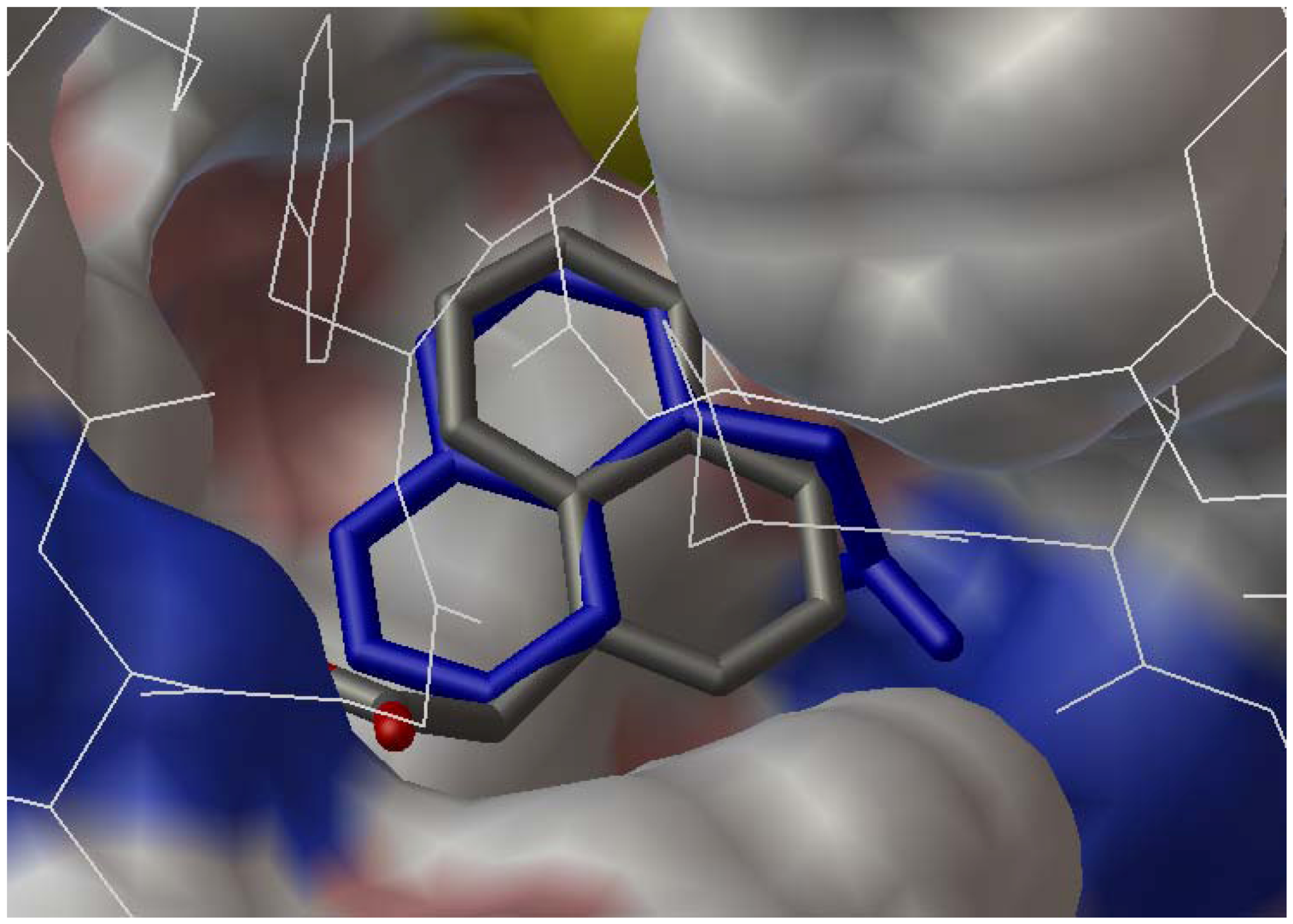
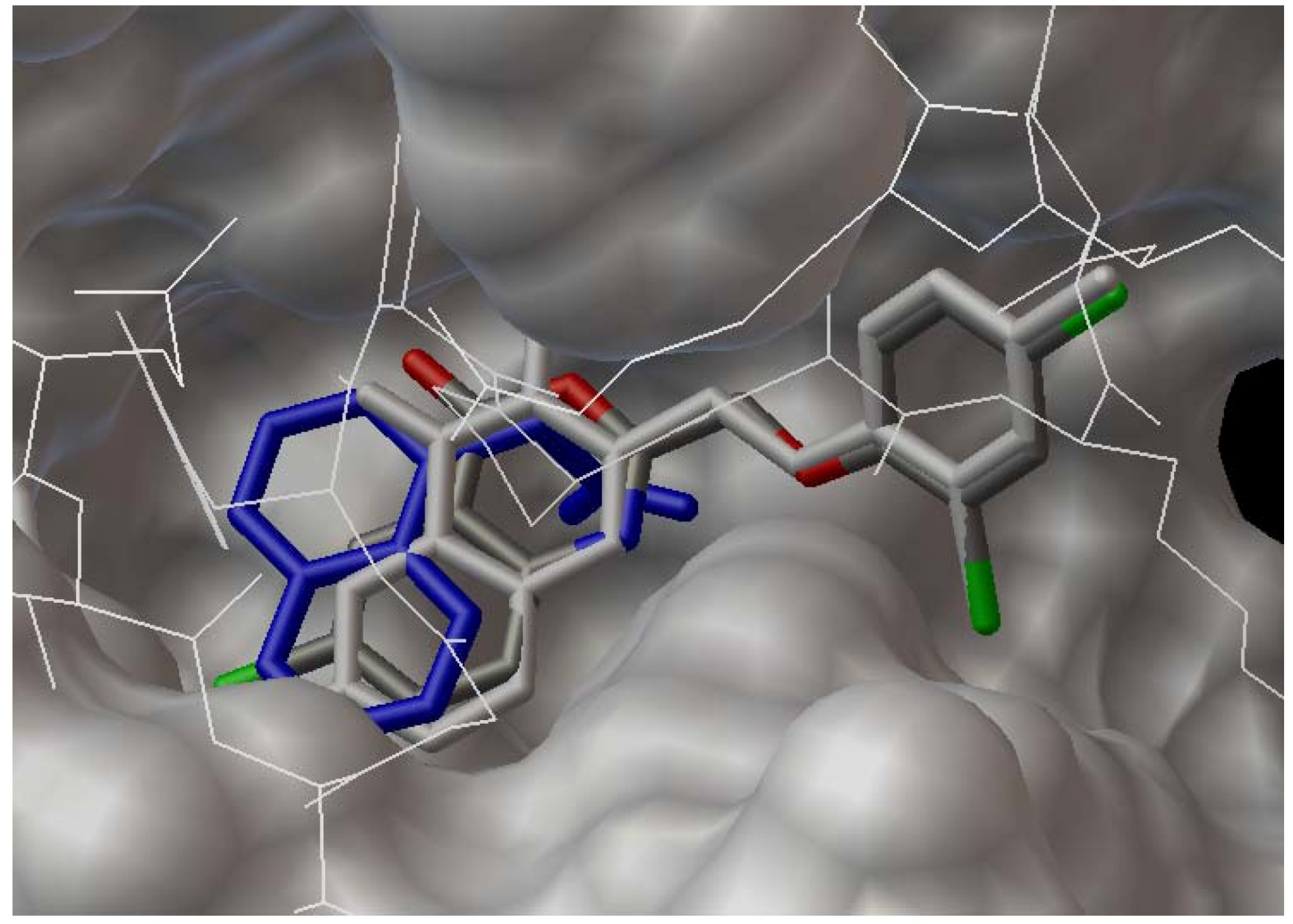
3. Experimental
3.1. General
3.2. Synthesis
| No. | R1 | R2 | R3 | R4 | R5 | Appearance | Mp/°C | Yield/% |
|---|---|---|---|---|---|---|---|---|
| 3a | H | H | H | H | H | pale yellow solid | 87.9~92.1 | 85.6 |
| 3b | Cl | H | H | H | H | white solid | 118.3~120.9 | 87.1 |
| 3c | H | Cl | H | H | H | pale yellow solid | 113.1~115.2 | 79.8 |
| 3d | H | H | Cl | H | H | pale yellow solid | 117.3~119.9 | 87.1 |
| 3e | H | CH3 | H | H | H | pink solid | 133.3~135.1 | 85.2 |
| 3f | H | OCH3 | OCH3 | H | H | pale yellow solid | 132.1~134.3 | 76.1 |
| 3g | H | H | H | H | Cl | white solid | 147.5~149.2 | 89.1 |
| 3h | Cl | H | H | H | Cl | white solid | 144.3~145.7 | 80.1 |
| 3i | H | Cl | H | H | Cl | pale yellow solid | 153.2~154.6 | 79.5 |
| 3j | H | H | Cl | H | Cl | pale yellow solid | 189.9~191.8 | 83.3 |
| 3k | H | CH3 | H | H | Cl | pale yellow solid | 143.7~145.6 | 92.3 |
| 3l | H | OCH3 | OCH3 | H | Cl | white solid | 118.5~119.3 | 90.8 |
| 3m | H | H | H | Cl | Cl | white solid | 118.5~119.3 | 73.5 |
| 3n | Cl | H | H | Cl | Cl | white solid | 161.9~163.2 | 81.1 |
| 3o | H | Cl | H | Cl | Cl | white solid | 157.9~160.1 | 78.6 |
| 3p | H | H | Cl | Cl | Cl | white solid | 139.6~142.5 | 88.1 |
| 3q | H | OCH3 | OCH3 | Cl | Cl | white solid | 161.9~163.2 | 90.2 |
| 3r | H | H | H | H | F | white solid | 112.5~114.6 | 92.2 |
| 3s | Cl | H | H | H | F | white solid | 155.9~156.7 | 77.3 |
| 3t | H | Cl | H | H | F | white solid | 159.8~161.7 | 87.1 |
| 3u | H | H | Cl | H | F | white solid | 123.6~124.9 | 89.9 |
| 3v | H | CH3 | H | H | F | white solid | 130.8~131.9 | 91.4 |
| 3w | H | OCH3 | OCH3 | H | F | white solid | 144.7~146 | 96.2 |
| No. | R1 | R2 | R3 | R4 | R5 | R6 | Appearance | Mp/°C | Yield/% |
|---|---|---|---|---|---|---|---|---|---|
| 4a | Cl | H | H | H | Cl | NH2 | white solid | 191.8~193.3 | 70.1 |
| 4b | H | Cl | H | H | Cl | NH2 | white solid | 178.1~179.4 | 60.7 |
| 4c | H | H | Cl | H | Cl | NH2 | white solid | 129.5~131.6 | 59.1 |
| 4d | H | CH3 | H | H | Cl | NH2 | white solid | 177.3~178.6 | 69.2 |
| 4e | H | OCH3 | OCH3 | H | Cl | NH2 | white solid | 202.8~204.1 | 81.3 |
| 4f | H | H | H | Cl | Cl | NH2 | white solid | 214.6~215.7 | 80.5 |
| 4g | Cl | H | H | Cl | Cl | NH2 | white solid | 201.5~202 | 76.4 |
| 4h | H | Cl | H | Cl | Cl | NH2 | white solid | 223.6~224.9 | 90.5 |
| 4i | H | H | Cl | Cl | Cl | NH2 | white solid | 129.1~131.3 | 85.6 |
| 4j | H | OCH3 | OCH3 | Cl | Cl | NH2 | white solid | 272.3~273.1 | 89.3 |
| 4k | Cl | H | H | H | Cl | CH3 | white solid | 184.9~185.7 | 65.1 |
| 4l | H | Cl | H | H | Cl | CH3 | white solid | 179.9~181.2 | 71.1 |
| 4m | H | H | Cl | H | Cl | CH3 | white solid | 202.4~203.9 | 67.3 |
| 4n | H | CH3 | H | H | Cl | CH3 | white solid | 196.4~197.6 | 68.3 |
| 4o | H | OCH3 | OCH3 | H | Cl | CH3 | white solid | 201.8~202.7 | 70.9 |
| 4p | H | H | H | Cl | Cl | CH3 | white solid | 172.1~173.4 | 81.7 |
| 4q | H | Cl | H | Cl | Cl | CH3 | white solid | 240.1~241.8 | 84.6 |
| 4r | H | H | Cl | Cl | Cl | CH3 | white solid | 210.4~211.4 | 78.9 |
| 4s | H | OCH3 | OCH3 | Cl | Cl | CH3 | white solid | 243.9~245.2 | 91.3 |
3.3. Herbicidal Acitivity Evaluation
3.4. Molecular Docking
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds from the series 3 and 4 are available from the authors.
References and Notes
- Gierl, A.; Frey, M. Evolution of benzoxazinone biosynthesis and indole production in maize. Planta 2001, 213, 493–498. [Google Scholar] [CrossRef]
- Macias, F.A.; Siqueira, J.M.D.; Chinchilla, N.; Marin, D.; Varela, R.M.; Molinillo, J.M.G. New herbicide models from benzoxazinones: Aromatic ring functionalization effects. J. Agric. Food Chem. 2006, 54, 9843–9851. [Google Scholar]
- Huang, M.; Luo, F.; Mo, H.; Ren, Y.; Wang, X.; Ou, X.; Lei, M.; Liu, A.; Huang, L.; Xu, M. Synthesis and herbicidal activity of isoindoline-1,3-dione substituted benzoxazinone derivatives containing a carboxylic ester group. J. Agric. Food Chem. 2009, 57, 9585–9592. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Hwang, T.-L.; Wu, C.-C.; Chang, F.-R.; Wang, T.-W.; Wu, Y.-C. The evaluation of 2,8-disubstituted benzoxazinone derivatives as anti-inflammatory and anti-platelet aggregation agents. Bioorg. Med. Chem. Lett. 2005, 15, 2786–2789. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Yu, H.-P.; Chang, Y.-J.; Hwang, T.-L. Synthesis and evaluation of benzoxazinone derivatives on activity of human neutrophil elastase and on hemorrhagic shock-induced lung injury in rats. Eur. J. Med. Chem. 2010, 45, 3111–3115. [Google Scholar] [CrossRef]
- Anderson, R.; Breazeale, S.; Elich, T.; Lee, S.-F. Modulators of acetyl-coenzyme a carboxylase and methods of use thereof. U.S. Patent US20100009982A1, 14 January 2010. [Google Scholar]
- Stephen, M.G.; Chen, Y.-C.S.; Fabbri, B.J.; Yalamanchili, G.; Hamper, B.C.; Walker, D.M.; Brookfield, F.A.; Boyd, E.A.; Ashton, M.R.; Yarnold, C.J.; CaJacob, C.A. The carboxy terminal processing protease of D1 protein Herbicidal activity of novel inhibitors of the recombinant and native spinach enzymes. Pestic. Biochem. Physiol. 2007, 88, 1–13. [Google Scholar] [CrossRef]
- Lisurek, M.; Rupp, B.; Wichard, J.; Neuenschwander, M.; von Kries, J.P.; Frank, R.; Rademann, J.; Kühne, R. Design of chemical libraries with potentially bioactive molecules applying a maximum common substructure concept. Mol. Divers. 2010, 14, 401–408. [Google Scholar] [CrossRef]
- The World of Herbicides According to HRAC classification on mode of action 2010, a free poster designed and produced by Syngenta. Available online: http://www.hracglobal.com (accessed on 28 February 2012).
- Krantz, A.; Spencer, R.W.; Tam, T.F.; Liak, T.J.; Copp, L.J.; Thomas, E.M.; Rafferty, S.P. Design and synthesis of 4H-3,1-benzoxazin-4-ones as potent alternate substrate inhibitors of human leukocyte elastase. J. Med. Chem. 1990, 33, 464–479. [Google Scholar]
- Shariat, M.; Abdollah, S. Synthesis of benzoxazinone derivatives: A new route to 2-(N-phthaloylmethyl)-4H-3,1-benzoxazin-4-ones. Molecules 2004, 9, 705–712. [Google Scholar] [CrossRef]
- Shishoo, C.J.; Shirsath, V.S.; Rathod, I.S.; Yande, V.D. Design, synthesis and antihistaminic (H1) activity of some condensed 3-aminopyrimidin-4(3H)-ones. Eur. J. Med. Chem. 2000, 35, 351–358. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Wu, C.; Li, H.B.; Zou, X.M. Design, synthesis and quantitative structure-activity relationship study of herbicidal analogues of pyrazolo[5,1-d][1,2,3,5]tetrazin-4(3H)-ones. J. Agric. Food Chem. 2007, 55, 1364–1369. [Google Scholar] [CrossRef]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Tan, X.; Zheng, N.; Hatate, T.; Kimura, Y.; Kepinski, S.; Nozaki, H. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5632–5637. [Google Scholar]
- Tarasov, A.V.; Strikanova, O.N.; Moskvichev, Y.A.; Timoshenko, G.N. Synthesis of 3-(4-Oxo-4H-3,1-benzoxazin-2-yl)-1-benzenesulfonyl Chloride and Its Reactivity toward Amines. Russ. J. Org. Chem. 2002, 38, 87–89. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Al-Omar, M.A.; Abdel-Aziz, A.A.-M.; Abdel-Aziz, N.I.; El-Sayed, M.A.-A.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Abdel-Hamide, S.G. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur. J. Med. Chem. 2010, 45, 4188–4198. [Google Scholar]
- Zhu, Y.Q.; Wu, C.; Li, H.B.; Zou, X.M. Design, synthesis, and quantitative structure−activity relationship study of herbicidal analogues of pyrazolo[5,1-d][1,2,3,5] tetrazin-4(3H)-ones. J. Agric. Food Chem. 2007, 55, 1364. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aibibuli, Z.; Wang, Y.; Tu, H.; Huang, X.; Zhang, A. Facile Synthesis and Herbicidal Evaluation of 4H-3,1-Benzoxazin-4-ones and 3H-Quinazolin-4-ones with 2-Phenoxymethyl Substituents. Molecules 2012, 17, 3181-3201. https://doi.org/10.3390/molecules17033181
Aibibuli Z, Wang Y, Tu H, Huang X, Zhang A. Facile Synthesis and Herbicidal Evaluation of 4H-3,1-Benzoxazin-4-ones and 3H-Quinazolin-4-ones with 2-Phenoxymethyl Substituents. Molecules. 2012; 17(3):3181-3201. https://doi.org/10.3390/molecules17033181
Chicago/Turabian StyleAibibuli, Zumuretiguli, Yufeng Wang, Haiyang Tu, Xiaoting Huang, and Aidong Zhang. 2012. "Facile Synthesis and Herbicidal Evaluation of 4H-3,1-Benzoxazin-4-ones and 3H-Quinazolin-4-ones with 2-Phenoxymethyl Substituents" Molecules 17, no. 3: 3181-3201. https://doi.org/10.3390/molecules17033181
APA StyleAibibuli, Z., Wang, Y., Tu, H., Huang, X., & Zhang, A. (2012). Facile Synthesis and Herbicidal Evaluation of 4H-3,1-Benzoxazin-4-ones and 3H-Quinazolin-4-ones with 2-Phenoxymethyl Substituents. Molecules, 17(3), 3181-3201. https://doi.org/10.3390/molecules17033181




