1. Introduction
In recent years, various preparations based on Danshen (roots of red sage,
Salvia miltiorrhiza Bge.) have become popular for patients with cardiovascular diseases both in China and other countries including the United States [
1,
2,
3]. This herb is one of the most important and highly valued Traditional Chinese Medicines (TCMs) and it has been used in the treatment of numerous ailments, including cardiovascular disease, for about 2,000 years. Its pronounced efficacies in improving microcirculation, causing coronary vasodilatation, suppressing the formation of thromboxane, inhibiting platelet adhesion and aggregation, and protecting against myocardial ischemia have attracted worldwide attention [
1,
2,
3]. The bioactivity of this herb is ascribed to an array of components, including hydrophilic caffeic acid derivatives (CaDs) and dozens of lipophilic tanshinones (TNs) [
4,
5,
6,
7]. Both of them contribute to its cardioprotective effects, but show significant mechanistic and temporal differences [
1,
8]. The predominant CaDs is salvianolic acid B (SaB, 1) and the major TNs are dihydrotanshinone I (dTN, 2), cryptotanshinone (cTN, 3), tanshinone I (TNI, 4) and tanshinone IIA (TNIIa, 5) (
Figure 1) [
7,
9].
Figure 1.
Structures of the five major compounds of Danshen: (1) salvianolic acid B (SaB); (2) dihydrotanshinone I (dTN); (3) cryptotanshinone (cTN); (4) tanshinone I (TNI); (5) tanshinone IIA (TNIIa).
Figure 1.
Structures of the five major compounds of Danshen: (1) salvianolic acid B (SaB); (2) dihydrotanshinone I (dTN); (3) cryptotanshinone (cTN); (4) tanshinone I (TNI); (5) tanshinone IIA (TNIIa).
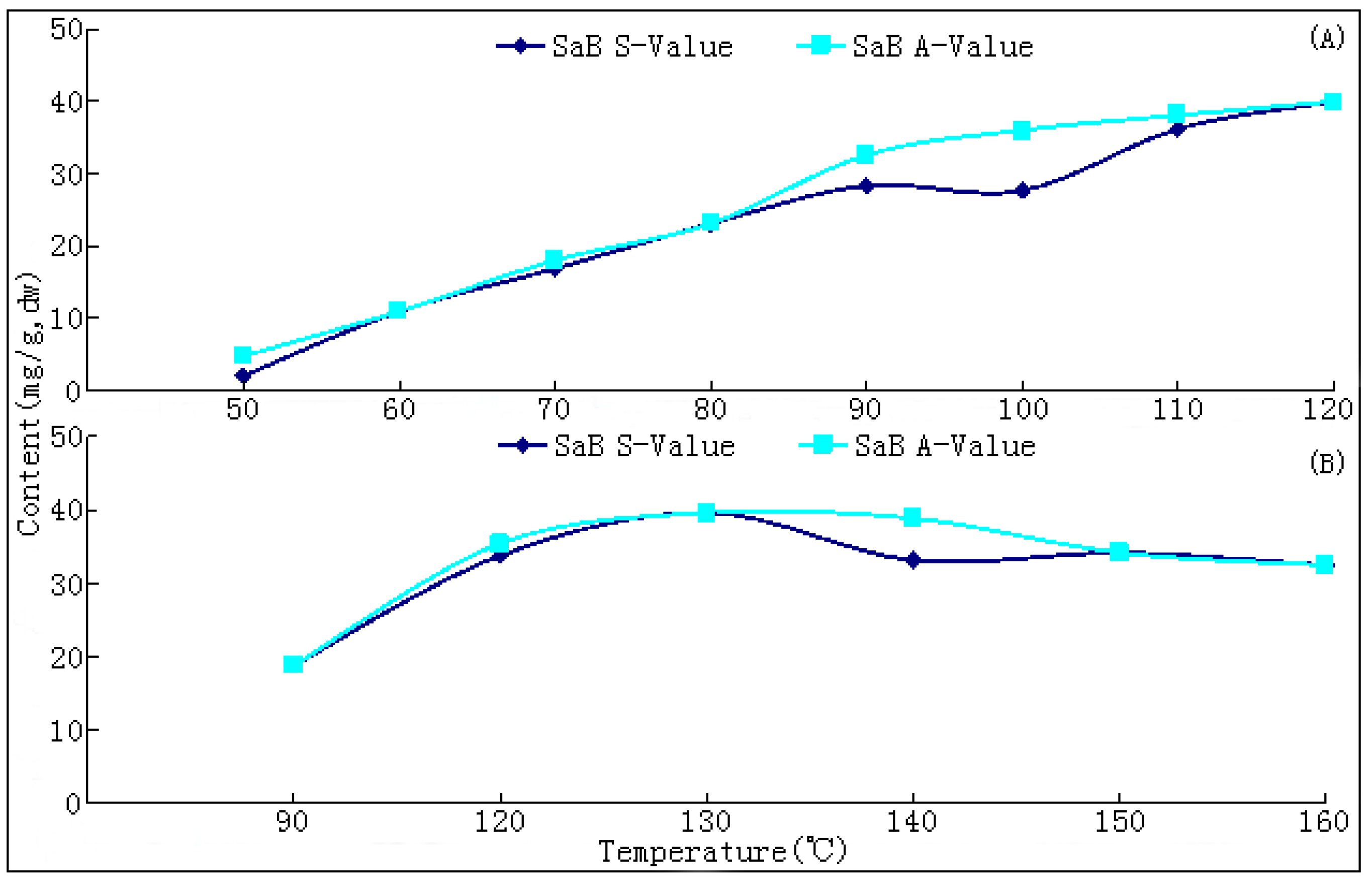
The commercial materials of this herb are standardized by values of SaB (≥3.0%) and TNIIa (≥0.2%) according to the 2010 Chinese Pharmacopoeia [
10]. Up to now, a number of reports on quality evaluations of Danshen and its preparations have been published [
7]. These reports demonstrated that values of SaB and TNIIa as well as other ingredients in roots of
S. miltiorrhiza and its based preparations varied significantly [
7,
9,
11]. Reasons for these variations were usually ascribed to differences in germplasms and environmental/climate factors of the cultivation [
12], or were blamed to the sensitivity of CaDs and TNs to light and temperature [
13]. The great variation in qualities of Danshen would certainly affect the qualities and clinical efficacies of its products and derived preparations, and therefore has become one of the most important problems pharmacognostic researches tried to overcome.
Drying is the most common and elementary procedure in the post-harvest process which affects the quality and value of medicinal plants [
14,
15]. The general belief is that levels of bioactive components in medicinal plants were a pre-harvest accumulation and were decreased in the post-harvest drying process along as the temperature increased and the duration was prolonged [
16,
17]. Therefore, the fundamental target of research on drying processes for medicinal plants up to now was how to best retain the initial levels of bioactive ingredients, and hence the freeze-drying protocol was recommended as the most suitable method [
18]. However, from the view of plant physiology, the newly harvested fresh plant materials, especially roots, are still physiologically active organs and the drying process is a
bona fide dehydration stress to these organs. Thus, the post-harvest drying process, especially at its early stage, could induce a series of anti-dehydration mechanisms including the production or increase of related secondary metabolites of these organs [
19,
20]. That is to say, pharmacologically important ingredients of medicinal plants might emerge or increase during a certain period of drying in post-harvest processing. The drying-induced increase of bioactive components might especially be true for some root materials and some types of secondary metabolites with important ecological functions [
19,
20]. This physiological peculiarity of the post-harvest plant materials has not be documented aside from the mention of its role in preventing water loss in the drying of fresh ginseng [
21].
To test this hypothesis, we carried out a series of exploratory works on the post-harvest TCM drying process. Here, we displayed one of the important findings on roots of
S. miltiorrhiza. Like most other TCMs, the universally applied way to dry this herb is through a sun-curing process. Usually, this process will be prolonged for at least one month to reach the standard level of moisture (≤13%) as documented in the Chinese Pharmacopoeia [
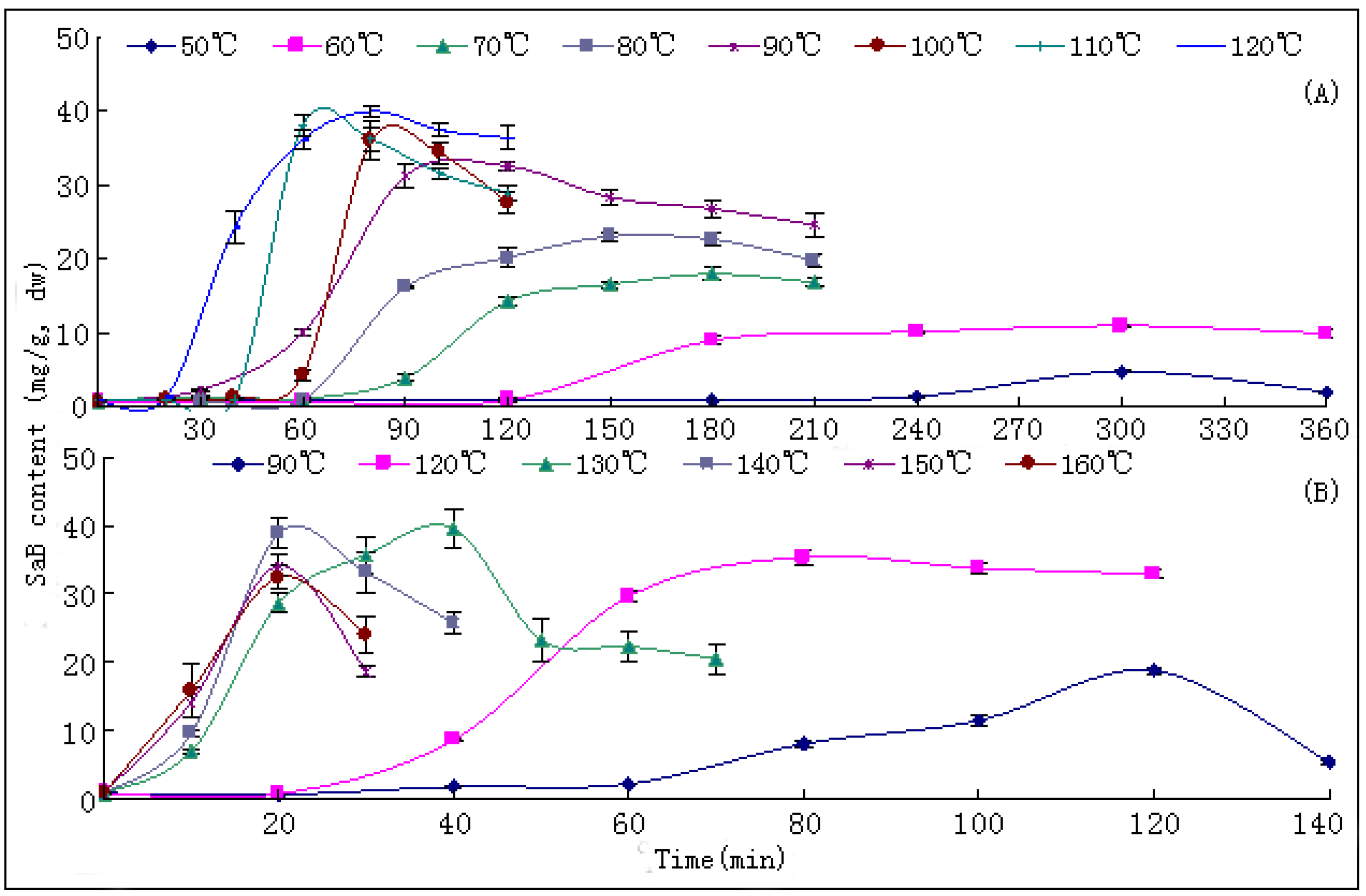
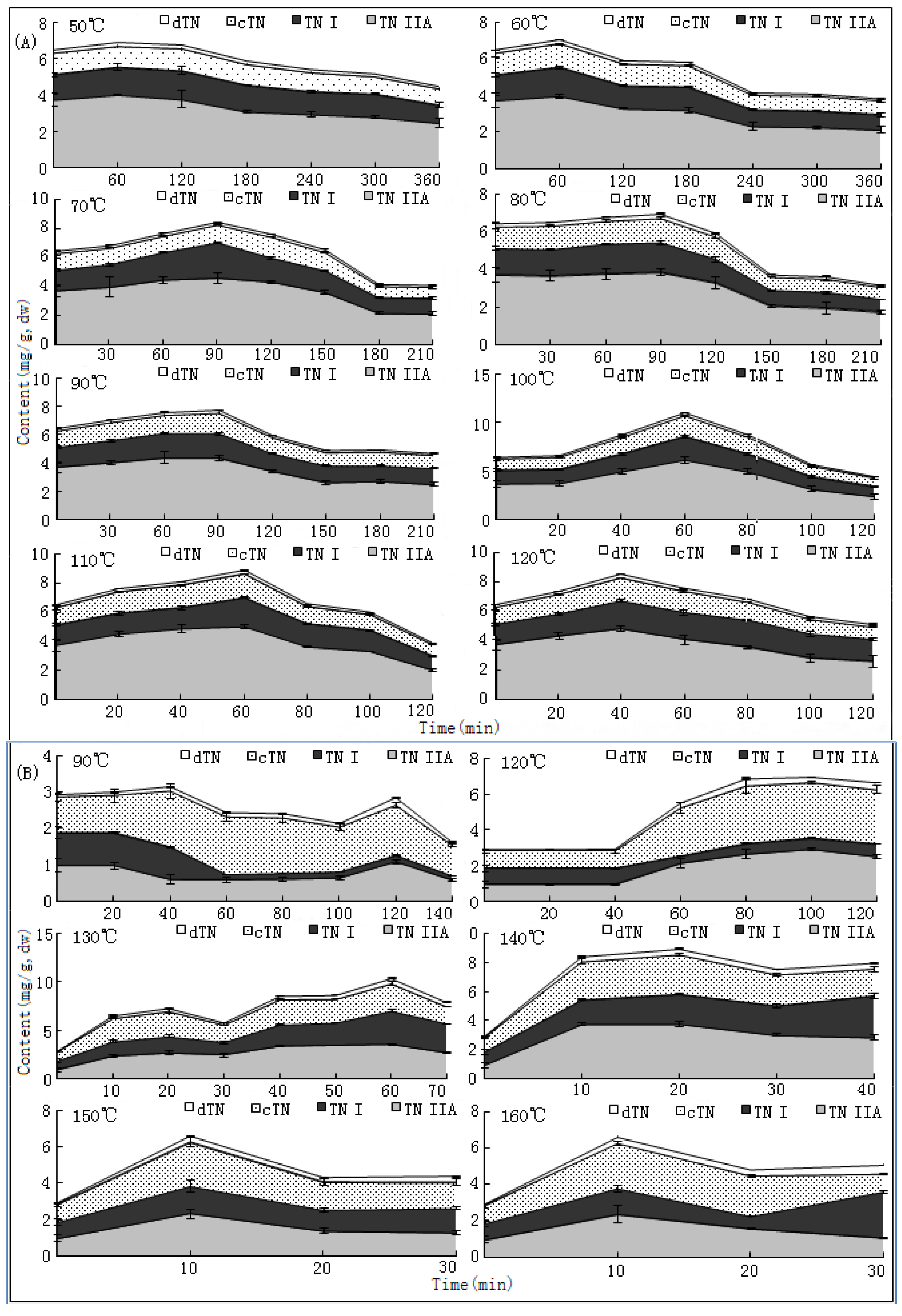
10]. The changing trends of bioactive components during this process have not been reported hitherto, nor have researches on the ideal method for drying this herb. In the present paper, we report that SaB, the most abundant and important active ingredient of Danshen, was unexpectedly a product of the post-harvest drying process. The values of major tanshinones were also obviously increased during the drying process period. The results were of great value for promoting and stabilizing the quality of Danshen, and were also helpful for guiding similar investigations on other TCMs.
3. Experimental
3.1. Plant Materials
Experiments in this paper were separately conducted by two graduate students, Mr. Xiao-Bing Li (T1) and Mr. Wei Wang (T2), during 2007~2010. Fresh root samples of S. miltiorrhiza (each about 20 Kg) were collected from Linqu County, Shandong Province of China, during the harvest season in October 1~15 of 2007 and 2009. The fresh roots were taken to the laboratory within two days and immediately sliced in 2~3 mm pieces. The sliced materials were mixed well and stored at −20 °C in a refrigerator until assay. The samples were authenticated by Dr. Tong-Shui Zhou and the voucher specimens were deposited in the Herbarium of Fudan University, Shanghai, China.
3.2. Drying Process and Temperature Design
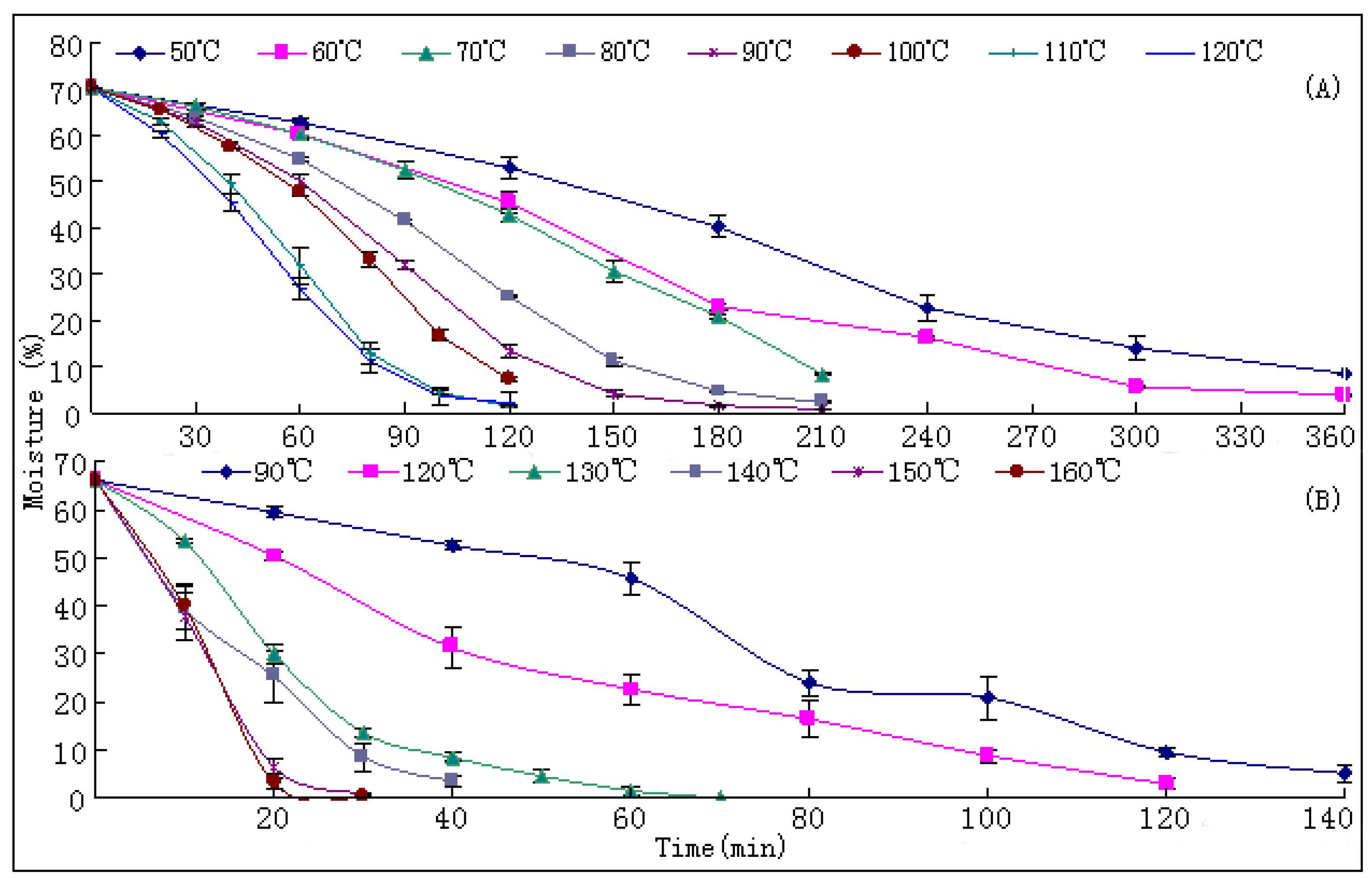
The drying process of the frozen sliced materials was conducted in a thermostatic oven. In T1, eight groups of samples were dried at temperatured from 50~120 °C in 10 °C intervals, respectively. Accurately weighted materials (30.0 g) were evenly placed in a ф 9.0 cm watch-glass. The oven was preheated to the set temperature, put into the materials and the experimental duration was started. The amount of materials and watch-glasses for each test group were carefully designed according to the sampling interval and frequency at each drying temperature. For each sampling point, two batches each in three watch-glasses were randomly extracted from the oven during the drying process. The sampled watch-glasses were placed in a desiccator for cooling and then, the contained samples were pulverized separately (80 mesh) and weighed accurately. These samples were used for the determination of moisture and contents of analytes, respectively. The sampling interval and frequency at each drying temperature were determined according to the difference of drying efficiency at each temperature, i.e.,1 h intervals in total 6 h for 50 and 60 °C; 30 min intervals in total 3.5 h for 70, 80 and 90 °C; and 20 min intervals in total 2 h for 100, 110 and 120 °C. In T2, six further groups of tests from 90~160 °C were designed to determinate the inflection point of SaB change. The drying and sampling procedures were consistent with T1.
3.3. Determination of Moisture
Moisture of samples from different sampling points was determined in triplicate by Method I as described in the 2010 Chinese Pharmacopoeia [
10]. Accurately weighed powdered samples were dried at 105 °C in an oven at least for 5 h until a constant weight was obtained. After cooling in a desiccator, they were accurately weighed and the loss of water calculated.
3.4. Determination of Analytes
The contents of five major bioactive components in Danshen, namely SaB (
1), dTN (
2), cTN (
3), TNI (
4) and TNIIa (
5), were determined by high performance liquid chromatography (HPLC). Details of the method have been published previously [
22], and are briefly described below:
Standard chemicals and reagents. High purity (>98%) cTN, dTN, TNI and TNIIa (purified from the dried roots of S. miltiorrhiza in our laboratory) and SaB (purchased from Shanghai R&D Center for Standardization of TCMs, China) were used as standards for quantitative analysis. HPLC grade acetonitrile and trifluoroacetic acid (TFA) were products of Merck (Darmstadt, Germany). Triple distilled water produced using an 1810D water distiller (Shanghai Shenke Ltd., Shanghai, China) was used for all extraction and separation procedures. All other reagents were of analytical grade and obtained from local companies.
Apparatus and chromatographic conditions. An Agilent Series 1100 LC instrument (Agilent Technologies, Waldbronn, Germany) equipped with an on-line degasser, a quaternary pump, a diode-array detector (DAD) and a 20 µL sample loop manual injector was used for sample analysis. The equipment was automatically controlled by ChemStation (Rev.A. 07.01) software. The column configuration was an YMC-Pack Pro C18 fast analysis column (3 µm, 4.6 mm × 150 mm) connected to an Industries C18 guard column (5 µm, 4.0 mm × 20 mm). The mobile phase consisted of solvent A (acetonitrile) and B (0.1% aqueous trifluoroacetic acid (aTFA), v/v). A gradient elution program was used as follows: 20–27% A (v/v) at 0–14 min, 27–47% A (v/v) at 14–15 min, 47–52% A (v/v) at 15–31 min and 52–85% A (v/v) at 31–44 min. The flow rate was 1.0 mL/min and the injection volume was 20 µL. Re-equilibration duration was 10 min between individual runs. The diode-array detector was set at 280 nm for SaB and 254 nm for tanshinones, respectively.
Calibration and method validation. Standard stock solutions of the five analytes were prepared by dissolving the accurately weighed dTN (2.1 mg), cTN (5.2 mg), TNI (3.2 mg), TNIIa (2.6 mg) and SaB (8.5 mg) in a 10 mL volumetric flask with 70% aqueous methanol (aM). The solution was then diluted with 70% aM to appropriate concentrations for the assessment of linearity and method validation. Seven concentration levels were prepared for calibration and linear analysis of each standard compound. Peak area (Y) and concentration (X) for each compound were analyzed to calculate the calibration curve and correlation coefficient (r). The LODs and LOQs under the present chromatographic conditions were determined at a signal-to-noise (S/N) ratio of 3 and 10, respectively. The recovery tests were assessed with an appropriate amount of herb sample spiked with three different quantities (low, medium and high) of authentic standards. The intra-day variability was examined five times within one day, and the inter-day precision was calculated from nine determinations over three days (three determinations per day) for low, medium and high concentrations of authentic standard solutions. All standard solutions of various concentrations were stored at 4 °C in a refrigerator until assayed. Each test was analyzed in triplicate.
Sample assay. Accurately weighed (~0.50 g) powders (80 mesh) of samples were extracted twice with 70% aM (20 mL) in an ultrasonic bath at room temperature for 20 min. The supernatants after centrifugation (3000 rpm for 10 min) were combined and diluted with 70% aM to 50 mL. The solutions were filtered through a 0.45 µm nylon syringe filter (Millex-HN, Massachusetts, USA) before HPLC analysis. Triplicate samples for each sampling point were analyzed.
3.5. Statistic Analysis
All data were the mean values of three independent experiments. The statistic analysis, i.e., the least significance difference and the bivariate correlation, was conducted using the SPSS 11.0 program.